Agricultural Sciences
Vol.08 No.04(2017), Article ID:75686,25 pages
10.4236/as.2017.84020
Effect of Plant Growth Stimulants on Alfalfa Response to Salt Stress
Mahmoud El-Sharkawy1, Talaat El-Beshsbeshy1, Rania Al-Shal1, Ali Missaoui2*
1Soil Science and Water Department, Faculty of Agriculture, Tanta University, Tanta, Egypt
2Department of Crop and Soil Sciences, Institute of Plant Breeding Genetics and Genomics, University of Georgia, Athens, USA

Copyright © 2017 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/



Received: March 17, 2017; Accepted: April 24, 2017; Published: April 27, 2017
ABSTRACT
Salinity is a major impediment to crop production. This study was undertaken to compare the effect of seaweed extract, humic acid, and potassium sulfate nanoparticles in alleviating salt stress in Alfalfa (Medicago sativa L.). Seeds of ten alfalfa genotypes were germinated in a growth chamber at five salt concentrations (0%, 0.5%, 1.0%, 1.5%, and 2.00%). Salt concentrations above 1% reduced seed germination by more than 70% in most genotypes. One salt tolerant genotype (Mesa-Sirsa) and one salt sensitive (Bulldog 505) were selected and planted in greenhouse pots containing 2 kg of sand and subjected to two salt levels (10 and 15 dS・m−1). Four treatments consisting of 1) control (Hoagland solution, no-salt), 2) seaweed extract at 4 Kg・ha−1, 3) humic acid at 28 L・ha−1, and 4) potassium sulfate at 300 Kg・ha−1. Plant biomass was reduced under both salt concentrations in both genotypes, with a greater magnitude in the salt sensitive genotype. Application of seaweed extract resulted in higher relative water content and proline under both salt concentrations (10 and 15 dS・m−1) in the salt sensitive genotype, and lower electrolyte leakage in both salt tolerant and salt sensitive genotypes, under both salt concentrations. Seaweed extract also resulted in higher catalase and SOD activities in both genotypes under 10 dS・m−1. Catalase and SOD activities were associated with significantly (p < 0.01) reduced electrolyte leakage and increased shoot dry weight. Overall, seaweed extract seemed to have a positive effect in alleviating salt stress in alfalfa.
Keywords:
Biological Growth Stimulants, Humic Acid, Salt Stress, Seaweed Extract, Potassium Nanoparticles

1. Introduction
About 900,000 ha of Egypt’s agricultural lands are suffering from salinity build- up problem [1] . The majority of salt-affected areas are located in the northern- central part of the Nile Delta and on its eastern and western sides. Sixty percent of the cultivated land of the Northern Delta region, 20% of the Southern Delta and Middle Egyptian region, and 25% of the Upper Egypt regions are salt-affec- ted [2] . Salinity is one of the most limiting factors to crop production in arid and semiarid regions of the world. Globally, it affects an estimated 6% of the world’s land area or 12,780 million hectares (Mha), in addition to an estimated 20% of irrigated land impacted by secondary salinization resulting from irrigation [3] [4] . Salinity reduces plant growth and production by affecting physiological processes, including disruption of ionic equilibrium, water status, mineral nutrition, stomatal behavior, and photosynthetic efficiency [5] . Disparity in Osmotic potential leads to water deficit, reduced leaf area expansion and stomatal closure, which ultimately reduces photosynthesis and plant growth [6] . The ionic disequilibrium causes excessive accumulation of Na+ and Cl− in the older leaves, leading to their premature senescence [6] [7] , in addition to creating an ionic imbalance that reduces the uptake of beneficial ions such as K+, Ca2+, and Mn2+ [8] and inhibits photosynthesis and enzyme activities [9] .
Several studies carried out to elucidate salinity tolerance mechanisms included the application of biological stimulants and organic acids [10] . Seaweed extract is considered a source of organic matter and nutrients and is used as a soil conditioner [11] [12] . Seaweed extracts have been used in agriculture and horticulture as bio-stimulants to promote plant growth and increase crop yields [13] . Zhang and Ervin [14] provided evidence that plant performance was improved under water stress conditions upon treatment with seaweed extracts. Seaweeds have been reported to possess plant-growth promoting activity which made them relevant in agriculture and horticulture as organic fertilizers [15] . Fike et al. [16] reported that seaweed extract derived from A. nodosum contains various compounds including amino acids and micronutrients. The application of Seaweed extract on vegetables and forage grasses increased root length, leaf area, and root and shoot biomass in response to drought stress, in addition to increasing chlorophyll and carotenoids [17] . In general, seaweed extracts are capable of inducing an array of physiological plant responses, such as the promotion of plant growth, improvement of flowering and yield, and enhancing nutritional quality of edible products as well as shelf life, even at low concentrations. Furthermore, applications of different extract types have been reported to enhance plants’ tolerance to a wide range of abiotic stresses, such as salinity, drought, and temperature extremes [12] . The effect of seaweed extracts in alleviating salt stress in Alfalfa has not been investigated. Having been derived from a renewable resource, the bioactive extracts from seaweed could be useful on a large scale to improve alfalfa productivity and sustainability of agricultural systems if proven effective in overcoming salt stress.
Humic substances have shown anti-stress effects under abiotic stress conditions such as unfavorable temperature, salinity, and low pH [18] . Humic acid promotes plant growth by enhancing the uptake of nutrients and reducing the uptake and accumulation of some toxic elements. Increasing cell membrane permeability, oxygen uptake, photosynthesis, phosphate uptake, and root cell elongation are some of the factors suggested to explain the positive effect of humic acid on plant growth [19] . Humic acid has the ability to chelate various nutrients including Na, K, Mg, Zn, Ca, Fe, and Cu in order to overcome nutrient deficiencies [20] [21] .
Plants require K for a number of important physiological processes including the activation of various enzymes, the synthesis and metabolism of carbohydrates, protein synthesis, and the opening and closing of stomata thus controlling gas exchange and photosynthesis [22] . The application of K+ has been shown to mitigate the adverse effects of salinity through its roles in stomatal regulation, osmotic adjustment, and maintenance of the membrane ion-charge balance, cellular-energy status, and protein synthesis [23] .
Alfalfa (Medicago sativa L.), is an important forage crop grown over 32 million hectares globally [24] . It is a high yielding perennial forage grown in different climates all over the world [25] . Alfalfa is highly sought after for its high crude protein content and total digestible nutrients [26] [27] . Alfalfa has been characterized as moderately sensitive to salts with an electrical conductivity (EC) of 2.0 dS・m−1 (1280 ppm) and a threshold of 1.5 bars (1 bar = 0.987 atmospheres) osmotic potential of soil solution at field capacity [28] .
The primary objective of this study was to explore the effect of different growth-enhancing substances, such as seaweed extract, humic acid, and potassium sulfate, on alfalfa growth and physiological response under salt stress.
2. Materials and Methods
2.1. Alfalfa Seed Germination under Salt
Alfalfa seeds were germinated in 100-mm petri plates containing a single piece of Whatman No. 2 filter paper imbibed with two types of saline solutions. The first solution consisted of NaCl and the second was a mixture of (NaCl, MgCl2・6H2O and Na2SO4), the most common active ingredients in seawater. Each saline solution was added in five concentrations 0%, 0.5%, 1%, 1.5% and 2% (wt/wt) in deionized water. Twenty-five scarified seeds from each of ten alfalfa genotypes that were described as salt tolerant (Malone, Mesa-Sirsa, Rambler, and Saranac) and salt sensitive (Barricade, Bulldog 505, Hybriforce 2600, Magna 801FQ, 3010, and CW1010) were placed in each petri plate. Four and a half (4.5) mL of each salt solution with the appropriate concentration was added to each plate. The experimental design was a split-plot in a randomized complete block design with three treatments. Salt solutions represented the main plots and the increasing concentrations represented the sub-plots. The germination test was conducted in a dark growth chamber at 25˚C for 14 days. Seed germination percentage (GP) was calculated as:
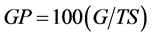 (1)
(1)
where G = number of germinated seeds, and TS = total number of seeds.
2.2. Whole-Plant Response to Salt Stress
One alfalfa salt-tolerant genotype (Mesa-Sirsa) and one salt-sensitive (Bulldog 505) were selected based on the results of the seed germination experiment described above. Six seeds from each genotype were planted in pots containing 2 kg sand and lined with plastic bags. Seedlings within each pot were thinned after four weeks from planting to keep four plants per pot. The plants were gradually subjected to two levels of salt concentrations starting at five weeks after planting. Calcium chloride (CaCl2・2H2O) and sodium chloride (NaCl) were mixed in a 2:1 proportion (CaCl2:NaCl) and added to Hoagland solution to make two saline nutrient solutions of 10 and 15 dS・m−1 electrical conductivity. The moisture level in the pots was kept at field capacity. Four treatments consisting of 1) control (Hoagland solution), 2) seaweed extract from Ascophyllum nodosum (Organic Approach, Lancaster, PA) at 4 Kg・ha−1, (3) humic acid (KELP4LESS, Idaho Falls, ID) at 28 L・ha−1, and 4) potassium sulfate nanoparticles at 300 kg・ha−1. Humic acid and seaweed extract were applied once at one week after the start of salt treatments, whereas potassium sulfate was added in 2 split applications, the first application was one week following salt application and the second at bud stage. All treatments were irrigated with Hoagland solution when needed. Plants were harvested 90 days after planting. Soil characteristics and chemical composition were recorded at the beginning of the study (Table 1) and at the end of the study. Plant biomass was measured as shoot and root dry weights using a digital scale with 0.001 g sensitivity. Root length in cm, number of tillers, and relative yield were also recorded.
Relative water content (RWC) of shoots was measured according to Turner [29] using the equation:
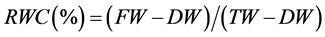 (2)
(2)
where, FW = fresh weight, TW = turgor weight, and DW = dry weight. Dry weight was estimated by drying the samples in a convection oven at 80˚C for 48 h. Turgor weight was determined by floating the shoots on water at room
Table 1. Physical characteristics and chemical composition of the soil used in the evaluation of two Alfalfa genotypes grown under three salt levels (0, 10, and 15 dS・m−1 EC) and three treatments of growth-enhancing substances (seaweed extract, humic acid, and potassium sulfate).
1EC: Electrical conductivity in dS・m−1. 2CEC: Cation exchange capacity in meq.100 g−1 soil. 3OM: Organic matter.
temperature for 48 h. Relative yield was determined according to Isla & Aragüés [30] by dividing the actual yield in each saline treatment by the highest yield observed.
2.2.1. Physiological Response to Salt Stress
Free proline content in plant tissue was determined according to the methods of Bates et al. [31] , where 100 mg of plant tissue were homogenized in 2 ml of 3% aqueous sulfosalicylic acid. The homogenate was centrifuged at 13,000 ×g for 10 mn. Then, 1 ml of supernatant was placed in a test tube and reacted with 1 ml of acid ninhydrin and 1 ml glacial acetic acid. The test tubes were heated in boiling water in a warm bath for 1 h, and the reaction was terminated by placing the test tubes in an ice bath. The reaction mixture was extracted with 2 ml toluene and mixed vigorously by vortexing. The toluene layer was separated at room temperature and the absorbance of chromophore containing toluene was measured at 520 nm on a Varian Cary 50 UV-Vis spectrophotometer (Agilent Technologies, Santa Clara, CA), using pure toluene as the blank. Standard curves were prepared for each assay using standard proline in 3% sulfosalicylic acid solution. The proline content was expressed as micromoles per gram of fresh weight plant material (µmol・g−1 FW).
Electrolyte leakage (EL) was determined as described by Lutts et al. [32] . Fresh leaves (200 mg) from the alfalfa plants were placed in test tubes containing 10 ml of deionized water. The tubes were incubated at 25˚C on a rotary shaker for 24 hours and, subsequently, the electrical conductivity of the solution (Lt) was measured. The samples were then autoclaved at 120˚C for 20 mn, and the final electrical conductivity (L0) was determined after equilibration at 25˚C. Measurements of electrical conductivity were made using H1993310 conducti-meter (HANA Instruments, Romania). Electrolyte leakage was expressed as:
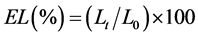 (3)
(3)
2.2.2. Antioxidant Enzymes Analysis
Tissue samples of 0.2 g from each leaf were homogenized with 50 mM sodium phosphate buffer (pH 7.0) containing 1 mM ethylenediaminetetraacetic acid (EDTA) and 2% (w/v) polyvinylpyrrolidone (PVP). The entire extraction procedure was carried out at 4˚C. The homogenate was centrifuged at 10,000 g for 15 mn at 4˚C and the supernatant was collected and used for assaying enzyme activity.
Catalase (CAT, EC 1.11.1.6) activity was measured according to Bergmeyer & Gawehn (1970) [33] as the rate of H2O2 disappearance at 240 nm by adding 100 μl leaf crude extract to the solution mixture containing 50 mM sodium phosphate buffer (pH 7.0) and 2% H2O2. Enzyme activity was calculated as units of H2O2 consumed per minute and per gram fresh weight (mmol H2O2 min−1・g−1 FW).
Superoxide dismutase (SOD, EC 1.15.1.1) activity was assayed spectrophotometrically as the inhibition of photochemical reduction of nitro-blue tetrazolium (NBT) at 560 nm according to the method of Beauchamp & Fridovich (1971) [34] . The reaction mixture of 3 ml volume, consisted of 50 mM sodium phosphate buffer (pH 7.8), 13 mM L-methionine, 75 μM NBT, 10 μM EDTA, 2.0 μM riboflavin and 0.3 ml enzyme extract. The test tubes containing reaction mixtures were weighed for 10 mn under 4000 lx at 35˚C. One-unit SOD activity was defined as the amount of enzyme required to cause a 50% inhibition of the rate of NBT reduction measured at 560 nm.
2.3. Statistical Analysis
Analysis of variance (ANOVA) was conducted on the data from the two experiments using PROC GLM of SAS 9.4 (SAS Institute Inc., Cary, NC). Replications were considered random and all other variables were considered fixed effects. Means of all variables were separated using Fisher’s protected LSD test.
3. Results and Discussion
3.1. Alfalfa Seed Germination under Increasing Salt Concentrations
There were significant differences between genotypes in their response to the type of salt solution and to the increase in salt concentration. Increasing salt concentrations significantly affected (p < 0.01) seed germination after two weeks in the 10 alfalfa genotypes (Table 2). The germination percentage under NaCl salt solution decreased at a faster pace in all genotypes with increasing salt concentration compared to the mixed salts solution (Table 3). At the lower salt levels, the reduction in germination varied from 0% to 78.05% at the 0.5% NaCl concentration and from 58.69% to 95.52% at the 1.0% NaCl level compared to no-salt control. Increasing NaCl concentration to 1.5 % resulted in a decrease of germination ranging from 70.83% to 100% compared to no-salt control. At 2% salt level, the magnitude of reduction in germination varied from 93.75% to 100% compared to the no-salt control. Under mixed salts solutions, the response in germination was different from NaCl solution, as 0.5% and 1.0% mixed salt concentrations enhanced the germination percentage by 14.03% and 9.52% more
Table 2. Mean squares and significance of seed germination of ten alfalfa genotypes after two weeks, in response to two salt solutions of NaCl and mixed salts (NaCl, MgCl2・6H2O and Na2SO4) at five different concentrations (0%, 0.5%, 1%, 1.5%, and 2%) each.
*, ** Significant at 0.05 and 0.01 probability level.
Table 3. Percent seed germination of ten alfalfa genotypes after two weeks, in response to two salt solutions (NaCl and mixed NaCl, MgCl2・6H2O and Na2SO4) at five different concentrations (0%, 0.5%, 1%, 1.5%, and 2%) each.
than the no-salt control (Table 3). At 1.5% mix salts concentration, the reduction in seed germination percentage varied from 52.78% to 95.52%, while at 2% concentration the decrease ranged from 93.75% to 100% compared to the no-salt control. This trend was observed in other crops exposed to salt stress [35] [36] [37] . The genotype Mesa-Sirsa exhibited the highest tolerance to increasing salt concentrations, whereas Bulldog 505 genotype was the most susceptible of the 10 genotypes tested. Germination percentage of Mesa-Sirsa decreased from 96% under no-salt to 28% and 6% at 1.5% and 2% NaCl solution, and to 45.33% and 6% at 1.5% and 2% mixed salt solution after 2 weeks. Whereas Bulldog 505, grown under 1.5% and 2% NaCl or mixed salts showed no germination after 2 weeks (Table 3).
3.2. Greenhouse Experiment
3.2.1. Soil Properties
An overall change in soil properties was observed at the end of the study, relative to the no-salt control (Table 4). There was a significant difference (p < 0.01) among genotypes in pH, Ca, K, Mg, B, Zn, and Fe. Salt concentrations had a significant effect (p < 0.01) on pH, EC, CEC, Ca, K, Mg, Na, B, and Zn. The application of growth enhancing treatments resulted in significant changes (p < 0.01) in EC, Ca, K, Mg, Zn, Fe, and Mn (Table 4). The application of humic acid reduced the pH under both salt levels (10 and15 dS・m−1), with Bulldog recording 6.7 and 6.63 respectively, compared to the no-salt control, while seaweed extract resulted in the lowest pH with the tolerant genotype Mesa-Sirsa under both salt levels (6.57 and 6.48, respectively). The EC increased by 3 to 4 folds with increasing salinity levels under the 10 dS・m−1 salt level and by 4 to 6 folds under the 15 dS・m−1 salt level, regardless of the growth enhancing treatment applied (Table 5). Aydin et al. (2012) [19] reported increases in EC of the soil with increasing salt concentrations with the highest increase was at 120 mM NaCl concentration. Potassium sulfate enhanced CEC of the soil under both alfalfa geno-
Table 4. Mean squares for various soil properties and mineral concentrations (mg・kg−1) measured after the evaluation of two Alfalfa genotypes grown under three salt levels (0, 10 and 15 dS・m−1 EC) and three treatments of growth-enhancing substances (seaweed extract, humic acid, and potassium sulfate).
*, **Significant at 0.05 and 0.01 probability level. 1Treatments: growth-enhancing substances (seaweed extract, humic acid, potassium sulfate). 2DF: Degrees of freedom. 3EC: Electrical conductivity in dS・m−1. 4CEC: Cation exchange capacity in meq 100 g−1 soil. 5LSD: Least significant difference at α = 0.05.
types at 10 dS・m−1 recording 69.4% with Bulldog, and 80.4% with Mesa-Sirsa, compared to no-salt control. Under 15 dS・m−1, seaweed extract increased the CEC of the soil under the susceptible genotype (Bulldog) recording 9.2 meq 100 g−1, while humic acid treatment recorded the highest CEC with the tolerant genotype (Mesa-Sirsa) with a value of 7.5 meq 100 g−1. The application of potassium sulfate resulted in accumulations of Ca, Mg, K, P, Na, B, Zn, Fe and Mn in the soil under both alfalfa genotypes at10 dS・m−1, while under 15 dS・m−1, seaweed extract resulted in accumulation of Ca, Mg, K, P, Na, B, Zn, Fe and Mn in the soil under both alfalfa genotypes.
3.2.2. Plant Tissue Chemical Characteristics
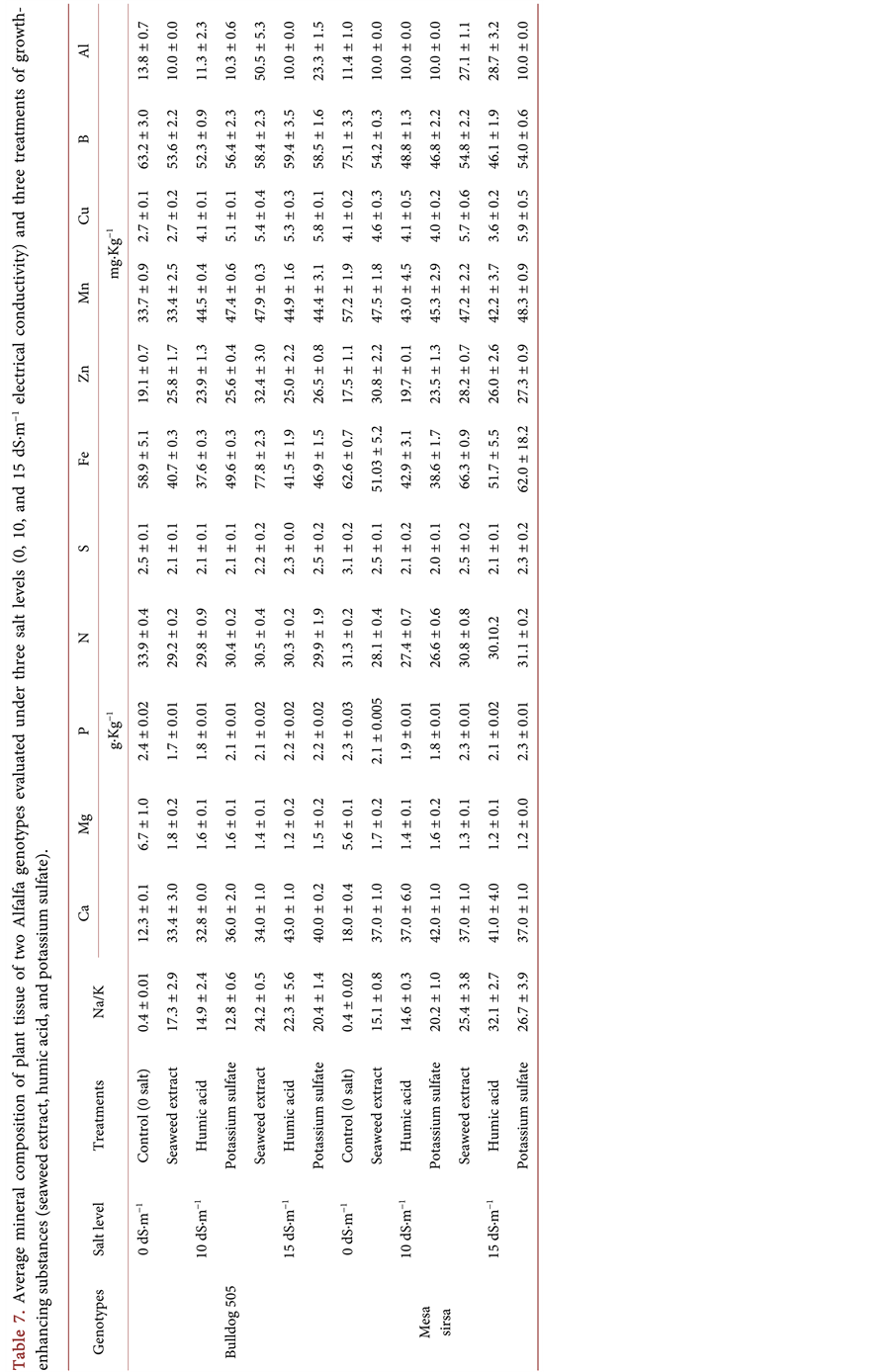
Salt concentrations significantly affected (p < 0.01) the mineral composition of plant tissue in both genotypes and increased the Na/K ratio compared to the no-salt control (Table 6). Increasing the salt concentration to 10 dS・m−1 resulted in an increase in the Na/K by a 32 fold in the susceptible genotype and 36 fold in the tolerant genotype, compared to the no-salt control (Table 7). A similar trend was reported in rice grown under salt stress [38] . Na/K ratio is an important consideration in salt tolerance and salt-stress alleviation because reducing Na+ uptake and transport from roots to shoots and increasing retention of K+ in the cytosol are considered key factors in conferring salt tolerance in plants [39] [40] .
There was a significant difference (p < 0.01) among the genotypes in Na/K and the concentration of Ca, Mg, N, S, Mn, Cu and Al in plant tissue. In the sensitive genotype, the application of potassium sulfate under salt stress at 10 dS・m−1 enhanced the uptake of K ions rather than Na+ ions leading to a lower Na/K compared to the other treatments. Humic acid treatment reduced the Na/K ratio under the higher salt concentration (15 dS・m−1). In the salt tolerant genotype, the Na/K ratio was lower than other treatments under the 10 dS・m−1 salt level,
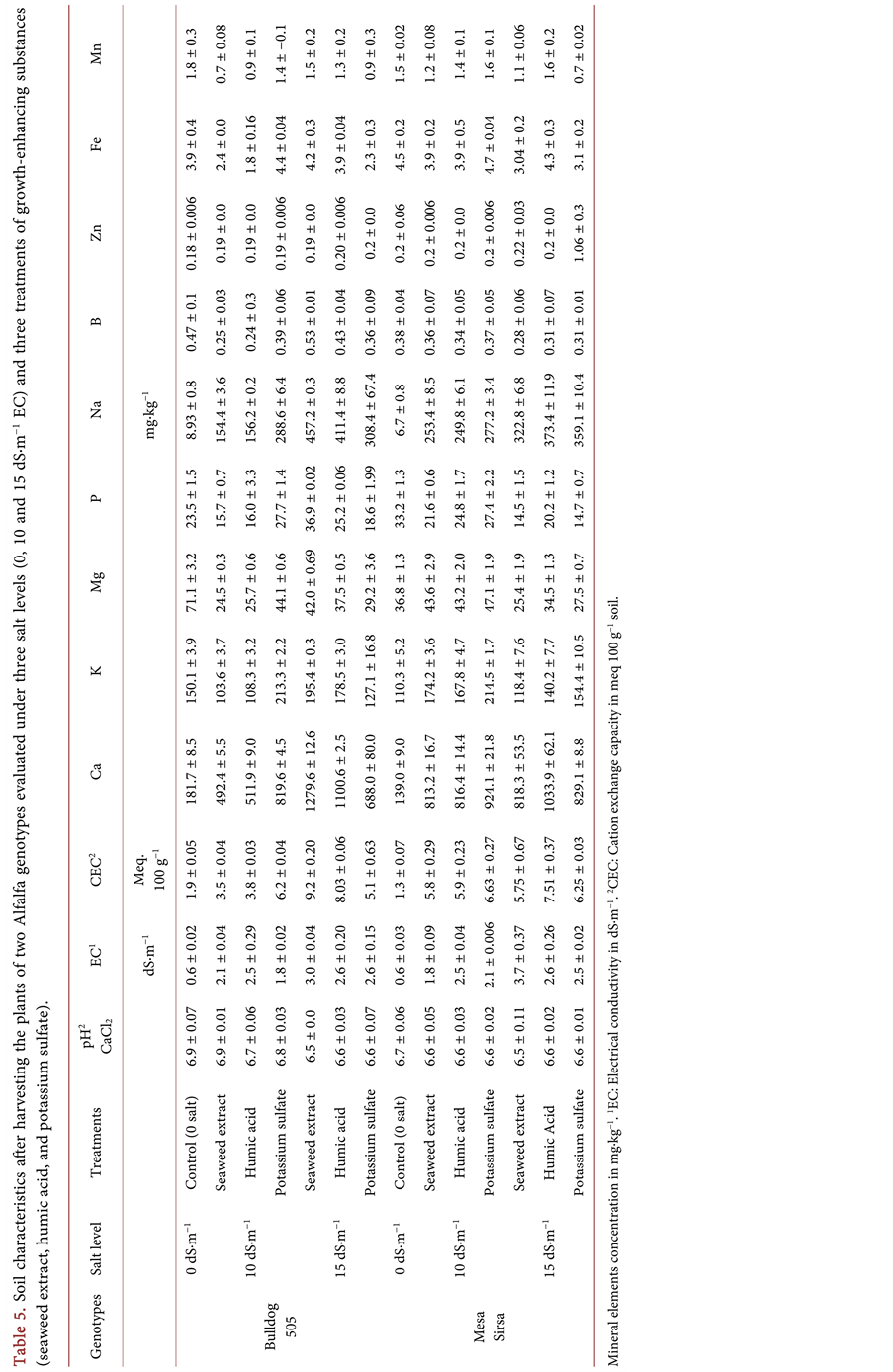
Table 6. Mean squares for chemical composition of the plant tissue of two Alfalfa genotypes evaluated under three salt levels (0, 10, and 15 dS・m−1 electrical conductivity) and three treatments of growth-enhancing substances (seaweed extract, humic acid, and potassium sulfate).
*, **Significant at 0.05 and 0.01 probability level. 1DF: Degrees of freedom. 2Treatments: seaweed extract, humic acid, and potassium sulfate. 3LSD: Least significant difference at α = 0.05.
following the application of humic acid, whereas under the higher salt level (15 dS・m−1), the application of seaweed extract resulted in the lowest Na/K ratio (Table 7). The lower Na/K ratio in the salt-tolerant genotype is a possible indication of higher Na exclusion in this genotype. The higher content of Ca in the two genotypes under both salt concentrations 10 and 15 dS・m−1, and under all the growth enhancing treatments compared to the no-salt control was possibly attributable to the fact that calcium was one of the components of the saline solution. Magnesium, P, N, S, Fe and B concentrations were reduced in both genotypes with increasing salt levels, and Zn, Mn, Cu and Al were slightly increased (Table 7). There were significant interactions (p < 0.01) between genotypes and salt levels for Ca, N, Mn, Al and B. The application of potassium sulfate to the salt-sensitive genotype resulted in the highest plant uptake of macro/micro elements under 10 dS・m−1 compared the other two growth enhancing treatments. Alfalfa growth and development depends upon the availability of adequate amounts of potassium, which increases crops overall productivity [41] . Similar results of the effect of potassium sulfate application under salt stress were reported in wheat [42] . Application of seaweed extract to the salt-tolerant genotype boosted the accumulation of Mg, P, N, S, Fe, Zn, Mn, Cu, B and Al in plant tissue under both salt levels (10 and 15 dS・m−1). Similar observations were reported following the foliar application of seaweed extract on alfalfa plants at a rate of 300 g, which resulted in increased plant macro/micro elements such as N, P, K, Fe, Mn, and Zn [43] . These results suggest that seaweed extract may have the ability to improve stress tolerance [44] [45] by increasing nutrient uptake from the soil [46] . The beneficial effect of seaweed extract may be the result of many components working synergistically at different concentrations [47] especially those derived from Ascophyllum nodosum, which were reported to have
high concentrations of total phenolics, a complex group known for its strong chelating activities [48] [49] .
3.2.3. Plant Biomass
Salt concentrations had significant effects (p < 0.01) on shoot and root dry weights. Plant biomass was reduced under both salt concentrations in both genotypes, with a greater magnitude in the salt-sensitive Bulldog 505, compared with the no-salt control (Table 9). Growth reduction in beans (Phaseolus vulgaris) treated with 50 and 100 mM sodium chloride were reported [50] . Barley plants exposed to salt levels of 0.75 and 13 dS・m−1 with and without potassium application also showed a similar response [51] and so did wheat plants under salt stress [42] . There was a significant difference (p < 0.01) between the two alfalfa genotypes in their response to salt levels and growth-stimulant treatments applied (Table 8). Application of seaweed extract to the salt sensitive genotype resulted in higher shoot and root dry weights compared with the other treatments under 10 dS・m−1 recording, 1.68 and 2.97 g, respectively (Table 9). The application of sea algae extracts) as organic bio-stimulants is becoming an accepted practice in horticulture industry, helping plants becoming more disease resistant and enabling rapid root development [52] . At the higher salt level (15 dS・m−1), the salt sensitive genotype responded with higher shoot and root dry weights following the application of K2SO4 nanoparticles. Adequate availability of potassium increased dry mass production of plants under salt and drought stress compared to lower concentrations of potash fertilizer [41] . The addition of humic acid increased plant biomass of the salt-tolerant genotype Mesa-Sirsa under both 10 and 15 dS・m−1 compared with the other treatments (Table 9). The increased growth observed following the application of humic acid might be attributed to its positive effects on the soil, which led to an increase in CEC and
Table 8. Mean squares and significance of shoot shoot dry weight (gm), plant height (cm), root length (cm), and root dry weight (gm) in two alfalfa genotypes grown under three salt levels (0, 10 and 15 dS・m−1 EC) and three treatments of growth-enhancing substances (seaweed extract, humic acid, and potassium sulfate).
*, **Significant at 0.05 and 0.01 probability level.
Table 9. Shoot dry weight (gm), plant height (cm), root length (cm), and root dry weight (gm) of two alfalfa genotypes grown under three salt levels (0, 10 and 15 dS・m−1 EC) and three treatments of growth-enhancing substances (seaweed extract, humic acid, and potassium sulfate).
nutrients (Table 5). Humic acid resulted in higher rates of K uptake and low Na/K ratio (Table 7), which may lead to overcoming the stress induced by alterations in the balance of endogenous hormones. Humic substances might exhibit anti-stress effects under abiotic stress conditions such as salinity stress [18] [53] [54] . It has been suggested that humic acid increases root cell elongation by acting on growth hormones, resulting in increased cell membrane permeability, oxygen uptake, respiration, and photosynthesis [55] [56] [57] [58] .
3.2.4. Physiological Responses
1) Relative water content (RWC)
Relative water content is an index describing the amount of water in plant tissue and indicates the ability of a plant to maintain adequate water under stress conditions [59] . There was a significant effect (p < 0.01) of salt concentrations on RWC. In both genotypes, RWC was lower under 10 and 15 dS・m−1 compared with the no-salt control, regardless of the growth-stimulant compound applied, and decreased with increasing salt level (Table 11). Similar reports were reported by Chen et al. [60] . Application of plant growth stimulants to the two alfalfa genotypes under the high salt levels affected significantly (p < 0.01) plant relative water content (Table 10). In the salt sensitive genotype Bulldog, RWC was higher following the application of seaweed extract compared with humic acid and potassium sulfate treatments under both salt levels 10 and 15 dS・m−1 (Table 11). Seaweed extracts are known to contain osmolytes such as mannitol, an important protectant compound under abiotic stress [12] that enhances root growth as well as soil water-holding capacity [11] . In the salt tolerant genotype,
Table 10. Means squares of relative water content (RWC), electrolyte leakage (EL) and proline in response to the application of three growth-enhancing treatments (seaweed extract, humic acid, and potassium sulfate) to alfalfa genotypes grown under two salt levels (10 and 15 dS・m−1 electrical conductivity).
*, **Significant at 0.05 and 0.01 probability level.
Table 11. Relative water content (RWC), Proline, and Electrical leakage (EL) in two alfalfa genotypes grown under three salt levels (0, 10 and 15 dS・m−1 EC) and three treatments of growth-enhancing substances (seaweed extract, humic acid, and potassium sulfate).
Mesa-Sirsa, the highest RWC was achieved by the addition of humic acid under 10 dS・m−1 (46.03%) and K2SO4 nanoparticles under 15 dS・m−1 (40.71%). The increase in the RWC following the application of humic acid may be attributed to enhanced root biomass (root length and dry weight) that leads to absorbing more water under salt stress, while potassium acts as an osmo-regulator in the cell [61] [62] [63] .
2) Electrolyte leakage
Electrolyte leakage (EL) is an indirect assessment of the degree of cell membrane integrity [64] , and is considered a reliable physiological indicator of the degree of plant cell injury under stress conditions [65] . Increasing salt concentrations resulted in significant increases (p < 0.01) in EL in both genotypes compared to the no-salt control (Table 10). Application of plant growth stimulants to the two alfalfa genotypes resulted in significantly different (p < 0.01) responses in electrolyte leakage under the two salt concentrations. Application of seaweed extract enhanced the response of both alfalfa genotypes to salt stress by decreasing EL at both salt levels, 10 and 15 dS・m−1 (Table 11). Seaweed extract was reported to significantly improve water use efficiency, increase leaf water content, and improve salt stress tolerance [66] [67] .
3) Proline
Proline accumulation is a sensitive physiological index of plant response to various abiotic stresses [68] . It plays a positive role in the salt tolerance of many crops by contributing to membrane stability [69] . Proline also induces the expression of salt stress-responsive proteins and may improve the plant adaptation to salt stress [70] . There was a significant difference (p < 0.01) between the alfalfa genotypes in proline accumulation in plant tissue in response to salt concentrations. In the salt sensitive genotype bulldog, the application of potassium sulfate resulted in the highest increase in proline content with 3.16 µmol・g−1 FW under 10 dS・m−1 (Table 11), while seaweed extract resulted in the highest increase (3.61 µmol・g−1 FW) under 15 dS・m−1 compared to 2.90 µmol・g−1 FW in the no-salt control. In the salt tolerant genotype Mesa-Sirsa, the highest proline accumulation was observed following the application of potassium sulfate at the 10 dS・m−1 (2.67 µmol・g−1 FW) and following the application of humic acid under 15 dS・m−1 (4.12 µmol・g−1 FW) compared to 1.83 µmol・g−1 FW in the no-salt control (Table 11).
4) Proline
Proline accumulation is a sensitive physiological index of plant response to various abiotic stresses [68] . It plays a positive role in the salt tolerance of many crops by contributing to membrane stability [69] . Proline also induces the expression of salt stress-responsive proteins and may improve the plant adaptation to salt stress [70] . There was a significant difference (p < 0.01) between the alfalfa genotypes in proline accumulation in plant tissue in response to salt concentrations. In the salt sensitive genotype bulldog, the application of potassium sulfate resulted in the highest increase in proline content with 3.16 µmol・g−1 FW under 10 dS・m−1 (Table 11), while seaweed extract resulted in the highest increase (3.61 µmol・g−1 FW) under 15 dS・m−1 compared to 2.90 µmol・g−1 FW in the no-salt control. In the salt tolerant genotype Mesa-Sirsa, the highest proline accumulation was observed following the application of potassium sulfate at the 10 dS・m−1 (2.67 µmol・g−1 FW) and following the application of humic acid under 15 dS・m−1 (4.12 µmol・g−1 FW) compared to 1.83 µmol・g−1 FW in the no-salt control (Table 11).
3.2.5. Antioxidant Enzymes
There was a significant difference (p < 0.01) between the genotypes in their CAT activity following the increase in salt concentrations and the application of growth stimulant treatments (Table 12). Increasing salt concentration resulted in significant increases (p < 0.01) in CAT activity in both genotypes compared to the no-salt control. (Table 12, Figure 1). Application of growth stimulants resulted in significant increases (p < 0.01) in CAT activity in both genotypes. Application of seaweed extract resulted in the highest increase of CAT in both genotypes under 10 dS・m−1 salt level and was three times higher than the control in the salt sensitive bulldog and four times higher than the control in the salt tolerant Mesa-Sirsa. Under the 15 dS・m−1 salt level, seaweed extract also resulted in the highest CAT activity in the salt tolerant genotype and was similar to humic acid in the salt sensitive genotype (Figure 1). Application of humic acid and potassium sulfate resulted in no change in CAT activity at 10 dS・m−1 in both genotypes compared to the no-salt control. However, they resulted in steep increases in CAT in both genotypes under 15 dS・m−1 salt level (Figure 1). A similar trend was observed for SOD activity. The application of growth stimulants resulted in significant increases (p < 0.01) in SOD in both genotypes at both salt levels compared to the no-salt control (Table 12). The response of the genotypes to the application of growth stimulants was similar under both salt levels. The application of seaweed extract resulted in the highest increase in SOD activity at the 10 dS・m−1 salt level in both genotypes, and was 92% higher in the bulldog genotype and 16% higher in the Mesa-Sirsa genotype compared to the control (Figure 2).
Table 12. Means squares of catalase (mmol H2O2 min−1・g−1 fresh tissue) and SOD (Super oxide dismutase in U・g−1 fresh tissue) in two alfalfa genotypes grown under three salt levels (0, 10 and 15 dS・m−1 EC) and three treatments of growth-enhancing substances (seaweed extract, humic acid, and potassium sulfate).
*, **Significant at 0.05 and 0.01 probability level.
Figure 1. Catalase activity (mmol H2O2 min−1・g−1 fresh tissue) in one salt sensitive (Bulldog) and one salt tolerant (Mesa-Sirsa) alfalfa genotypes grown under three salt levels (0, 10 and 15 dS・m−1 EC) and three treatments of growth-enhancing substances (seaweed extract, humic acid, and potassium sulfate).
Figure 2. Superoxide dismutase (U・g−1 fresh tissue) in one salt sensitive (Bulldog) and one salt tolerant (Mesa-Sirsa) alfalfa genotypes grown under three salt levels (0, 10 and 15 dS・m−1 EC) and three treatments of growth-enhancing substances (seaweed extract, humic acid, and potassium sulfate).
Potassium sulfate resulted in the highest increases in SOD activity in both genotypes under the 15 dS・m−1 salt level, but was not much different from seaweed extract (Figure 2). Seaweed extract is known to contain betaines, including gamma-aminobutyric acid betaine, 6-aminovaleric acid betaine, and glycine betaine, which play an important role in enhancing chlorophyll and antioxidant enzymes [71] .
There is evidence that seaweed extract increases tolerance to oxidative stress and protects against adverse environmental conditions by enhancing the activity of the antioxidant enzymes SOD and Ascorbate peroxidase (ASP) [16] [72] . Potassium plays a key role in plants through the activation of enzymes by stabilizing the pH between 7 and 8 and changing the conformation of enzymes by binding to their surfaces [73] . Tripathi et al. [74] reported that proteins, such as thioredoxin, glutaredoxin, and cyclophilin, facilitated the regeneration of the catalytically active form of peroxiredoxins that played an important role in reducing the formation of reactive oxygen species in plants under biotic and abiotic stress.
3.3. Correlation between Physiological and Phenotypic Responses
There was a significant positive correlation (r = 0.42, p < 0.01) between proline content in the plant tissue and relative water content (Table 13). Proline content was also negatively correlated (r = −0.18, p < 0.05) with electrolyte leakage. Proline plays important roles as an osmo-protectant and acts as an antioxidant as
Table 13. Correlation between shoot dry weight (g), plant height (cm), root length (cm), root dry weight (g), relative water content (%), electrolyte leakage (%), Proline (µmol・g−1 FW), of two alfalfa genotypes evaluated under three salt levels (0, 10, and 15 dS・m−1 electrical conductivity) and three treatments of growth-enhancing substances (seaweed extract, humic acid, and potassium sulfate).
well as a source of energy [75] [76] . It is generally considered an important biomolecule that has a protective role in tolerance of plant to abiotic stresses [77] [78] leading to a higher water potential, and hence improving the water uptake and growth under stress [79] . This explains the negative correlation observed in this study between proline and electrolyte leakage and the positive correlation with relative water content. The accumulation of proline under stress is an adaptation mechanism, regulating membrane water permeability in cells and influencing water movement among tissue and organs [80] , leading to enhanced plant growth. This may explain the positive correlations observed between RWC and shoot dry weight (r = 0.57, p < 0.01) and the positive correlation (r = 0.62, p < 0.01) between shoot dry weight and plant root dry weight. Catalase activity showed a significant negative correlation with electrolyte leakage (r = −0.40, p < 0.01) and significant positive correlations with shoot dry weight (r = 0.34, p < 0.05) and plant height (r = 0.37, p < 0.05). SOD activity showed positive correlations with shoot dry weight (r = 0.50, p < 0.01) and plant height (r = 0.55, p < 0.01), but a low correlation with root dry weight (r = 0.17, p > 0.05). It also showed a significant positive correlation with relative water content (r = 0.44, p < 0.01). The significant positive correlation between SOD and CAT activity (r = 0.47, p < 0.01) is an indication that the two enzymes act synergistically to enhance alfalfa tolerance to salt stress.
4. Conclusion
Increasing salt concentration reduced germination in all alfalfa genotypes. Above 1% salt in the solution reduced seed germination by more than 70% in most genotypes. Growing alfalfa plants under salt concentration of 10 dS・m−1 and above, resulted in significant decreases in plant shoot and root growth, with a lesser magnitude in salt tolerant genotypes. Application of plant growth stimulants (seaweed extract, humic acid, and potassium sulfate) to one salt tolerant and one salt sensitive genotype grown under two salt levels (10 and 15 dS・m−1) resulted in overall changes in soil properties relative to the no-salt control. Application of humic acid improved the growth of the salt-tolerant genotype under both salt concentrations, while seaweed extract and potassium sulfate were more effective in the salt sensitive genotype under 10 and 15 dS・m−1. Overall, potassium sulfate was more effective in maintaining a low Na/K ratio in the susceptible genotype under 10 dS・m−1 and humic acid under 15 dS・m−1, while in the salt tolerant genotype, humic acid was more effective in maintaining a low Na/K ratio under 10 dS・m−1 and seaweed extract under 15 dS・m−1. Seaweed extract resulted in higher RWC and proline under both salt concentrations (10 and 15 dS・m−1) in the salt sensitive genotype and lower electrolyte leakage in both salt tolerant and salt sensitive genotypes under both salt concentrations. Seaweed extract also enhanced CAT and SOD activities in both genotypes under 10 dS・m−1. Application of seaweed extract seemed to have an overall positive effect in alleviating salt stress in the salt-sensitive alfalfa genotype, while the application of humic acid and potassium sulfate seemed to have an overall positive effect in maintaining growth under salt stress in the salt-tolerant alfalfa genotype.
Cite this paper
El-Sharkawy, M., El-Beshsbeshy, T., Al-Shal, R. and Missaoui, A. (2017) Effect of Plant Growth Sti- mulants on Alfalfa Response to Salt Stress. Agricultural Sciences, 8, 267-291. https://doi.org/10.4236/as.2017.84020
References
- 1. Doaa, A.I.M., Ibrahim, M.M., Ramadan, H.M. and Mahmoud, E.K. (2012) Effect of Organic Amendment and Potassium Fertilizing on Improvement of a Salt Affected Soil and Wheat Yield. M.Sc. Thesis, Faculty of Agriculture, Tanta University, Tanta.
- 2. El-Banna, I.M.M., Abou El-Defan, T.A., Selem, M.I. and El-Maghraby, T.A. (2004) Potassium Fertilization and Soil Amendments Interactions and Their Effect on Wheat with Different Water Qualities. Journal of Agricultural Science, Mansoura University, 29, 5953-5963.
- 3. Munns, R. (2002) Comparative Physiology of Salt and Water Stress. Plant, Cell & Environment, 25, 239-250.
https://doi.org/10.1046/j.0016-8025.2001.00808.x - 4. Chinnusamy, V., Jagendorf, A. and Zhu, J.K. (2005) Understanding and Improving Salt Tolerance in Plants. Crop Science, 45, 437-448.
https://doi.org/10.2135/cropsci2005.0437 - 5. Munns, R. (1993) Physiological Processes Limiting Plant Growth in Saline Soils: Some Dogmas and Hypotheses. Plant, Cell & Environment, 16, 15-24.
https://doi.org/10.1111/j.1365-3040.1993.tb00840.x - 6. Roy, S.J., Negrao, S. and Tester, M. (2014) Salt Resistant Crop Plants. Current Opinion in Biotechnology, 26, 115-124.
https://doi.org/10.1016/j.copbio.2013.12.004 - 7. Munns, R. and Tester, M. (2008) Mechanisms of Salinity Tolerance. Annual Review of Plant Biology, 59, 651-681.
https://doi.org/10.1146/annurev.arplant.59.032607.092911 - 8. Hasegawa, P.M., Bressnan, R.A., Zhu, J.K. and Bohnert, H.J. (2000) Plant Cellular and Molecular Responses to High Salinity. Annual Review of Plant Physiology and Plant Molecular Biology, 51, 463-499.
https://doi.org/10.1146/annurev.arplant.51.1.463 - 9. Muchate, N.S., Nikalje, G.C., Rajurkar, N.S., Suprasanna, P. and Nikam, T.D. (2016) Plant Salt Stress: Adaptive Responses, Tolerance Mechanism and Bioengineering for Salt Tolerance. The Botanical Review, 82, 371-406.
https://doi.org/10.1007/s12229-016-9173-y - 10. Silva, M., Souza, J., Barreiro-Neto, J.G. and Silva, M.J.V. (1992) Selecao de três cultivares de algodoeiro para tolerancia a germinacao em condicoes salinas. Pesquisa Agropecuária Brasileira, Brasília, 27, 655-659.
- 11. Khan, W., Rayirath, U.P., Subramanian, S., Jithesh, M.N., Rayorath, P., Hodges, D. M., Critchley, A.T., Craigie, J.S., Norrie, J. and Rithiviraj, B.P. (2009) Seaweed Extracts as Biostimulants of Plant Growth and Development. Journal of Plant Growth Regulation, 28, 386 399.
https://doi.org/10.1007/s00344-009-9103-x - 12. Battacharyya, D., Babgohari, M.Z., Rathor, P. and Prithiviraj, B. (2015) Seaweed Extracts as Biostimulants in Horticulture. Scientia Horticulturae, 196, 39-48.
- 13. Stamatiadis, S., Evangelou, L., Yvin, J., Tsadilas, C., Mina, J.M.G. and Cruz, F. (2015) Responses of Winter Wheat to Ascophyllum nodosum (L.) Le Jol. Extract Application under the Effect of N Fertilization and Water Supply. Journal of Applied Phycology, 27, 589-600.
https://doi.org/10.1007/s10811-014-0344-0 - 14. Zhang, X. and Ervin, E.H. (2004) Cytokinin-Containing Seaweed and Humic Acid Extracts Associated with Creeping Bentgrass Leaf Cytokinins and Drought Resistance. Crop Science, 44, 1737-1745.
https://doi.org/10.2135/cropsci2004.1737 - 15. Craigie, J.S. (2011) Seaweed Extract Stimuli in Plant Science and Agriculture. Journal of Applied Phycology, 23, 371-393.
https://doi.org/10.1007/s10811-010-9560-4 - 16. Fike, J.H., Allen, V.G., Schmidt, R.E., Zhang, X., Fontenot, J.P., Bagley, C.P., et al. (2001) Tasco-Forage: I. Influence of a Seaweed Extract on Antioxidant Activity in Tall Fescue and in Ruminants. Journal of Animal Science, 79, 1011-1021.
https://doi.org/10.2527/2001.7941011x - 17. Neily, W., Shishkov, L., Nickerson, S., Titus, D. and Norrie, J. (2010) Commercial Extract from the Brown Seaweed Ascophyllum nodosum (Acadian (R)) Improves Early Establishment and Helps Resist Water Stress in Vegetable and Flower Seedlings. HortScience, 45, 105-106.
- 18. Kulikova, N.A., Stepanova, E.V. and Koroleva, O.V. (2005) Mitigating Activity of Humic Substances: Direct Influence on Biota. In: Perminova, I.V., Eds., Use of Humic Substances to Remediate Polluted Environments: From Theory to Practice, NATO Science Series IV: Erath and Environmental Series, Kluwer Academic Publishers, USA, 285-309.
https://doi.org/10.1007/1-4020-3252-8_14 - 19. Aydin, A., Kant, C. and Turan, M. (2012) Humic Acid Application Alleviate Salinity Stress of Bean (Phaseolus vulgaris L.) Plants Decreasing Membrane Leakage. African Journal of Agricultural Research, 7, 1073-1086.
- 20. Khaled, H. and Fawy, H.A. (2011) Effect of Different Levels of Humic Acids on the Nutrient Content, Plant Growth, and Soil Properties under Conditions of Salinity. Soil and Water Research, 6, 21-29.
- 21. Khalesro, S., Salehi, M. and Mahdavi, B. (2015) Effect of Humic Acid and Salinity Stress on Germination Characteristic of Savory (Satureja hortensis L.) and Dragonhead (Dracocephalum moldavica L.). Biological Forum—An International Journal, 7, 554-561.
- 22. Lissbrant, S., Berg, W.K., Volenec, J.J., Brouder, S.M., Joern, B.C., Cunningham, S. M. and Johnson, K.D. (2009) Phosphorus and Potassium Fertilization of Alfalfa. AY-331-W, 6 p.
- 23. Dawood, M.G., Abdelhamid, M.T. and Schmidhalter, U. (2014) Potassium Fertilizer Enhances the Salt-Tolerance of Common Bean (Phaseolus vulgaris L.). The Journal of Horticultural Science and Biotechnology, 89, 185-192.
https://doi.org/10.1080/14620316.2014.11513067 - 24. Mohamed, A.B., Haddad, M. and Ferchichi, A. (2009) Diversity of Lucerne (Medicago sativa L.) Populations in South Tunisia. Pakistan Journal of Botany, 41, 2851-2861.
- 25. Lancefield, G.D., Ball, D., Hannock, M.D., Andrae, J. and Smith, R. (2009) Growing Alfalfa in the South. National Alfalfa and Forage Alliance.
https://www.alfalfa.org/pdf/alfalfainthesouth.pdf - 26. Barnes, D.K., Golen, B.P. and Baylor, J.E. (1980) Highlights in the USA and Canada. In: Alfalfa and Alfalfa Improvement, No. 29, Agronomy Series, Crop Science Society of America, Madison, WI.
- 27. McDonald, W., Nikandrow, A., Bishop, A., Lattimore, M., Gardner, P., Williams, R. and Hysons, L. (2003) Lucerne for Pasture and Fodder. Agfact.2.2.2.25, 3rd Edition, NSW, Agriculture, Orange, 1-39.
- 28. Maas, E.V. and Hoffman, G.J. (1977) Crop Salt Tolerance, Current Assessment. Journal of the Irrigation and Drainage Division, 103, 115-134.
- 29. Turner, N.C. (1981) Techniques and Experimental Approaches for the Measurement of Plant Water Status. Plant and Soil, 58, 339-366.
https://doi.org/10.1007/BF02180062 - 30. Isla, R. and Aragüés, R. (2009) Response of Alfalfa (Medicago sativa L.) to Diurnal and Nocturnal Saline Sprinkler Irrigations. II: Shoot Ion Content and Yield Relationships. Irrigation Science, 27, 507-513.
https://doi.org/10.1007/s00271-009-0166-z - 31. Bates, L., Waldren, R. and Teare, I. (1973) Rapid Determination of Free Proline for Water-Stress Studies. Plant and Soil, 39, 205-207.
https://doi.org/10.1007/BF00018060 - 32. Lutts, S., Kinet, J. and Bouharmont, J. (1996) NaCl-Induced Senescence in Leaves of Rice (Oryza sativa L.) Cultivars Differing in Salinity Resistance. Annals of Botany, 78, 389-398.
https://doi.org/10.1006/anbo.1996.0134 - 33. Bergmeyer, H.U. and Gawehn, K. (1970) Methoden der enzymatischen Analyse. Verlag Chemie, Weinheim.
- 34. Beauchamp, C. and Fridovich, I. (1971) Superoxide Dismutase: Improved Assays and an Assay Applicable to Acrylamide Gels. Analytical Biochemistry, 44, 276-287.
- 35. Epstein, E., Norlyn, J.D., Rush, D.W., Kingsbury, R.W., Kelley, D.B., Cunningham, G.A. and Wrona, A.F. (1980) Saline Culture of Crops: A Genetic Approach. Science, 210, 399-404.
https://doi.org/10.1126/science.210.4468.399 - 36. El-Madidi, S., El-Baroudi, B. and Aameur, F.B. (2004) Effects of Salinity on Germination and Early Growth of Barley (Hordeum vulgare L.) Cultivars. International Journal of Agriculture & Biology, 6, 767-770.
- 37. Abbas, M.K., Ali, A.S., Hasan, H.H. and Ghal, R.H. (2012) Salt Tolerance Study of Six Cultivars of Rice (Oryza sativa L.) during Germination and Early Seedling Growth. Journal of Agricultural Science, 5, 250.
https://doi.org/10.5539/jas.v5n1p250 - 38. Morales, S.G., Trejo-Téllez, L.I., Merino, F.C.G., Caldana, C., Espinosa-Victoria, D. and Cabrera, E.H. (2012) Growth, Photosynthetic Activity, and Potassium and Sodium Concentration in Rice Plants under Salt Stress. Acta Scientiarum. Agronomy, 34, 317-324.
- 39. Garthwaite, A.J., von Bothmer, R. and Colmer, T.D. (2005) Salt Tolerance in Wild Hordeum Species Is Associated with Restricted Entry of Na+ and Cl¯ into the Shoots. Journal of Experimental Botany, 56, 2365-2378.
https://doi.org/10.1093/jxb/eri229 - 40. Zepeda-Jazo, I., Shabala, S., Chen, Z. and Pottosin, I.I. (2008) Na-K Transport in Roots under Salt Stress. Plant Signaling & Behavior, 3, 401-403.
https://doi.org/10.4161/psb.3.6.5429 - 41. Wang, M., Zheng, Q., Shen, Q. and Guo, S. (2013) The Critical Role of Potassium in Plant Stress Response. International Journal of Molecular Sciences, 14, 7370-7390.
https://doi.org/10.3390/ijms14047370 - 42. Kausar, A. and Gull, M. (2014) Effect of Potassium Sulphate on the Growth and Uptake of Nutrients in Wheat (Triticum sativum L.) under Salt Stressed Conditions. Journal of Agricultural Science, 6, 101-112.
https://doi.org/10.5539/jas.v6n8p101 - 43. El Sayed, S.A.A., Hellal, F.A., Nofal, O.A., EL-Karamany, M.F. and Bakry, B.A. (2015) Influence of Algal Extracts on Yield and Chemical Composition of Moringa and Alfalfa Grown under Drought Condition. International Journal of Environment, 4, 151-157.
- 44. Zhang, X. and Schmidt, R.E. (2000) Hormone Containing Products’ Impact on Antioxidant Status of Tall Fescue and Creeping Bentgrass Subjected to Drought. Crop Science, 40, 1344-1348.
https://doi.org/10.2135/cropsci2000.4051344x - 45. Zhang, X., Ervin, E.H. and Schmidt, E.R. (2003) Plant Growth Regulators Can Enhance the Recovery of Kentucky Bluegrass Sod from Heat Injury. Crop Science, 43, 952-956.
https://doi.org/10.2135/cropsci2003.9520 - 46. Turan, M. and Kose, C. (2004) Seaweed Extracts Improve Copper Uptake of Grapevine. Acta Agriculturae Scandinavica, Section B. Soil & Plant Science, 54, 213-220.
- 47. Fornes, F., Sánchez-Perales, M. and Guadiola, J.L. (2002) Effect of a Seaweed Extract on the Productivity of “de Nules” Clementine Mandarin and Navelina Orange. Botanica Marina, 45, 486-489.
https://doi.org/10.1515/bot.2002.051 - 48. Laetitia, A., Fauchon, M., Blanc, N., Hauchard, D. and ArGall, E. (2010) Phenolic Compounds in the Brown Seaweed Ascophyllum nodosum: Distribution and Radical-Scavenging Activities. Phytochemical Analysis, 21, 399-405.
https://doi.org/10.1002/pca.1210 - 49. Balboa, E.M., Enma, C., Moure, A., Falqué, E. and Domínguez, H. (2013) In Vitro Antioxidant Properties of Crude Extracts and Compounds from Brown Algae. Food Chemistry, 138, 1764-1785.
- 50. Gama, P.B., Inanaga, S., Tanka, K. and Nakaza R. (2007) Physiological Response of Common Bean (Phaseolus vulgaris) Seedlings to Salinity Stress. African Journal of Biotechnology, 6, 79-88.
- 51. Endris, S. and Mohammad, M. (2007) Nutrient Acquisition and Yield Response of Barley Exposed to Salt Stress under Different Levels of Potassium Nutrition. International Journal of Environmental Science & Technology, 4, 323-330.
https://doi.org/10.1007/BF03326289 - 52. El-Khateeb, M.A., Nasr, E.A., Fahmy, A.N. and Dorgham, A.H.H. (2010) Effect of GA3 and Growth Biostimulants on Growth and Chemical Composition of Calia Secundiflora Plants. Journal of Horticultural Science & Ornamental Plants, 2, 118-124.
- 53. Marschner, H. (1995) Mineral Nutrition of Higher Plants. 2nd Edition, Academic Press, London, 889.
- 54. Xudan, X. (1986) The Effect of Foliar Application of Fulvic Acid on Water Use, Nutrient Uptake and Wheat Yield. Australian Journal of Agricultural Research, 37, 343-350.
https://doi.org/10.1071/AR9860343 - 55. Vaughan, D. (1985) Effect of Humic Substances on Metabolic Processes in Plants. In: Burns, R.G., Dell’Agnola, S., Miele, S., Nardi, G., Savoini, G., Schnitzer, M., Sequi, P., Vaughan, D. and Visser, S.A., Eds., Humic Substances Effects on Soil and Plants, Reda, Roma, 54-77.
- 56. Cacco, G. and Dell’Agnolla, G. (1984) Plant Growth Regulator Activity of Soluble Humic Substances. Canadian Journal of Soil Science, 64, 25-28.
https://doi.org/10.4141/cjss84-023 - 57. Russo, R.O. and Berlyn, G.P. (1990) The Use of Organic Biostimulants to Help Low Input Sustainable Agriculture. Journal of Sustainable Agriculture, 1, 19-42.
https://doi.org/10.1300/J064v01n02_04 - 58. Masciandaro, G., Ceccanti, B., Ronchi, V., Benedicto, S. and Howard, L. (2002) Humic Substances to Reduce Salt Effect on Plant Germination and Growth. Communications in Soil Science and Plant Analysis, 33, 365-378.
https://doi.org/10.1081/CSS-120002751 - 59. Rad, J.S., Karimi, J., Mohsenzadeh, S., Rad, M.S. and Moradgohli, J. (2014) Evaluation SiO2 Nanoparticles Effects on Developmental Characteristic and Photosynthetic Pigment Contents of Zea mays L. Bulletin of Environment, Pharmacology and Life Sciences, 3, 194-201.
- 60. Chen, C.C.S. and Plant, A.L. (1999) Salt-Induced Protein Synthesis in Tomato Roots: The Role of ABA. Journal of Experimental Botany, 50, 677-687.
https://doi.org/10.1093/jxb/50.334.677 - 61. Marschner, H., Kuiper, P.J.C. and Kylin, A. (1981) Genotypic Differences in the Response of Sugar-Beet Plants to Replacement of Potassium by Sodium. Physiologia Plantarum, 51, 239-244.
https://doi.org/10.1111/j.1399-3054.1981.tb02705.x - 62. Lawrence, D.T. and Zeiger, E. (1996) Central Roles for Potassium and Sucrose in Guard-Cell Osmoregulation. Plant Physiology, 111, 1051-1057.
https://doi.org/10.1104/pp.111.4.1051 - 63. Subbarao, G.V., Wheeler, R.M., Stutte, G.W. and Levine, L.H. (1999) How Far Can Sodium Substitute for Potassium in Red Beet? Journal of Plant Nutrition, 22, 1745-1761.
https://doi.org/10.1080/01904169909365751 - 64. Mo, Y., Liang, G., Shi, W. and Xie, J. (2011) Metabolic Responses of Alfalfa (Medicago sativa L.) Leaves to Low and High Temperature Induced Stresses. African Journal of Biotechnology, 10, 1117-1124.
- 65. Gao, Z., Zhu, H., Gao, J., Yang, C., Mu, C. and Wang, D. (2011) Germination Responses of Alfalfa (Medicago sativa L.) Seeds to Various Salt-Alkaline Mixed Stress. African Journal of Agricultural Research, 6, 3793-3803.
- 66. Neily, W., Shishkov, L., Tse, T. and Titus, D. (2008) Acadian LSC Helps Reduce Salinity Stress in Pepper Seedlings cv. California Wonder. PGRSA Newsl, 1, 14.
- 67. Little, H. and Neily, W. (2010) Commercial Extracts of the Brown Seaweed Ascophyllum nodosum Improve Plant Water Use and Drought Stress Resistance in the Greenhouse and Field. Oral Presentation. Western Plant Growth Regulator Society Annual Meeting, Davis, California.
- 68. Shi, D.C. and Yin, L.J. (1992) Strain Responses in Na2CO3 Stressed Leymus chinensis Seedlings and Their Mathematical Analysis. Acta Botanica Sinica, 34, 386-393.
- 69. Ashraf, M. and Harris, P.J.C. (2004) Potential Biochemical Indicators of Salinity Tolerance in Plants. Plant Science, 166, 3-16.
- 70. Khedr, A.H., Abbas, M.A., Wahid, A.A., Quick, W.P. and Abogadallah, G.M. (2003) Proline Induces the Expression of Salt-Stress-Responsive Proteins and May Improve the Adaptation of Pancratium maritimum L. to Salt-Stress. Journal of Experimental Botany, 54, 2553-2562.
https://doi.org/10.1093/jxb/erg277 - 71. Blunden, G., Crips, A.L., Gordon, S.M., Masson, T.G. and Turner, C.H. (1986) The Characterization and Quantitative Estimate of Betaines in Commercial Seaweed Extracts. Botanica Marina, 8, 138-143.
- 72. Schmidt, L.E. (2005) Age and Paracetamol Self-Poisoning. Gut, 54, 686-690.
https://doi.org/10.1136/gut.2004.054619 - 73. Marschner, H. (2002) Mineral Nutrition of Higher Plants. 2nd Edition, Academic press, Amsterdam, Boston, Heidelberg, London, New York, Oxford, Paris, San Diego, San Francisco, Singapore, Sydney, Tokyo.
- 74. Tripathi, B.N., Bhatt, I. and Dietz, K.J. (2009) Peroxiredoxins: A Less Studied Component of Hydrogen Peroxide Detoxification in Photosynthetic Organisms. Protoplasma, 235, 3-15.
https://doi.org/10.1007/s00709-009-0032-0 - 75. Siddiqui, M.H., Al-Whaibi, M.H. and Basalah, M.O. (2011) Interactive Effect of Calcium and Gibberellin on Nickel Tolerance in Relation to Antioxidant Systems in Triticum sativum L. Protoplasma, 248, 503-511.
https://doi.org/10.1007/s00709-010-0197-6 - 76. Siddiqui, M.H., Mohammad, F., Khan, M.M. and Al-Whaibi, M.H. (2012) Cumulative Effect of Nitrogen and Sulphur on Brassica juncea L. Genotypes under NaCl Stress. Protoplasma, 249, 139-153.
https://doi.org/10.1007/s00709-011-0273-6 - 77. Matysik, J., Alia, B.B. and Mohanty, P. (2002) Molecular Mechanisms of Quenching of Reactive Oxygen Species by Proline under Stress in Plants. Current Science, 82, 525-532.
- 78. Zhao, Y. (2011) Cadmium Accumulation and Antioxidative Defenses in Leaves of Triticum sativum L. and Zea mays L. African Journal of Biotechnology, 10, 2936-2943.
https://doi.org/10.5897/AJB10.1230 - 79. Nayyar, H. (2003) Variation in Osmoregulation in Differentially Drought Sensitive Wheat Genotypes Involves Calcium. Biologia Plantarum, 47, 541-547.
https://doi.org/10.1023/B:BIOP.0000041059.10703.11 - 80. Ashraf, M., Mueen-Ud-Din, M. and Warrich, N. (2003) Production Efficiency of Mung Bean Vigna radiata L. as Affected by Seed Inoculation and NPK Application. International Journal of Agriculture and Biology, 5, 179-180.




