Agricultural Sciences
Vol.5 No.4(2014), Article ID:43553,7 pages DOI:10.4236/as.2014.54028
Regulation of Photoassimilate Distribution between Source and Sink Organs of Crops through Light Environment Control in Greenhouses
Lina Wang1,2*, Xiaoyu Yang3, Zhonghai Ren1,2, Xiufeng Wang1,2
1College of Horticulture Science and Engineering, Shandong Agricultural University, Tai’an, China
2State Key Laboratory of Crop Biology, Tai’an, China
3School of Life Sciences, Faculty of Science, The Chinese University of Hong Kong, Hong Kong, China
Email: *lnwang007@163.com
Copyright © 2014 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/


Received 10 January 2014; revised 10 February 2014; accepted 20 February 2014
ABSTRACT
Photosynthesis, the most important physiological process in plants, can produce not only ATP and NADPH used in other processes but also carbohydrate, the key factor for crop yield. Production of photoassimilates is often influenced by various environmental factors such as light, temperature, CO2, water, mineral elements and leaf stage and position. Here we focused on the light-mediated regulation of photoassimilate translocation in plants and the application of light environment control in greenhouse production. We also reviewed the effects of other factors including leaf age and position, air temperature, CO2 concentration and water and mineral element supply on photoassimilate translocation in plants. Finally some perspectives have been proposed.
Keywords: Light; Photoassimilate Translocation; Plant

1. Introduction
As sessile organisms, plants may suffer from many unfavorable environmental conditions such as drought, mineral insufficiency, low light intensity and chilling, which all can significantly influence their growth and productivity via disturbance of the normal physiological processes in plants. In these processes, capacity to produce high amounts of photoassimilates and efficient partitioning of carbon compounds towards harvestable organs have been shown a major impact on crop yield [1] [2] . Therefore, a series of studies on the relationship between environmental factors (e.g. light, air temperature, relative humidity and CO2 concentration) and crop production have been done to optimize the environmental conditions for stimulating photoassimilate translocation to harvestable organs. Among these environmental factors, light is thought to be the most important one. Here, recent advances about the involvement of light environment, including light intensity, photoperiod and light quality, in photoassimilate translocation have been reviewed. Supplemental lighting is thus considered as a powerful approach and has been applied widely in greenhouse production to improve crop yield and quality. In addition, the effects of leaf stage and position, air temperature, CO2 concentration and water and mineral element supply have also been introduced on the partitioning of photosynthetic product from source to sink organs in plants. Finally some perspectives in this field have been proposed as well.
2. Light Environment and Photoassimilate Translocation
2.1. Light Intensity
Light intensity is a fundamental factor for the translocation of photoassimilates in plants. The export percentage of carbon from source leaves is stable, but the amount of carbon per unit leaf area mainly depends on the carbon pool [3] . The carbon pool of a leaf is determined by light intensity, leaf photosynthetic capacity and leaf features such as area and thickness [4] . Robbins and Pharr reported that the photoassimilate translocation from leaves of cucumbers is significantly promoted with alteration of light intensity from 380 μmol m−2∙s−1 to 650 μmol m−2∙s−1 [5] . Nishizawa et al. reported that the amount of carbon exported is higher in tomato seedlings raised under 414 μmol m−2∙s−1 than those raised under 166 μmol m−2∙s−1 PPF [3] . The reason for these differences in photoassimilate translocation might be attributed to both the direct positive effects of light intensity on the activities of enzymes related to photoassimilate translocation and its indirect positive effects on stomata opening [6] [7] .
2.2. Photoperiod
The influence of photoperiod on partitioning of photoassimilates between soluble sugar and starch is considered to determine the concentrations of carbohydrate available for growth of sink organs [8] -[10] . Previous studies showed that the extension of photoperiod can significantly increase the export of photoassimilates from source leaves to sink organs [5] [11] . This can be explained by the results of recent proteomic analysis, which uncover that enzymes involved in reduction phase of Calvin-Benson cycle are more abundant in plants under long day treatment than those under short day treatment [12] . However, it does not mean “the longer, the better”. Moe et al. reported that continuous lighting (24 h) results in reduced yield and serious leaf yellowing of cucumbers [13] .
2.3. Light Quality
Translocation of photoassimilates in crops also responds to light quality. Compared with broad spectrum ones such as daylight fluorescence lamp, red-biased light sources such as high pressure sodium (HPS) lamp enhance carbohydrate levels in the leaves of Digitaria decumbens and Asplenium australasicum [14] [15] . Blue light can stimulate the accumulation of photoassimilates in cucumber source leaves, while photoassimilate export is significantly increased to sink organs by red light [16] . The stimulation of photoassimilate translocation from source leaves to fruits by UV-B light has also been reported [17] . All evidence indicates that the influence of light quality on photoassimilate translocation might be specie-specific.
2.4. Supplemental Lighting
In winter, natural light intensity level is too low to produce fruit vegetables in the region of rigid and temperature zones. Therefore, artificial light has been applied to improve light environment in crop canopy. Now supplemental lighting is an important technology in greenhouse production in the region of middle and high latitudes [18] -[21] . Supplemental lighting is almost exclusively applied on the top of crop canopy (Figure 1). However, top lighting (TP) might not be the optimal, because it provides unequal irradiation distribution, with the top of the canopy receiving more irradiation than the lower parts [22] . Consequently, the lower leaves may at times be below light compensation point, while the upper leaves may be approaching the saturation point of photo-
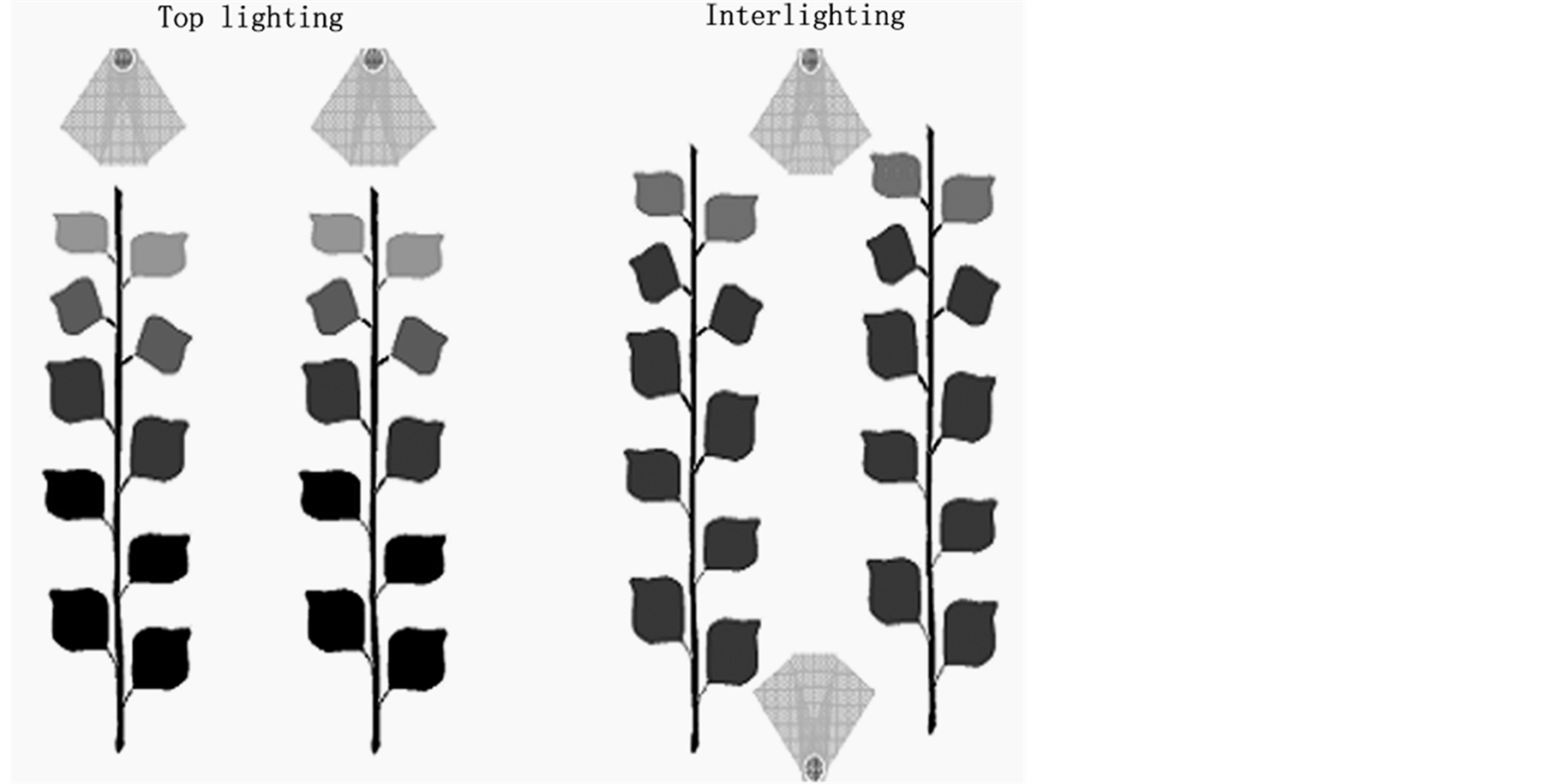
Figure 1.Models for top lighting and interlighting in greenhouses.
synthesis [22] . The insufficient supply of photoassimilates, which results in the competition for assimilates between fruits and vegetative organs, is considered to be an important reason for fruit abortion and retarded growth, which severely influence cucumber yield [23] . Interlighting (IL), which means that part of supplemental lighting is installed between crop rows instead of completely on the top of crop canopy (Figure 1), has raised interests not only among researchers but also among growers of greenhouse vegetables in recent years [24] . Many horticultural crops such as tomato and cucumber have been shown to benefit from interlighting because of the increase of photoassimilate supply from the leaves at the bottom layer (Table 1).
3. Other Factors for Photoassimilate Translocation
3.1. Leaf Age and Leaf Position
Nishizawa and Hori investigated the effects of leaf age and position on photoassimilate translocation from leaves of strawberry plants at the vegetative stage . The results showed that the export of 14C-assimilates increases with the increase of leaf age and reaches to the peak at 40 days after full expansion. In the strawberry plants with 7 fully expanded leaves, where Leaf-7 is the uppermost, the contribution percentage to the total production of photoassimilates is the greatest for Leaf-5 followed by Leaf-6, although the photosynthetic capacity differs little from Leaf-1 to Leaf-5 and is somewhat lower in Leaf-6 and Leaf-7. These variations might be associated with light intercepting efficiency of individual leaf at different nodes.
3.2. Air Temperature
Translocation of photoassimilates in crops is largely influenced by air temperatures . Toki et al. reported that during dark period photoassimilates in mature cucumber leaves are exported within 2 h at 20˚C and 4 h at 16˚C of air temperature . At 10˚C of air temperature, translocation of photoassimilates is strongly inhibited. Miao et al. reported that compared to cucumbers grown at 22˚C of night air temperature, sucrose, stachyose and galactinol contents increase in mature leaves, while sucrose, glucose and fructose contents in fruits remain unchanged at 12˚C . In peduncles, where stachyose is catabolized to sucrose after long-distance transport, cold night simultaneously induces a significant increase of stachyose and a decrease of sucrose, indicating that the metabolic step from stachyose to sucrose is significantly inhibited in peduncles of cold-night grown cucumbers.
3.3. CO2 Concentration
Positive effects of CO2 availability on plant growth and development have been reported in previous studies
Table 1. Effects of different lighting regimes on crop production.
. Peet et al. reported that cucumber leaf starch concentration, sucrose synthase (SS) and sucrose phosphate synthase (SPS) activities are higher under 1000 μmol mol−1 of CO2 concentration than those under 350 μmol mol−1 , indicating that the increasing of CO2 concentration might have positive effects on photoassimilate translocation in cucumbers. Some negative effects by CO2 enrichment on plants have also been reported due to the disturbance of the balance between nitrate and carbon metabolism at the same time .
3.4. Water and Mineral Elements
The supply of water and mineral elements can be involved in the regulation of photoassimilate translocation from source to sink organs of plants as well. Water deficiency is an important factor restricting crop development and yield. Mild water deficiency inhibits shoot growth only and, consequently, can cause plant architecture modification . As a result of this modification, net photosynthesis may decrease and thus the photoassimilate translocation to different sink organs is affected . Mineral deficiency can stimulate photoassimilate translocation from source leaves to mineral element acquisition organs either directly through the enhancement of phloem loading and transport or indirectly through the depression of the need of other sink organs . Consequently, a lager root surface and higher sugar concentration can be observed in plants under mineral element deficiency . However, it is still unclear whether the increased sugar concentration is a stress response and/or a stress signal .
4. Conclusions and Future Prospects
Light is critical for the synthesis and translocation of assimilates from mature leaves to harvestable organs of greenhouse crops. Light environment can be controlled through adjustment of light intensity, photoperiod and light quality by supplemental lighting, which now has become an effective technology for the production of vegetables, fruits, flowers and so on. But there are still some problems which limit the application of light environment control in greenhouse production.
The basic knowledge about light-mediated photoassimilate translocation in plants is still poor because of the remaining ambiguity in the underlying mechanism of source-sink relationship and its interaction with environmental factors. For example, a mismatch between the increase in photosynthesis by supplemental lighting and the increase in resulted yield has been widely reported in previous studies due to the deficiency of sink capacity to fully utilize the increased supply of photoassimilates [41] . Hence, actual crop yield in a given crop-season depends on a complex interplay of light interception, photosynthetic efficiency, sink strength and harvest index. There is ample evidence of heritable variations between different crops and between different cultivars for the characters of light interception, photosynthetic efficiency, sink strength and harvest index, but yet there are still few studies that report the genetic basis of these characters either individually or in combination. If the underlying mechanism for this complex interplay can be illuminated, we can better understand the source-sink relationship in plants and thus optimize the present light environment control technology.
The defects in present supplemental lighting systems are restricting the further application of light environment control technology in greenhouse production as well. The most significant factor is the high capital cost for the design and installation of artificial lighting systems in greenhouses, especially in developing countries such as China. Another problem is that the light spectrum of present artificial light sources such as HPS lamp is not well consistent with the optimal light spectrum of photosynthetic pigments [42] . This mismatch reduces the utilization efficiency of artificial lighting and thus increases the production cost in greenhouses. In order to extend the application of artificial lighting in greenhouse production, people should reduce the price of the present lighting systems through technique innovation and develop new artificial light sources which can be well consistent with plant needs. LED lamp is a kind of solid artificial light source with longer lifespan, less heat emission and narrower spectrum compared with the traditional lamps and recently has been introduced into greenhouse production [43] -[45] . In theory we can develop lighting systems with optimal spectrum for specific crops through combination of different LED lamps, but still there are few studies about how LEDs are combined and how crops respond to these combinations.
Light environment control can be realized not only by supplemental lighting but also by cover materials such as plastic film and shade net in greenhouses [46] . Especially in middle-latitude or low-latitude areas such as Central China and South China, cover materials might be a more economic approach than supplemental lighting because light intensity in these areas is enough to grow most of horticultural crops in greenhouses even in winter. However, environmental changes including not only light but also temperature and relative humidity in greenhouses by cover materials are much more complex than those in greenhouses with supplemental lighting [47] [48] . As a result, the responses of crops might be quite different to cover materials from those to artificial light sources. More studies should thus be done in order to promote the application of functional cover materials in greenhouse production in the future.
Acknowledgements
This work was supported by Research Award Fund for Outstanding Middle-Aged and Young Scientist of Shandong Province (NO. BS2011NY010), Research Fund for the Doctoral Program of Higher Education of China (20113702120008) and the China Agriculture Research System (CARS-25-D).
References
- Pandey, M., Srivastava, A.K., D’Souza, S.F. and Penna, S. (2013) Thiourea, a ROS Scavenger, Regulates SourceTo-Sink Relationship to Enhance Crop Yield and Oil Content in Brassica juncea (L.). PLoS One, 8, e73921. http://dx.doi.org/10.1371/journal.pone.0073921
- Ruan, Y.L., Patrick, J.W., Shabala, S. and Slewinski, T.L. (2013) Uptake and Regulation of Resource Allocation for Optimal Plant Performance and Adaptation to Stress. Frontier in Plant Science, 4, 455.
- Nishizawa, T., Shishido, Y. and Murakami, H. (2009) Effect of Temporary Changes in Light Intensity on Carbon Transport, Partitioning and Respiratory Loss in Young Tomato Seedlings Raised under Different Light Intensities. Physiologia Plantarum, 136, 351-357. http://dx.doi.org/10.1111/j.1399-3054.2009.01241.x
- Grodzinski, B., Jiao, J., Knowles, V.L. and Plaxton, W.C. (1999) Photosynthesis and Carbon Partitioning in Transgenic Tobacco Plants Deficient in Leaf Cytosolic Pyruvate Kinase. Plant Physiology, 120, 887-895. http://dx.doi.org/10.1104/pp.120.3.887
- Robbins, N.S. and Pharr, D.M. (1987) Regulation of Photosynthetic Carbon Metabolism in Cucumber by Light Intensity and Photosynthetic Period. Plant Physiology, 85, 592-597. http://dx.doi.org/10.1104/pp.85.2.592
- Hashimoto-Sugimoto, M., Higaki, T., Yaeno, T., Nagami1, A., Irie, M., Fujimi, M., Miyamoto, M., Akita, K., Negi, J., Shirasu, K., Hasezawa, S. and Iba, K. (2013) A Munc13-Like Protein in Arabidopsis Mediates Hþ-ATPase Translocation that Is Essential for Stomatal Responses. Nature Communication, 4, 2215. http://dx.doi.org/10.1038/ncomms3215
- Yonekura, M., Aoki, N., Hirose, T., Onai, K., Ishiura, M., Okamura, M., Ohsugi, R. and Ohto, C. (2013) The Promoter Activities of Sucrose Phosphate Synthase Genes in Rice, OsSPS1 and OsSPS11, Are Controlled by Light and Circadian Clock, but Not by Sucrose. Frontiers in Plant Science, 4, 31. http://dx.doi.org/10.3389/fpls.2013.00031
- Andriotis, V.M.E., Pike, M.J., Schwarz, S.L., Rawsthorne, S., Wang, T.L. and Smith, A.M. (2012) Altered Starch Turnover in the Maternal Plant Has Major Effects on Arabidopsis Fruit Growth and Seed Composition. Plant Physiology, 160, 1175-1186. http://dx.doi.org/10.1104/pp.112.205062
- Stitt, M. and Zeeman, S.C. (2012) Starch Turnover: Pathways, Regulation and Role in Growth. Current Opinion in Plant Biology, 15, 282-292. http://dx.doi.org/10.1016/j.pbi.2012.03.016
- Martins, M.C.M., Hejazi, M., Fettke, J., Steup, M., Feil, R., Krause, U., Arrivault, S., Vosloh, D., Figueroa, C.M., Ivakov, A., Yadav, U.P., Piques, M., Metzner, D., Stitt, M. and Lunn, J.E. (2013) Feedback Inhibition of Starch Degradation in Arabidopsis Leaves Mediated by Trehalose 6-Phosphate. Plant Physiology, 163, 1142-1163. http://dx.doi.org/10.1104/pp.113.226787
- Shishido, Y., Seyama, N., Imada, N. and Hori, Y. (1990) Effect of the Photosynthetic Light Period on the Carbon Budget of Young Tomato Leaves. Annals of Botany, 66, 729-735.
- Victor, K.J., Fennel, A.Y. and Jérôme Grimplet, J. (2010) Proteomic Analysis of Shoot Tissue during Photoperiod Induced Growth Cessation in V. riparia Michx. Grapevines. Proteome Science, 8, 44. http://dx.doi.org/10.1186/1477-5956-8-44
- Moe, R., Grimstad, S.O. and Gislerǿd, H.R. (2006) The Use of Artificial Light in Year Round Production of Greenhouse Crops in Norvey. Acta Horticulturae, 711, 35-42. http://www.actahort.org/books/711/711_2.htm
- Britz, S.J., Hungerford, W.E. and Lee, D.R. (1985) Photoperiodic Regulation of Photosynthate Partitioning in Leaves of Digitaria decumbens. Plant Physiology, 78, 710-714. http://dx.doi.org/10.1104/pp.78.4.710
- Leong, T.Y., Goodchild, D.J. and Anderson, J.M. (1985) Effect of Light Quality on the Composition, Function, and Structure of Photosynthetic Thylakoid Membranes of Asplenium australasicum (Sm.) Hook. Plant Physiology, 78, 561-567. http://dx.doi.org/10.1104/pp.78.3.561
- Wang, Z., Kong, Y., Cheng, J. and Yang, R. (2008) Effects of Supplied Light on Photosynthetic Characteristics and Translocation of 14C-Assimilates of Cucumber Growing under Solar Greenhouse. Transactions of the CSAE, 24, 203-206. http://en.cnki.com.cn/Article_en/CJFDTOTAL-NYGU200809044.htm
- Yu, N., Li, D., Tan, Q., Zhang, H. and Gao, D. (2013) Effect of UV-B Radiation on Assimilate Translocation and Distribution in Fruiting Shoot of Protected Peach. Chinese Journal of Applied and Environmental Biology, 19, 157-163. http://www.cibj.com/en/oa/DArticle.aspx?type=view &id =201112022
- Blancharda, M.G., Runklea, E.S. and Fisherb, P.R. (2011) Modeling Plant Morphology and Development of Petunia in Response to Temperature and Photosynthetic Daily Light Integral. Scientia Horticulturae, 129, 313-320. http://dx.doi.org/10.1016/j.scienta.2011.03.044
- Kjaera, K.H., Ottosena, C.O. and Jørgensenb, B.N. (2012) Timing Growth and Development of Campanula by Daily Light Integral and Supplemental Light Level in a Cost-Efficient Light Control System. Scientia Horticulturae, 143, 189-196. http://dx.doi.org/10.1016/j.scienta.2012.06.026
- Lu, N., Maruo, T., Johkan, M., Hohjo, M., Tsukagoshi, S., Ito, Y., Ichimura, T. and Shinohara, Y. (2012) Effects of Supplemental Lighting with Light-Emitting Diodes (LEDs) on Tomato Yield and Quality of Single-Truss Tomato Plants Grown at High Planting Density. Environment Control in Biology, 50, 63-74. https://www.jstage.jst.go.jp/article/ecb/50/1/50_63/_pdf http://dx.doi.org/10.2525/ecb.50.63
- Hidaka, K., Dan, K., Imamura, H., Miyoshi, Y. and Takayama, T. (2013) Effect of Supplemental Lighting from Different Light Sources on Growth and Yield of Strawberry. Environment Control in Biology, 51, 41-47. https://www.jstage.jst.go.jp/article/ecb/51/1/51_41/_pdf http://dx.doi.org/10.2525/ecb.51.41
- Hovi, T., Näkkilä, J. and Tahvonen, R. (2004) Interlighting Improves Production of Year-Round Cucumber. Scientia Horticulturae, 102, 283-294. http://dx.doi.org/10.1016/j.scienta.2004.04.003
- Marcelis, L.M.F. (1992) The Dynamics of Growth and Dry Matters Distribution in Cucumber. Annals of Botany, 69, 487-492. http://aob.oxfordjournals.org/content/69/6/487
- Hovi-Pekkanen, T. and Tahvonen, R. (2008) Effects of Interlighting on Yield and External Fruit Quality in YearRound Cultivated Cucumber. Scientia Horticulturae, 116, 152-161. http://dx.doi.org/10.1016/j.scienta.2007.11.010
- Gómez, C., Morrow, R.C., Bourget, C.M., Massa, G.D. and Mitchell, C.A. (2013) Comparison of Intracanopy LightEmitting Diode Towers and Overhead High-Pressure Sodium Lamps for Supplemental Lighting of Greenhouse-Grown Tomatoes. HortTechnology, 23, 93-98. http://horttech.ashspublications.org/content/23/1/93.full.pdf+html
- Hao, X., Zheng, J.M., Little, C. and Khosla, S. (2012) LED Inter-Lighting in Year-Round Greenhouse Mini-Cucumber Production. Acta Horticulturae, 956, 335-340. http://www.actahort.org/books/956/956_38.htm
- Nishizawa, T. and Hori, Y. (1986) Translocation of 14C-Assimialtes from Leaves of Strawberry Plants in the Vegetative Stage as Affected by Leaf Age and Leaf Position. Journal of the Japanese Society for Horticultural Science, 54, 467-476. https://www.jstage.jst.go.jp/article/jjshs1925/54/4/54_4_467/_pdf http://dx.doi.org/10.2503/jjshs.54.467
- Lundmark, M., Cavaco, A.M., Trevanion, S. and Hurry, V. (2006) Carbon Partitioning and Exporting Transgenic Arabidopsis Thaliana with Altered Capacity for Sucrose Synthesis Grown at Low Temperature: A Role for Metabolite Transporters. Plant, Cell and Environment, 29, 1703-1714. http://dx.doi.org/10.1111/j.1365-3040.2006.01543.x
- Thorpe, M.R., Furch, A.C., Minchin, P.E., Föller, J., VanBel, A.J. and Hafke, J.B. (2010) Rapid Cooling Triggers Forisome Dispersion Just before Phloem Transport Stops. Plant, Cell and Environment, 33, 259-271. http://dx.doi.org/10.1111/j.1365-3040.2009.02079.x
- Toki, T., Ogiwara, H. and Aoki, H. (1978) Effect of Varying Night Temperature on the Growth and Yields in Cucumber. Acta Horticulturae, 87, 233-237. http://www.actahort.org/books/87/87_24.htm
- Miao, M.M., Xu, X.F., Chen, X.H., Xue, L.B. and Cao, B.S. (2007) Cucumber Carbohydrate Metabolism and Translocation under Chilling Night Temperature. Journal of Plant Physiology, 164, 621-628. http://dx.doi.org/10.1016/j.jplph.2006.02.005
- Lee-Ho, E., Walton, L.J., Reid, D.M., Yeung, E.C. and Kurepin, L.V. (2007) Effects of Elevated Carbon Dioxide and Sucrose Concentrations on Arabidopsis Thaliana Root Architecture and Anatomy. Canadian Journal of Botany, 85, 324-330. http://dx.doi.org/10.1139/B07-009
- Peet, M.M., Huber, S.C. and Patterson, D.T. (1986) Acclimation to High CO2 in Monoecious Cucumbers. II Carbon Exchange Rates, Enzyme Activities, and Starch and Nutrient Concentrations. Plant Physiology, 80, 63-67. http://dx.doi.org/10.1104/pp.80.1.63
- Pleijel, H. and Uddling, J. (2012) Yield vs. Quality Trade-Offs for Wheat in Response to Carbon Dioxide and Ozone. Global Change Biology, 18, 596-605. http://dx.doi.org/10.1111/j.1365-2486.2011.2489.x
- Courtois, B., Mclaren, G., Sinha, P.K., Prasad, K., Yadav, R. and Shen, L. (2000) Mapping QTLs Associated with Drought Avoidance in Upland Rice. Molecular Breeding, 6, 55-66. http://link.springer.com/article/10.1023%2FA%3A1014805625790#page-1
- Lebon, E., Pellegrino, A., Louarn, G. and Lecoeur, J. (2006) Branch Development Controls Leaf Area Dynamics in Grapevine (Vitis vinifera) Growing in Drying Soil. Annual of Botany, 98, 175-185.
- Lemoine, R., La Camera, S., Atanassova, R., Dédaldéchamp, F., Allario, T., Pourtau, N., Bonnemain, J.L., Laloi, M., Coutos-Thévenot, P., Maurousset, L., Faucher, M., Girousse, C., Lemonnier, P., Parrilla, J. and Durand, M. (2013) Source-To-Sink Transport of Sugar and Regulation by Environmental Factors. Frontiers in Plant Science, 4, 272. http://dx.doi.org/10.3389/fpls.2013.00272
- Marschner, H., Kirkby, E.A. and Cakmak, I. (1996) Effect of Mineral Nutritional Status on Shoot-Root Partitioning of Photoassimilates and Cycling of Mineral Nutrients. Journal of Experimental Botany, 47, 1255-1263. http://dx.doi.org/10.1093/jxb/47.Special_Issue.1255
- Hermans, C., Hammond, J.P., White, P.J. and Verbruggen, N. (2006) How Do Plants Respond to Nutrient Shortage by Biomass Allocation? Trends in Plant Science, 11, 610-617. http://dx.doi.org/10.1016/j.tplants.2006.10.007
- Peuke, A.D. (2010) Correlations in Concentrations, Xylem and Phloem Flows, and Partitioning of Elements and Ions in Intact Plants. A Summary and Statistical Reevaluation of Modeling Experiments in Ricinus communis. Journal of Experimental Botany, 61, 635-655. http://dx.doi.org/10.1093/jxb/erp352
- Zhu, X.G., Long, S.P. and Ort, D.R. (2010) Improving Photosynthetic Efficiency for Greater Yield. Annual Review of Plant Biology, 61, 235-261. http://dx.doi.org/10.1146/annurev-arplant-042809-112206
- Liu, Z.L., Ma, C.W. and Yang, Q.C. (2004) Review on Controlling the Ratio of Red Light to Far-Red Light in Protected Environment. Journal of Transactions of the CSAE, 20, 270-273. http://d.wanfangdata.com.cn/periodical_nygcxb200401065.aspx
- Wang, X., Guo, W., Gao, G. and Shen, G. (2005) Gate of Dawn-LED Will Illuminate the Future. Advanced Display, 53, 15-19.
- Wang, X., Wu, G., Jiang, W., Bian, S. and Li, S. (2006) LED’s Principle and Its Application. Light and Lighting, 4, 32- 35. http://mall.cnki.net/magazine/Article/LAMP200604010.htm
- Zhang, H., Yang, Q., Hu, J., Fan, H., Dai, J. and Zhao, B. (2011) Self-Adaptive and Precise Supplementary Lighting System for Plant with Controllable LED Intensity. Journal of Transactions of the CSAE, 27, 153-158. http://d.wanfangdata.com.cn/periodical_nygcxb201109027.aspx
- Yang, X., Wang, X., Wang, L. and Wei, M. (2012) Control of Light Environment: A Key Technique for High-Yield and High-Quality Vegetable Production in Protected Farmland. Agricultural Sciences, 3, 923-928. http://dx.doi.org/10.4236/as.2012.37112
- Li, Q., Wang, X., Chu, M., Chen, X., Mi, Q., Wei, M., Shi, Q. and Yang, F. (2010) Effects of Neotype Greenhouse Film on Light and Temperature, Growth and Development of Tomato in Greenhouse. Shandong Agricultural Sciences, 3, 41-45. http://d.wanfangdata.com.cn/periodical_shandnykx201003011.aspx
NOTES

*Corresponding author.


