Advances in Enzyme Research
Vol.03 No.01(2015), Article ID:54778,9 pages
10.4236/aer.2015.31002
GABAA-Coupled Cl−/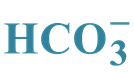 -ATPase from Plasma Membrane of the Rat Brain: Role of
-ATPase from Plasma Membrane of the Rat Brain: Role of
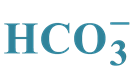 in the Enzyme Activation
in the Enzyme Activation
Sergey A. Menzikov*, Margarita N. Karpova, Lada V. Kuznetsova, Natalya Y. Klishina
Federal State Scientific Institution “Research Institute of General Pathology and Pathophysiology”, Moscow, Russia
Email: *menzikov@mail.ru
Copyright © 2015 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).


Received 19 February 2015; accepted 12 March 2015; published 18 March 2015

ABSTRACT
This work examines the influence of Cl− (2.5 - 125 mM) and
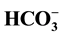 (2 - 30 mM) on the Cl−/
(2 - 30 mM) on the Cl−/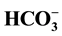 - ATPase complex of the neuronal membrane and this enzyme is a Cl−-pump that is coupled to GABAA receptors. The greatest (44%) activating effect on the enzyme is found with
- ATPase complex of the neuronal membrane and this enzyme is a Cl−-pump that is coupled to GABAA receptors. The greatest (44%) activating effect on the enzyme is found with
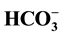 (20 - 30 mM), while the maximum activity occurs in the presence of a ratio of ~25 mM
(20 - 30 mM), while the maximum activity occurs in the presence of a ratio of ~25 mM
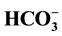 /~5mM Cl−. Blockers of the GABAA receptor, namely bicuculline (10 - 50 µM) and picrotoxin (50 - 100 µM), inhibit this anion activation, whereas the
/~5mM Cl−. Blockers of the GABAA receptor, namely bicuculline (10 - 50 µM) and picrotoxin (50 - 100 µM), inhibit this anion activation, whereas the
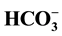 -ATPase activity is not sensitive to these ligands. Autoradiographic analysis of the spectrum of the partially purified enzyme phosphorylated with [γ-32P]ATP allowed us to distinguish three major 32P-labeled protein whose molecular weight are about 57, 53, and 48 kDa. In the presence of 5 mM Cl−/25mM
-ATPase activity is not sensitive to these ligands. Autoradiographic analysis of the spectrum of the partially purified enzyme phosphorylated with [γ-32P]ATP allowed us to distinguish three major 32P-labeled protein whose molecular weight are about 57, 53, and 48 kDa. In the presence of 5 mM Cl−/25mM
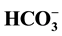 and 100 µM picrotoxin, the intensity of the phosphorylation of bands significantly decreased, thereby confirming the assump- tion about coupled of binding sites for anions and GABAA-ergic ligands. It was suggested scheme of Cl−-transport through the plasma membrane by utilizing neuronal Cl−/
and 100 µM picrotoxin, the intensity of the phosphorylation of bands significantly decreased, thereby confirming the assump- tion about coupled of binding sites for anions and GABAA-ergic ligands. It was suggested scheme of Cl−-transport through the plasma membrane by utilizing neuronal Cl−/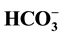 -ATPase in the low (5 mM) Cl− and high (25 mM)
-ATPase in the low (5 mM) Cl− and high (25 mM)
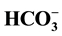 concentrations. The data demonstrated for the first time that the GABAA-coupled Cl−/
concentrations. The data demonstrated for the first time that the GABAA-coupled Cl−/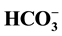 -ATPase from rat brain neuronal membranes is maximally activated at a Cl−/
-ATPase from rat brain neuronal membranes is maximally activated at a Cl−/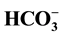 ratio of 1:5 and it remains stable at high concentrations of substrate and buffer.
ratio of 1:5 and it remains stable at high concentrations of substrate and buffer.
Keywords:
Rat Brain, Plasma Membrane, Mg2+-ATPase, Chloride, Bicarbonate, Mg2+-ATP, Picrotoxin,
Bicuculline, Autoradiography, Molecular Weight, Subunits

1. Introduction
The Cl−-ATPase/Cl−-pump (EC 3.6.3.11-Cl−-transporting ATPase) from the plasma membrane of different cells (including neurons) is a “molecular machine” that participates in Cl−-transport against an electrochemical gradient [1] -[3] . We have determined that neuronal membranes from animal brain contain a Cl−-ATPase that is functionally coupled with the GABAA/benzodiazepine receptor/Cl−-channel complex [4] -[6] . Cl−-transport through this ATPase in liposomal membranes depends on the presence of
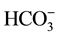 ions in the incubation medium [7] . In our preliminary studies, we relied on data from electrophysiological studies of GABAA receptor function to identify enzymatic activity. In particular, the literature showed that Cl− and
ions in the incubation medium [7] . In our preliminary studies, we relied on data from electrophysiological studies of GABAA receptor function to identify enzymatic activity. In particular, the literature showed that Cl− and
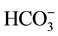 ions are transported through the GABAA receptor/Cl−-channel in a 5:1 ratio, respectively [8] . Indeed, the presence of Cl− and
ions are transported through the GABAA receptor/Cl−-channel in a 5:1 ratio, respectively [8] . Indeed, the presence of Cl− and
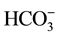 ions (in a 5:1 ratio) in the incubation medium resulted in greater Cl−/
ions (in a 5:1 ratio) in the incubation medium resulted in greater Cl−/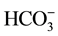 -ATPase activity than that was seen in the presence of either of these anions alone [5] [6] . Synergistic activation of the ATPase by anions was also observed in both low (~10 mM/~2mM) and high (~50 mM/~10mM) concentrations of Cl− and
-ATPase activity than that was seen in the presence of either of these anions alone [5] [6] . Synergistic activation of the ATPase by anions was also observed in both low (~10 mM/~2mM) and high (~50 mM/~10mM) concentrations of Cl− and






2. Materials and Methods
2.1. Animals
Male Wistar rats (180 - 200 g) were obtained from vivarium of the Federal State Scientific Institution “Research Institute of General Pathology and Pathophysiology”. Animals were housed in a climate-controlled room on a 12 - 22 hour light/dark cycle and where free access to water and food. The experiment was conducted under the “Rules of work with experimental animals” FSSI “RIGPP”, which complied with the World Society for the Protection of Animals (WSPA) and the European Convention for the protection of experimental animals.
2.2. Isolation of Plasma Membrane
All procedures were performed at 0˚C - 4˚C. After decapitation of animals, the brain was isolated, homogenized in 8 vol. of ice-cold buffer solution containing 0.25 M sucrose, 1 mM ethylenediaminetetraacetic acid-Tris (hydroxymethyl) aminomethane (EDTA-Tris, pH 7.4), 12.5 mM N-(2-Hydroxyethyl)piperazine-N’-(2-ethanesul- fonic acid) (HEPES-Tris, pH 7.4), 1 mM phenylmethylsulfonyl fluoride (PMSF) and 50 units/ml aprotinin and centrifuged in a Beckman ultracentrifuge (SW-28 bucket rotor) at 10,000 × g and 4˚C for 25 min. The supernatant was centrifuged at 100,000× g and 4˚C for 1 h. The supernatant was discarded and microsomal fraction enriched plasma membranes (pellet) was resuspended in 1 mM EDTA-Tris (pH 7.4), 12.5 mM HEPES-Tris (pH 7.4), stirred for 15 min and centrifuged (100,000 × g, 45 min). The resulting pellets were resuspended in 12.5 mM HEPES-Tris (pH 7.4) and frozen at −80˚C. This plasma membrane rich fraction was used for further measurements of the ATPase activity.
2.3. ATP Hydrolysis Assay
The enzyme preparation (20 - 25 μg) was added to 0.5 ml incubation medium containing 12.5 mM HEPES-Tris buffer (pH 7.4), 1.0 mM MgSO4, 1.0 mM ATP-Tris, 10 mM NaCl/2mM NaHCO3 and 60 mM NaNO3 (neutral salt) to measure enzyme activity. The specific activity of ATPase was estimated from the increase in the content of inorganic phosphorus (Pi) in 0.5 ml incubation medium at 30˚C for 30 min. Phosphorus concentration in samples was measured by the method of Chen and expressed in μmol Pi/h/mg protein [15] [16] . The activity of the “basal” Mg2+-ATPase was calculated as the difference between the ATPase activities in the presence and absence of MgSO4 in the incubation medium containing 12.5 mM HEPES-Tris buffer (pH 7.4), 1.0 mM MgSO4, 1.0 mM Tris-ATP and 60 mM NaNO3. The Cl−/

2.4. Protein Purification and Phosphorylation
To obtain soluble form of enzyme, plasma membranes were incubated with 1% sodium deoxycholate at room temperature for 20 min and centrifuged at 100,000 g and 4˚C for 30 min. Cl−,

Enzyme preparations were frozen at −20˚C and used within 30 days. The membrane preparation was phosphorylated in 30 μl of incubation medium containing 25 mM HEPES-Tris (pH 7.2), 3 mM MgSO4, and protein (~25 μg). The reaction of phosphorylation was started by the addition to the incubation medium of 70 μM [γ-32P]ATP (specific radioactivity, 5 × 10−6 dpm/nmol) (Amersham, Biosciences). The mixture was incubated at 0˚C - 1˚C for 2 min. To study the effect of 5 mM Cl/25 mM HCO3 and 100 μM picrotoxin on the phosphoprotein formation, the membrane preparation was preincubated with the ligands at 0˚C - 1˚C for 15 min [17] .
2.5. The Enzyme Identification by SDS-PAGE and Autoradiography
Briefly, phosphorylation of protein preparations was stopped by the addition of an equal volume of a buffer containing 100 mM Tris-HCl (pH 6.8), 2% SDS, 2% 2-mercaptoethanol, and 10% sucrose. Electrophoresis was performed in 12% SDS-containing polyacrylamide gel by the method of Laemmli at a current strength of 35 mA [6] . Electrophoretograms were stained with 0.1% Coomassie Brilliant Blue R-250. Stained and dried gels were placed in a chamber for autoradiography (Sigma, United States) on a Hyperfilm ™ MP film (Amersham, United States) and exposed at room temperature for 72 - 96 h. The film was developed using the standard developer to obtain the maximum contrast image. The molecular weight of proteins was determined by the conventional procedure by comparing their electrophoretic mobility with that of the standard protein markers (BSA, ovalbumin, chymotrypsinogen A, myoglobin, and cytochrome c).
2.6. Assay of Action of Chemicals on the Enzyme
The enzyme activity in the presence of GABAA-ergic chemicals (bicuculline, picrotoxin) was determined as described before [6] . Membrane samples were preincubated at 30˚C for 20 min with the relevant chemical in incubation medium containing 25 mM HEPES-Tris buffer (pH 7.4), 2.5 - 125 mM NaCl/2 - 30 mM NaHCO3 and 60 mM NaNO3. The reaction was started by addition of the substrate (Mg2+-ATP) to the incubation medium.
2.7. Chemicals
All drugs were prepared as stock solutions in water unless otherwise stated. Picrotoxin, bicuculline methochloride, EDTA, Tris, Hepes, PMSF, aptotixin, Na2ATP, sodium deoxycholate, albumin, ovalbumin, chymotrypsinogen A, myoglobin, and cytochrome c, sodium dodecyl sulfate BioUltra were by Sigma-Aldrich. Electrophoresis reagent Kit (Bio-Rad Laboratories, USA) and [γ-32P]ATP (5 × 10−6 dpm/nmol) (Amersham, Biosciences, United States Biochemical Corp.).
2.8. Statistics
The data are expressed with mean ± standard error where appropriate. The experimental data are statistically processed using one-way ANOVA test program “Statistica 7.0”. Evaluation of the significance of differences was carried out at p < 0.05 (n = 4).
3. Results and Discussion
We have previously found that the activity of the GABAA-coupled ATPase is a “basal” Mg2+-ATPase activity that is stimulated by anions [6] [7] . The activity of this “basal” Mg2+-ATPase from rat brain plasma membranes is 6.7 μmol Pi/h/mg protein. Addition of Cl− (0 - 125 mM) stimulates the activity of this enzyme (Figure 1(A)), with a maximal effect (9%) observed at 15 mM Cl−. At higher Cl− ion concentrations, no enzyme activation is observed. Addition of





Figure 1. (A) Effect of Cl− concentration on the the “basal” Mg2+-ATPase activity of rat brain plasma membranes in the absence or in the presence of 20 mM





A study of the combined action of these anions on the “basal” Mg2+-ATPase revealed a synergistic effect observed under conditions of low Cl− (2.5 - 7 mM) in the incubation medium. In this case, the stimulatory effect on the ATPase activity under the simultaneous action of Cl− +









Our preliminary studies showed that the stimulatory effect of Cl−/



Figure 2. (A) Effect of





Figure 3. Effect of Mg2+-ATP on the “basal” Mg2+-ATPase activity of rat brain plasma membranes in the absence and in the presence of 25 mM




of 7.4 in imidazole-HCl buffer (100 mM) [2] . At lower or higher concentrations of [H+], the enzyme activity is reduced. In our study, the maximum Cl−/

We confirmed that the enzyme activity under study is the GABAA-coupled ATPase by investigating the effect of antagonists of GABAA receptors (picrotoxin and bicuculline) on its activity (Figure 4). The GABAA receptor blockers, when supplied in a range of study concentrations (20 - 100 µM) stimulate the “basal” Mg2+-ATPase activity by ~30% (data not shown), whereas addition of picrotoxin (100 µM) and bicuculline (25 - 50 µM) completely eliminate the stimulatory effect of



Earlier studies of the Cl−/
Figure 4. Effect of picrotoxin (1,3) and bicuculline (2,4) on the Cl−/





We have previously shown that the Cl−/


Earlier studies that incorporated the purified of the enzyme into proteoliposomes showed a differentiation of the detected properties of the ATPase system and omnidirectional Cl−-transport that depended on the concentration of anions in the intracellular and extracellular medium [7] . In particular, the presence of high intracellular concentrations of Cl− and








Clearly, the hydrolytic activity of the ATPase under study plays an important role not only because this system provides metabolic energy but also because it provides a definite direction of flow of chloride ions, which depends not only on the intracellular ATP and Cl− concentrations, but also on the presence of a specific concentration of

Figure 5. Operational model of the GABAA-coupled Cl−/
4. Conclusion
In the present work, we described a new approach for the detection of anion-stimulated ATPase which is functionally coupled with GABAA/benzodiazepine receptor/Cl−-channel complex. The presence of physiological anion concentrations in the incubation medium not only increases the enzyme activity but also contributes to the stability of the activity in the presence of high concentrations of substrate and buffer molarity. This is an important aspect in the further study of molecular properties of the enzyme.
Cite this paper
Sergey A.Menzikov,Margarita N.Karpova,Lada V.Kuznetsova,Natalya Y.Klishina, (2015) GABAA-Coupled Cl-/ HCO3--ATPase from Plasma Membrane of the Rat Brain: Role of HCO3- in the Enzyme Activation。 Advances in Enzyme Research,03,9-18. doi: 10.4236/aer.2015.31002
References
- 1. Gerencser, G.A. and Zhang, J.L. (2003) Chloride ATPase Pumps in Nature: DO They Exist? Biological Reviews, 78, 197-218. http://dx.doi.org/10.1017/S146479310200605X
- 2. Inagaki, C., Hara, M. and Zeng, X.T. (1996) A Cl--Pump in Rat-Brain Neurons. The Journal of Еxperimental Zoology, 275, 262-268.
- 3. Pedersen, P.L. (2005) Transport ATPases: Structure, Motors, Mechanism and Medicine: A Brief Overview. Journal of Bioenergetics and Biomembranes, 37, 349-357. http://dx.doi.org/10.1007/s10863-005-9470-3
- 4. Menzikov, S.A. (2013) Neuronal Multifunctional ATPase. Biophysical Reviews and Letters, 8, 213-227. http://dx.doi.org/10.1142/S1793048013300065
- 5. Menzikov, S.A. and Menzikova, O.V. (2004) Effect of Chloride and Bicarbonate Ions on Mg2+-ATPase of Plasma Membranes of Bream (Abramis brama L.) Brain Sensitive to GABAA-Ergic Compounds. Ukrainskii Biokhimicheskii Zhurnal, 76, 53-58.
- 6. Menzikov, S.A. and Menzikova, O.V. (2007) Comparative Properties of Sensitive to GABAA-ergic Ligands, Cl-, HCO3--Activated Mg2+-ATPase from Brain Plasma Membranes of Fish and Rats. Zhurnal Evoliutsionnoi Biokhimii i Fiziologii, 43, 246-253.
- 7. Menzikov, S.A., Karpova, M.N. and Kalinina, M.V. (2011) Effect of HCO3- Ions on the ATP-Dependent GABAA Receptor-Coupled Cl-- Channel in Rat Brain Plasma Membranes. Bulletin of Experimental Biology and Medicine, 152, 38-42. http://dx.doi.org/10.1007/s10517-011-1448-z
- 8. Bormann, J., Hamill, O.P. and Sakmann, B. (1987) Mechanism Of Anion Permeation through Channels Gated by Glycine and Gamma-Aminobutyric Acid in Mouse Cultured Spinal Neurons. The Journal of Physiology, 385, 243-286. http://dx.doi.org/10.1113/jphysiol.1987.sp016493
- 9. Staley, K.J., Soldo, B.L. and Proctor, W.R. (1995) Ionic Mechanisms of Neuronal Excitation by Inhibitory GABAA Receptors. Science, 269, 977-981. http://dx.doi.org/10.1126/science.7638623
- 10. Staley, K.J. and Proctor, W.R. (1999) Modulation of Mammalian Dendritic GABAA Receptor Function by the Kinetics of Cl- and HCO3- Transpоrt. The Journal of Physiology, 519, 693-712.
- 11. Perkins, K.L. and Wong, R.K.S. (1996) Ionic Basis of the Postsynaptic Depolarizing GABA Response in Hippocampal Pyramidal Cells. Journal of Neurophysiology, 76, 3886-3894.
- 12. Perkins, K.L. (1999) Сl-Accumulation Does Not Account for the Depolarizing Phase of the Synaptic GABA Response in Hippocampal Pyramidal Cells. Journal of Neurophysiology, 82, 768-777.
- 13. Ashmarin, I.P., et al. (1999) Biochemistry of Brain. St. Petersburg State University, St. Petersburg.
- 14. Glebov, R.N. and Kryzhanovsky, G.N. (1978) The Functional Biochemistry of Synapses. Medicine, Moscow.
- 15. Bradford, M.M. (1976) A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principal of Protein-Dye Binding. Analytical Biochemistry, 72, 248-254. http://dx.doi.org/10.1016/0003-2697(76)90527-3
- 16. Chen, P.S., Toribara, T.Y. and Warner, H. (1956) Microdetermination of Phosphorus. Analytical Chemistry, 28, 1756- 1758. http://dx.doi.org/10.1021/ac60119a033
- 17. Menzikov, S.A. and Menzikova, O.V. (2006) Phosphorylation of Cl-, HCO3- -Activated Mg2+-ATPase from Carp (Cyprinus carpio L.) Brain Plasma Membranes. Doklady Biochemistry and Biophysics, 407, 64-67. http://dx.doi.org/10.1134/S1607672906020050
- 18. Menzikov, S.A. and Karpova, M.N. (2011b) Cl--Transporting Mechanisms in Neuronal Membranes and Role of ATP in the Functioning. Pathogenesis, 1, 4-10.
- 19. Lambert, N. and Grover, L. (1995) The Mechanism of Biphasic GABA Responses. Science, 269, 928-929. http://dx.doi.org/10.1126/science.7638614
- 20. Hyden, H., Cupello, A. and Rapallino, M.V. (1999) Chloride Permeation across the Deiters’ Neuron Plasma Membrane: Activation by GABA on the Membrane Cytoplasmic Side. Neuroscience, 89, 1391-1400. http://dx.doi.org/10.1016/S0306-4522(98)00357-1
- 21. Alvarez-Leefmans, F.J. and Delpire, E. (2009) Physiology and Pathology of Chloride Transporters and Channels in the Nervous System: From Molecules to Diseases. Elsevier-Academic Press, San Diego.
- 22. Menzikov, S.A., Karpova, M.N. and Kalinina, M.V. (2012) Effect of Pentylenetetrazole on the GABAA-Coupled Cl-, HCO3- -Activated Mg2+-ATPase Activity of the Plasma Membrane from Rat Brain both in Vitro and in Vivo Experiences. Pathological Physiology and Experimental Therapy, 98-102.
NOTES
*Corresponding author.








