Agricultural Sciences
Vol.04 No.03(2013), Article ID 28803, 6 pages
doi:10.4236/as.2013.43018
Action modes of Aloe vera L. extracts against Tetranychus cinnabarinus Boisduval (Acarina: Tetranychidae)
![]()
College of Plant Protection, Southwest University, Chongqing, China; *Corresponding Author: zhangyq80@tom.com, zhangyq881@126.com
Received 21 January 2013; revised 4 March 2013; accepted 15 March 2013
Keywords: Aloe vera L.; Acaricidal Activity; Repellent Activity; Fumigant Activity; Oviposition Inhibition Activity; Tetranychus cinnabaribus
ABSTRACT
Objective: This paper mainly determined the action modes of extract of Aloe vera L. against Tetranychus cinnabarinus Boisduval. Methods: The different action modes, contact action, repellent, fumigant, and oviposition inhibition property of the acetone extract of Aloe vera L. leaf against the carmine spider mite Tetranychus cinnabaribus (Boisduval) (Acarina: Tetranychidae) were investigated at 26˚C ± 1˚C, 75% - 80% relative humidity, and 14:10 light: day cycle in the laboratory. Results: Based on the established toxicity regression line of the Aloe vera L. acetone extract against female adult mites, the median lethal concentrations (LC50) were 0.836 and 0.167 mg/mL for 48 and 72 h, respectively. With processing time increased, the contact acaricidal activity increased and the repellent activity gradually decreased. The main modes of action of the extract against female adult mites were contact and repellent, and preferable effects were observed on adult mites. These results indicate that A. vera L. extract contains acaricidal and repellent bioactive components that may be useful in future control of the phytophagous mites.
1. INTRODUCTION
The carmine spider mite, Tetranychus cinnabarinus Boisduval is a globally important pest. This mite can harm 100 kinds of field, greenhouse crop, and fruit tree [1]. The yield and quality of plants are seriously affected by these pests, which are small, fast breeding, strongly adaptive, and can easily develop resistance. Indeed, this pest is very difficult to control and harmful [2]. The control of mites mainly depends on chemical pesticides, which are frequently and excessively used. Consequently, the drug-resistance of mite species is intensified. Pesticide abuse can also lead to the eradication of natural enemy insects and damage to the ecological balance, which result in the problems of resistance, resurgence, and residue. Therefore, the key to comprehensive mite control is to choose an acaricidal agent that is selective, environment friendly, and able to undergo natural degradation [3].
Aloe vera L., a member of the Liliaceae family, is a perennial, evergreen, herbaceous plant. Also known as medicinal aloe, the medicinal value of A. vera L. is widely recognized by the medical profession and traditional Chinese medicine practitioners. A. vera L. also has good antibacterial, acaricidal, and insecticidal activities; it plays an important role in killing pests in granaries [4]. However, only its ovicidal activity and contact toxicity have been confirmed [5]. Whether the mode of insectkilling action of the carmine spider mite is limited to its contact toxicity and ovicidal activity is undetermined. The existence of other modes of actions and the effects of such actions on other modes of action are also unknown. This article investigated four modes of actions, namely, contact, repellent, fumigant, and oviposition inhibition activities, of an aloe acetone extract against carmine female spider mites. The purpose was to lay the solid foundation for the development of a new plant source of an acaricidal agent.
2. MATERIALS AND METHODS
2.1. Plant Materials and Extraction
Fresh A. vera L. leaves were collected, chopped into small pieces, placed in an oven, and dried at 50˚C. The leaves were then pulverized into plant powder using a by crusher. The plant powder was weighed and placed in a 10 L grinding mouth bottle. The plant powder was immersed in 100% acetone with 1 g of ground material and 5.0 mL of acetone while intermittently shaking. The acetone extract was filtered and evaporated at 50˚C using a vacuum rotary evaporator. The plant extract was transferred in Petri dishes. The extract was washed three times and stored in a refrigerator at 4˚C until use.
2.2. Preparation of Mites
The mites used were from a T. cinnabarinus population sampled from young cowpea plants (Vigna unguiculata) in Beibei District, Chongqing, China. The mites were maintained in potted young cowpea plants in a laboratory at 26˚C ± 1˚C, 75% - 80% relative humidity (RH), and 14 h light/10h dark cycle with no acaricide exposure for more than 13 years. Voucher specimens, as part of an insect collection, were deposited at Southwest University.
2.3. Chemical Reagents
Analytical-grade acetone was purchased from Chengdu Kelong Chemical Reagents Co., Chengdu, China. Tween-80 F was purchased from Jiangsu Haian Petrochemical Plant, China.
2.4. Contact Toxicity Bioassay
The immersion method recommended by FAO [6] was used to examine the acaricidal activity of the acetone extract from A. vera L. against T. cinnabaribus engorged female adults. Double-sided adhesives were cut into 2 cm-long pieces and stuck to one end of the slides. A total of 35 female adult mites, which were uniform in size, color, and brightness, were stuck on their back onto double-sided gum (which does not stick onto mite feet, antennae, and mouth) using a zero brush. They were bred on the cushion of a wet sponge white disk and for 4 h before examination under a stereomicroscope (4× magnification). Then, some dead and unhealthy mites on the slides were removed.
About 5.00 mg of extract was mixed with and completely dissolved in a certain amount of acetone. Then, A 0.1% Tween-80 [7] was added to the mother aqueous solution, which was diluted into different concentrations (2.50, 1.25, 0.635, 0.3125 mg/mL) with water controls against T. cinnabarinus adults. The treatments were performed by dipping the slides with mites into each extract solution for 5 s. Any extra solution on the slides was carefully absorbed with filter paper after treatment. The controls were dipped in water only; they were subjected to the same conditions of 25˚C ± 1˚C, 65% - 80% RH, and 14 h light/10h dark photoperiod for 3 days. The mites were considered dead if their movement was imperceptible after repeated gentle probing with a fine brush. Mortality was assessed under a binocular microscope every 24 h. [8].

2.5. Repellency Bioassay
Based on the method of Hussen et al. [9], fresh cowpea seedling leaves were placed in Petri dishes. Half of the leaf surface was carefully wiped with treatment reagent; the other half was wiped with the same proportion of leaf solvent control. After the solution dried, 20 female adult mites were collected from each leaf. Each treatment was repeated three times. The number of repellency was assessed under a binocular microscope after every 12 h for 4 days. The repellency rates were calculated as follows:

2.6. Fumigation Bioassay
Based on the fumigate method of the tin method [10] with minor modifications, 20 female mites on each leaf were fresh cowpea seedlings. Leaves with mites, the absorbent cotton, and the filter in Petri dishes were placed in a 100 mL tin bottom. Then, three lethal concentrations of the leaf acetone extract to 30% (LC30), 50% (LC50), and 70% (LC70) of adults were injected. The same proportion of the solvent controls was also injected into the filter paper of the bottle caps as soon as the bottle caps were covered. The bottles were then moved into artificial climate indoors. Before observation, the bottle was kept open for 1 h, and the mites along with the cotton filter paper were removed for observation every 24 h. The mortality of the female mites was recorded. For each application, 15 replicates were tested. Continuous observation was conducted for 5 days. After the experiment, the corrected mortality was calculated as follows:

2.7. Oviposition Inhibition Bioassay
The leaf disk method was used to evaluate the oviposition inhibition activity of female carmine spider mites. Leaves from young cowpea plants, filter paper, and absorbent cotton were placed in Petri dishes. The back of leaves was carefully wiped with different concentrations of treatment reagent using swabs. Another three Petri dishes were set at the same proportion of solvent solution for comparison. After the solution dried, 20 female adult mites were collected from each leaf. The numbers of mites in the extract-treated and non-treated areas were counted every 12 h post-treatment under a stereomicroscope (4´ magnification). The experiment lasted for 6 days. When the experiment was finished, the oviposition inhibition rate was calculated as follows:
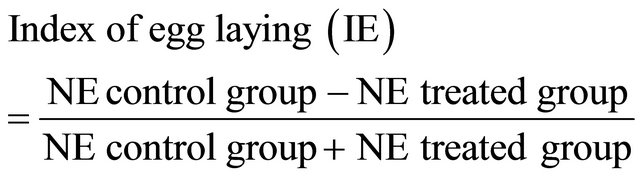

where NE is the number of eggs laid.
2.8. Data Analysis
All mortality results were corrected using Abbott’s formula in terms of the aloe acetone extracts with control. The data were analyzed with one-way ANOVA followed by Tukey’s multiple range test at P < 0.05 using SPSS 17.0 software. The LC50 and LC99 values, as well as their respective 95% confidence intervals (CIs), were calculated by probit analysis (Polo Plus software). The repellent and oviposition inhibition rates were analyzed with software of Microsoft Excel 2003 and SPSS 17.0.
3. RESULTS
3.1. Toxicity of the Acetone Extract of A. vera L. Leaf to T. cinnabarinus
The aloe acetone extract showed strong contact acaricidal activity against T. cinnabarinus female adults (Table 1). The LC50 was 0.836 mg/mL, and the 95% CI ranged from 0.716 mg/mL to 0.964 mg/mL. The LC30, LC50, and LC70 values were calculated at different processing times (Table 2). At 48 h after treatment, the LC30, LC50, and LC70 values were 0.473, 0.836, and 1.476 mg/mL. These three concentrations were used to measure the fumigant, repellent, and egg laying inhibition activities of the aloe acetone extract against T. cinnabarinus female adults.
3.2. Repellent Effect of the Acetone Extract of A. vera L. Leaf against T. cinnabarinus
The repellent rates after treatment for 96 h were obtained from the LC30, LC50, and LC70 values of the aloe acetone extract against T. cinnabarinus female adults. Figure 1 shows that after treatment for 12 h, remarkable repellent activities at the LC30, LC50, and LC70 values were observed. After testing for 12 and 48 h, the mites’ rate of treatment reached 50%. The repellent rates at the three concentrations showed more fluctuations with the processing time. With increased processing time, the repellent rates of LC30, LC50, and LC70 showed lower rates than that after 12 h of testing. The female carmine spider mites may control eating when food is abundant. However, under food shortage, the mites feed in other areas to guarantee their survival. At LC50 and LC70, low mortality was observed, which also explained the gradual decrease in the LC50 and LC70 values. In summary, the acetone extract of A. vera L. leaf had some repellent effect against T. cinnabarinus.
3.3. Fumigation Effect of the Acetone Extract of A. vera L. Leaf against T. cinnabarinus
The LC30, LC50, and LC70 values of the aloe acetone extract were determined. The corrected fumigating morTable 1. Toxicity regression line of the acetone crude extract from Aloe vera L. against Tetranychus cinnabarinus female adults in the laboratory.
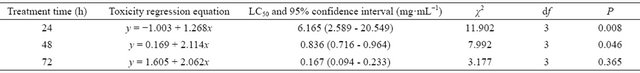
Table 2. Lethal concentrations (in mg·mL−1) of the acetone crude extract from Aloe vera L. against Tetranychus cinnabarinus female adult in the laboratory.
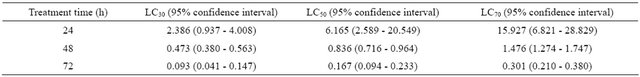
Note: LC30, LC50, and LC70 are the lethal concentrations that killed 30%, 50%, and 70% of T. cinnabarinus. Each extract solution had five concentrations, and tests were replicated three times. The control solution comprised water with 1% Tween-80. P < 0.05 (ANOVA, followed by Duncan).
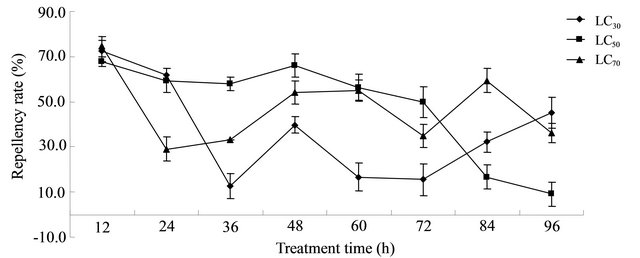
Figure 1. Repellent activity of the acetone extract of A. vera L. leaf against Tetranychus cinnabarinus adults.
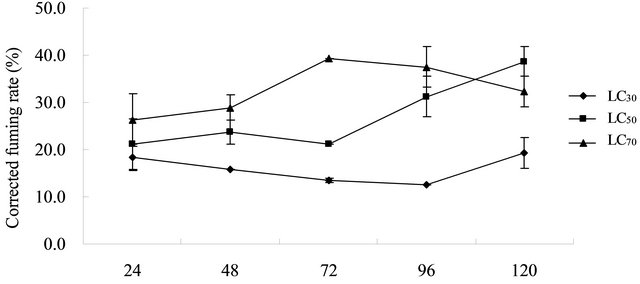
tality increased with increased concentration of aloe acetone extract (Figure 2). The maximum LC70 value fumigating mortality rate against T. cinnabarinus adults reached 39.39%, which also indicated that the LC70 value of the aloe acetone extract against T. cinnabarinus adults for 120 h. The average corrected fumigating mortality rates reached 32.88%. The average corrected fumigating mortality rates for LC30 and LC50 were 15.92% and 27.18%. In this experiment, the symptoms of mite death were their clinging back to cowpea leaves, pointed-up feet, and limb stiffness. Although the fumigating mortality rate slightly differed with prolonged processing time, the fumigating mortality result was not significant. The fumigating mortality rate was generally below 40% based on the overall results. Thus, the three concentrations of Aloe vera acetone extract against T. cinnabarinus adults under the same test conditions had minimal fumigating activity.
3.4. Egg-Laying Effect of the Acetone Extract of A. vera L. Leaf against T. cinnabarinus
The result of the oviposition inhibition activity was obtained from the LC30, LC50, and LC70 values of the acetone extract of A. vera L. leaf against T. cinnabarinus female mites (Figure 3). The oviposition inhibition rate of LC30 decreased with prolonged processing time. The concentration of the oviposition inhibition rate effect was not significant. For the LC30, LC50, and LC70 values, the average oviposition inhibition rates were 1.83%, 27.50%, and 62.86% for 6 days. The LC30 and LC50 values had some oviposition inhibition activity; however, the activity was low. Especially after 84 h, the oviposition inhibition activity of LC30 was not obvious. LC70 showed that the acetone extract of A. vera L. had a significantly higher oviposition inhibition activity against T. cinnabarinus adults, and the highest oviposition inhibition rate reached 83.33%. The aloe acetone extract against the cinnabar of female adult mites had a certain spawning inhibited activity. In summary, the acetone extracts of A. vera L. had some oviposition inhibition activity against T. cinnabarinus adults. However, the activities were not very strong and cannot last for a long time.
4. DISCUSSION
This study investigates the contact acaricidal, repellent, fumigant, and oviposition inhibition activities of the acetone extract of A. vera L. leaf against Tetranychus cinnabarinus. The aloe acetone extract was found to have good contact acaricidal activity against the cinnabar of female adult mites. Through the toxicity regression line of the aloe acetone extract against female carmine spider mites, the LC50 values to T. cinnabarinus were found to be 0.836 and 0.167 mg/mL for 48 and 72 h, respectively. Wei et al. [5] reported LC50 values of 0.614 and 0.099 mg/mL for 48 and 72 h, respectively. Various factors can influence the bioassay results, such as the mites’ physical condition, different geographical location, time of bioassay, environmental temperature, pharmaceutical activity, solubility, and method. All of these factors lead to different results.
This study confirmed that the main modes of action of the aloe acetone extract against female carmine spider mites were contact and repellent. A preferable effect was observed on adult T. cinnabarinus. With processing time increased, the contact acaricidal activity increased and the repellent activity gradually decreased. A significant change was not observed in the fumigant and oviposition inhibition activities. Therefore, during potion excogitation and development process, more attention should be paid to extend the persistence of agentia and increase the potential of slow release. After insecticidal application, the agentia can play a role in the repellent activity against female carmine spider mites in the first two days, and also restrain the female mites’ eggs. Then, drugs mainly exert contact acaricidal and repellent activities, which can effectively control the mite population.
Female carmine spider mites usually complete a gen-
Treatment time (h)
Figure 2. Fumigating effect of the acetone extract of A. vera L. leaf against Tetranychus cinnabarinus.
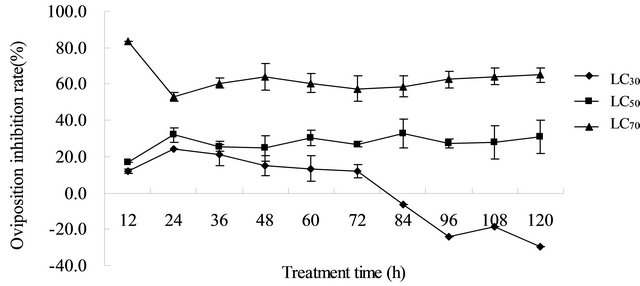
Figure 3. Oviposition inhibition activity of Aloe vera L. extracts against the carmine spider mite Tetranychus cinnabarinus after 120 h. Means followed by a different letter significantly differ from the control (P < 0.05).
eration for only a week or so. Serious generation overlaps may occur. The mites also have a high reproductive potential, and frequent acaricidal agent use can result in resistance [11]. Therefore, safe, efficient, and environment-friendly acaricidal agents from plants have emerged as desirable replacements for chemical pesticides.
Acaricidal agents from different plant sources control insects (mites) through a variety of functions and mechanisms [12]. Cheng found that the insecticide Rhodojaponin-III mainly prevents the food intake of and induces stomach toxicity in Spodoptera litura [13]. Its contact acaricidal activity is weak. This finding is similar to the repellent and oviposition inhibition activities of Azadirachta indica A. Juss against Polyphagotarsonemus latus female mites [14,15]. Mansour et al. [3] examined 29 kinds of local plant species with T. cinnabarinus, and found that 16 species have repellent and reproduction inhibition activities. The biological activity and seconddary metabolism of plants, as well as repellent and reproduction inhibition activities, have received more attention than other modes of action. George assayed seven kinds of plant essential oils that are toxic to pests, and found that they have repellent activity against Dermanyssus gallinae [16]. Among the oils, thyme essential oil has the best repellent effect, which can last until the experiment end (13 days). Kumral et al. also reported that Datura stramonium L. leaves and seeds ethanol extracts act against T. urticae Koch female adults via repellent and reproduction inhibition activities [17].
The aforementioned results indicate that plant insecticidal activity has a variety of mechanisms characterized by effect strengthening and decreased resistance. Thus, mites can be effectively controlled.
The contact acaricidal, repellent, fumigant, and oviposition inhibition activities of the A. vera L. leaf acetone extract have a good effect against Tetranychus cinnabarinus. These observations have not yet been reported. The application of an acaricidal agent from a plant source to control T. cinnabarinus has directive significance. The results of this study can aid the development of agricultural and biological foods. Indeed, A. vera L. is a valuable research tool because it contains potent materials. This study also provides a new approach to the research and development of pesticides from natural products.
5. ACKNOWLEDGEMENTS
This work was financially supported by the Doctoral New Teachers Fund of the Ministry of Education of China (20100182120021) and Chongqing Fund for Natural Science (cstc2011jjA80004).
![]()
![]()
REFERENCES
- Hazan, A., Gerson, U. and Tahori, A.S. (1974) Spider mite webbing I. The production of webbing under various environmental conditions. Acarologia, 16, 68-84.
- Zhang, Y.Q., Ding, W., Zhao, Z.M., Wu, J. and Fan, Y.H. (2008) Studies on acaricidal bioactivities of Artemisia annua L. extracts against Tetranychus cinnabarinus bois. (Acari: Tetranychidae). Agricultural Science in China, 7, 577-584. doi:10.1016/S1671-2927(08)60055-3
- Mansour, F., Azaizeh, H., Saad, B., Tadmor, Y., AboMoch, F. and Said, O. (2004) The potential of middle eastern flora as a source of new safe bio-acaricides to control Tetranychus cinnabarinus, the carmine spider mite. Phytoparasitica, 32, 66-72.
- Morsy, T.A., El-Ela, R.G.A., Nasser, M.M.I., Khalaf, S.A.A. and Mazyad, S.A.M. (2000) Evaluation of the invitro pediculicidal action of four known insecticides and three medicinal plant extracts. Journal of the Egyptian Society of Parasitology, 30, 699-708.
- Wei, J., Ding, W., Zhao, Y.G. and Patcharaporn, V. (2011) Acaricidal activity of Aloe vera L. leaf extracts against Tetranychus cinnabarinus (Boisduval) (Acarina: Tetranychidae). Journal of Asia-Pacific Entomology, 14, 353-356. doi:10.1016/j.aspen.2011.04.006
- FAO (2004) Resistance management and integrated parasites control in ruminants-guidelines. Module 1: Ticks: Acaricide Resistance, Diagnosis, Management and Prevention, Food and Agriculture Organization, Animal Production and Health Division, Rome, 25 p.
- Abbott, W.S. (1925) A method of computing the effectiveness of an insecticide. Journal of Economic Entomology, 18, 265-267.
- Helene, C., André, B., Noubar, B., Charles, V. and André, P. (2001) Acaricidal properties of Artemisia absinthium and Tanacetum vulgare (Asteraceae) essential oils obtained by three methods of extraction. Journal of Economic Entomology, 94, 57-59. doi:10.1603/0022-0493-94.1.167
- Hussen, H., Abou-Elella, M., Amer, S.A.A. and Momen, F.M. (2006) Repellency and toxicity of extracts from Capparis aegyptia L. to Tetranychus urticae Koch (Acari: Tetranychidae). Acta Phytopathologica et Entomologica Hungarica, 41, 331-340. doi:10.1556/APhyt.41.2006.3-4.15
- Zhao, S.H. (2001) Chemical protection of plants. China Agriculture Press, Beijing, 57 p.
- Sertkaya, E., Kaya, K. and Soylu, S. (2010) Acaricidal activities of the essential oils from several medicinal plants against the carmine spider mite (Tetranychus cinnabarinus Boisd) (Acarina: Tetranychidae). Industrial Crops and Products, 31, 107-112. doi:10.1016/j.indcrop.2009.09.009
- Knowles, C.O. and Ahmad, S. (1971) Mode of action studies with for metanate and form paranate acaricides. Pesticide Biochemistry and Physiology, 1, 445-452. doi:10.1016/0048-3575(71)90177-5
- Cheng, D.M., Zhang, Z.X. and Hu, M.Y. (2007) Mode of action of Rhodojaponin-III on the larvae of Spodoptera litura F. and its effect on amount glucose of body fluid. Journal of Huazhong Agricultural University, 26, 306- 309.
- Venzon, M., Rosado, M.C., Molina-Rugama, A.J., Duarte, V.S., Dias, R. and Pallini, A. (2008) Acaricidal efficacy of neem against Polyphago tarsonemuslatus (Banks) (Acari Tarsonemidae). Crop Protection, 27, 869-872. doi:10.1016/j.cropro.2007.10.001
- Tsolakis, H. and Ragusa, S. (2008) Effects of a mixture of vegetable and essential oils and fatty acid potassium salts on Tetranychus urticae and Phytoseiulus persimilis. Ecotoxicology and Environmental Safety, 70, 276-282. doi:10.1016/j.ecoenv.2007.10.001
- George, D.R., Sparagano, O.A.E., Port, G., Okello, E., Shiel, R.S. and Guy, J.H. (2009) Repellence of plant essential oils to Dermanyssus gallinae and toxicity to the non-target invertebrate Tenebrio molitor. Veterinary Parasitology, 162, 129-134. doi:10.1016/j.vetpar.2009.02.009
- Kumral, N.A., Cobanoglu, S. and Yalcin, C. (2010) Acaricidal, repellent and oviposition deterrent activities of Datura stramonium L. against adult Tetranychus urticae (Koch). Journal of Pesticide Science, 83, 173-180. doi:10.1007/s10340-009-0284-7

