Advances in Enzyme Research
Vol.1 No.2(2013), Article ID:33423,7 pages DOI:10.4236/aer.2013.12003
Thermostable alkaline protease production from Bacillus pumilus D-6 by using agro-residues as substrates
![]()
School of Biotechnology, University of Jammu, Jammu, India; *Corresponding Author: bkbajaj1@rediffmail.com, bajajbijenderk@gmail.com
Copyright © 2013 Bijender Kumar Bajaj, Gaytri Jamwal. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received 6 April 2013; revised 15 May 2013; accepted 28 May 2013
Keywords: Alkaline protease; agro-residues; Bacillus pumilus; thermostable
ABSTRACT
Proteases due to their wide range of applications in biotechnological processes have been the focus of intense research for many decades. However, from industrial application view point most of the available proteases lack desired properties; therefore, search for better and efficient thermostable alkaline proteases are always on. Bacillus pumilus D-6, isolated from dairy plant soil sample, in the current study produced protease which showed activity and stability at high alkaline pH (8 - 12) and high temperatures (70˚C - 100˚C). Enzyme activity remained unfazed even in presence of inhibitors like Pb2+ and Hg2+ which are considered universal inhibitors of enzyme activity. Besides, the organism successfully utilized crude agriculture based substrates as carbon and nitrogen source and produced substantial enzyme titre.
1. INTRODUCTION
Recently, microbial enzymes due to their environmentally-friendly applications in industrial processes have become the focus of intense research by manufacturing biochemists and bioprocess engineers. Among industrial enzymes, proteases represent 60% of the total worldwide sales of enzymes, and alkaline proteases account for 89% of the total protease sale [1,2]. This huge market of the alkaline proteases is due to their vast applications in multifaceted industrial sectors such as detergent, food, and leather tanning industries. Besides, alkaline proteases are also used for the hydrolysis of hair, feather and horn for production of valuable products, in peptide synthesis, in resolution of racemic mixtures of amino acids, and in hydrolysis of gelatin layers of X-ray films for silver recovery [2,3]. Proteases may be obtained from plant, animal or microbial sources but microbial sources are the most preferred ones due to multiple advantages including their broad biochemical diversity and bioengineering potentiality [4]. In addition, exploration of microbial diversity represents the major driving force for development of novel biotechnological products and processes. For most of the applications, robust enzymes are stable and active under high temperature, acidic/alkaline pH and in the presence of surfactants, oxidizing agents or other inhibitory agents, are desired [4-7]. Furthermore, for successful industrial applications, it is necessary that bulk production of enzyme must be carried out in most cost-effective manner. Most of the studies on microbial proteases are confined to characterization of enzymes with relatively fewer reports on optimization of enzyme production [4]. One of the major cost-determining factors for bulk industrial enzyme production is substrate. It is envisaged that relatively low-cost agricultural by products may have great potential as substrates for enzyme production [4,8,9]. India, being blessed with rich agricultural heritage, produces tremendous quantum of agroresidues annually which can be employed as substrates for production of enzymes or other industrially important products, else the agro-wastes are causing environmental pollution and difficult to dispose.
Wide range of microorganisms including bacteria, moulds and yeasts have been used for production of proteases [4,10,11]. Currently, a large proportion of commercially available alkaline proteases which find a wide range of applications in laundry, dishwashing, textile, food processing, pharmaceuticals, leather, paper and pulp industries are derived from Bacillus spp. [3,5-7,12,13].
In the present study, Bacillus pumilus D-6, a bacterial isolate from dairy plant soil, was found to produce thermostable and broad range pH stable protease. Protease production was attempted by using agro-based crude carbon and nitrogen sources, and enzyme was partially purified and characterized.
2. MATERIALS AND METHODS
2.1. Isolation of Proteolytic Bacteria
The samples of soil were collected from slaughter house, local dairy and domestic waste disposal site. Soil samples (brown color, loose texture, organic matter rich, course, pH 6 - 8) were inoculated @ 2.0 g in 100 ml of nutrient broth (pH 8 - 10) and enrichment was carried out for 48 h at 37˚C under shaking @ 200 rpm (Innova, New Brunswick, USA). Then the enriched broth was appropriately diluted and spread plated on skim-milk-agar (SKMA) which consisted of (each, % w/v): skimmed milk powder (5), peptone (0.1), NaCl (0.5) and agar (2) at pH 8 - 10. The plates were incubated at 37˚C for 48 h and then examined for halo around the colonies as the sign of protease producing ability of the organism.
2.2. Fermentation for Protease Production
The bacterial isolates which showed significant proteolytic activity on SKMA plates under high alkaline conditions were selected and subjected to submerged fermentation for protease production. Protease production medium (PPM) consisted of: (each, % w/v) glucose (0.5), peptone (0.5), yeast extract (0.5), K2HPO4 (0.4), Na2HPO4 (0.1), CaCl2 (0.01), Na2CO3 (0.6) at pH 9. The culture was grown overnight under shaking at 37˚C in PPM and then inoculated into the same medium (PPM) @ 10% (v/v) and fermentation was conducted under shaking at 37˚C for 3 days. Appropriate volume of the fermented broth was centrifuged, periodically, and the supernatant obtained was considered as crude enzyme and was used for assaying the proteolytic activity.
2.3. Alternative Carbon and Nitrogen Sources for Protease Production
For studying the effect of various carbon source on protease production, glucose of the PPM was replaced with different carbon sources (@ 0.5%, w/v) which were either refined ones (lactose and sucrose) or crude substrates of agricultural origin viz. wheat bran, rice bran or rice husk. Similarly, peptone and yeast extract of the PPM were replaced with either complex nitrogen sources (soybean meal, gelatin), or simple ones (ammonium sulphate, urea) @ 1%, w/v, for examining the effect of nitrogen sources on protease production. Relative activity was determined by considering control as the standard reference.
2.4. Protease and Protein Assay
Protease activity was determined as described previously [4]. Enzyme assay mixture consisting of 1 ml of substrate (1% casein), 0.9 ml of Tris-HCl buffer (50 mM, pH 8) and 0.1 ml of appropriately diluted crude or partially purified enzyme, was incubated at 45˚C for 30 min. The reaction was stopped by adding 4 ml of TCA solution (10%, w/v), and the contents were allowed to stand on ice for 15 min. Then the contents were centrifuged at 10,000g for 10 min and 1 ml of supernatant was taken out in separate test tube and 5 ml of sodium carbonate (0.4 M) and 0.5 ml of Folin-Ciocalteu (FC) reagent was added into it. The absorbance of the solution was measured at 660 nm by spectrophotometer (Perkin-Elmer, Spectrophotometer, λ 35). A standard curve was developed by taking the various amounts of pure tyrosine (10 - 100 µmoles) along with 5 ml of sodium carbonate (0.4 M) and 0.5 ml of FC reagent. One unit of protease activity is defined as the amount of enzyme that releases 1 µmole of tyrosine per min at 45˚C under assay conditions.
Protein assay in the enzyme preparation was carried out by Lowry’s method [14] using bovine serum albumin V (BSA) as standard.
2.5. Partial Purification of Protease
The enzyme produced by fermentation in production medium with glucose as carbon source and urea as nitrogen source was subjected to ammonium sulphate precipitation at various saturation levels of 20% - 100%. The precipitated fractions were dialyzed and analysed for protease activity and protein content. The fraction which showed the highest activity was used for further analysis.
2.6. Effect of pH and Temperature on the Activity and Stability of Protease
The effect of temperature on the activity of protease was determined by carrying out the enzyme assay at different temperatures viz. 30˚C to 100˚C. The thermostability of enzyme was determined by pre-incubating the enzyme at different temperatures ranging from 30˚C to 100˚C for 30 min (pH 8) and then determining the residual activity.
For determining the effect of pH on protease activity, different buffers (50 mM) used in enzyme assay mixture were: phosphate buffer (pH 7 - 8), Tris-HCl buffer (pH 8 - 9), carbonate-bicarbonate buffer pH 10 - 11, and KClNaOH buffer (pH 12 - 13). For examining the pH stability of the protease the enzyme was pre-incubated with the buffer of appropriate pH for 30 min and then residual activity was assayed. Relative activity was determined by considering maximum activity as the standard reference.
2.7. Effect of Metal Ions on Activity of Protease
For determining the effect some divalent ions viz. Mg2+, Co2+, Hg2+, Zn2+, Pb2+, Ca2+ and Cu2+ on the activity of protease, either of the ion was included in the enzyme assay mixture at final concentration of 10 mM, and activity was assayed. Relative activity was determined by considering control as the standard reference.
All the analytical experiments were conducted in triplicates and results expressed are the mean of three different experiments.
3. RESULTS AND DISCUSSION
3.1. Proteolytic Bacteria
The bacterial isolates which showed considerable zone of hydrolysis on SKMA plates were subjected to submerged fermentation for examining their ability of producing enzyme under alkaline pH (8 - 10). Two isolates D-2 and D-6 (from dairy plant soil sample) showed the maximum proteolytic activity (Figure 1) on skim-milkagar plates as well as under submerged fermentation. Preliminary examination of proteases produced by the isolates D-2 and D-6 showed substantial alkalistability and thermostability. The isolate D-6 was selected for detailed investigation as it yielded higher enzyme titre as compared to the isolate D-2. The bacterial isolate was examined microscopically and found to be Gram-positive spore-former. Detailed identification of the organism
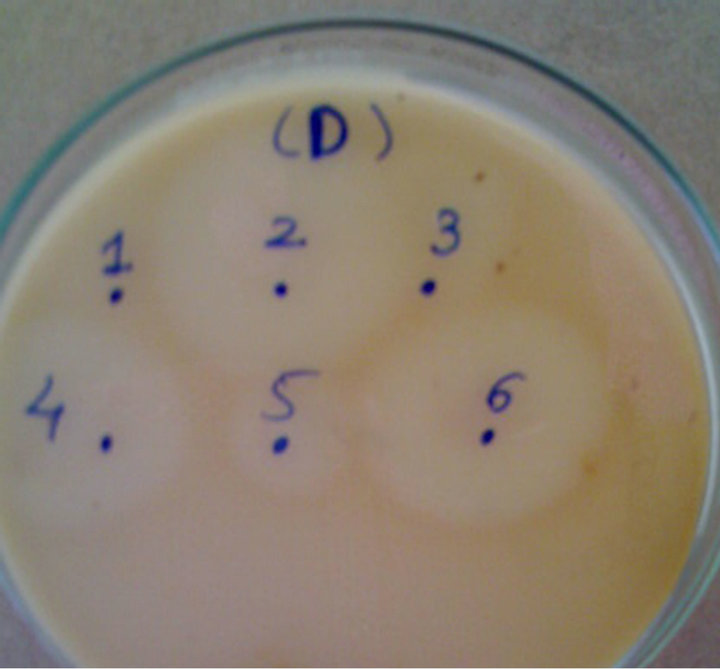
Figure 1. Screening of bacterial isolates for proteolytic activity. Bacterial isolates were spotted on skim-milk-agar plates, and plates were incubated at 37˚C for 24 h.
was done using Biolog System (Central Instrumentation Facility, University of Delhi South Campus (UDSC) and the bacterium was identified as Bacillus pumilus and designated as B. pumilus D-6. Protease production has been studied from bacterial isolates by various researchers [4]. B. pumilus CBS, an isolate from Tunisian soil produced protease which has compatibility with laundry detergents, and powerful dehairing, feather-degradation and blood/chocolate stains removal capabilities [1]. B. pseudofirmus was isolated from coastal region of Gujarat which produced halo-alkaline protease [12]. Hmidet et al. [15] isolated several Bacillus spp. from various natural sources (activated sludge reactor, soil near the detergent industry, polluted water from the local slaughter house and from marine water) which co-produced alkaline proteases and thermostable α-amylase. Organic solvent stable protease was reported from B. pumilus 115b isolate from contaminated soils of a wood factory [6]. B. subtilis P13, an isolate from Vajreshwari hot springs was reported to produce an extracellular serine protease that catalyses dehairing of hides as well as feather degradation [16].
Analysis of time-profile vs enzyme production showed that B. pumilus D-6 produced maximum protease titre after 48 h of fermentation, after this the protease activity remained almost constant. Incubation period for maximum protease production has been reported to vary from 24 - 48 h to a few days or weeks depending upon the type of microorganism and the cultural conditions employed [4]. B. pumilus CBS, B. pseudofirmus, and B. subtilis P13 showed maximum protease production after 24 h [1,12,16]. Qadar et al. [17] and Kumar et al. [18] reported 48 h as the optimum time for protease production from Bacillus sp. PCSIR EA-3, and from B. subtilis MTCC 9102, respectively.
3.2. Protease Production Using Different Carbon Sources
Glucose of the PPM was replaced with either refined carbon source (lactose or sucrose) or crude carbon source (rice bran, wheat bran or rice husk) and fermentation was conducted. It was found that lactose and sucrose supported significant level of protease production (68 and 65.3 U/ml respectively) and interestingly even the crude substrates like rice bran, wheat bran or rice husk also gave comparable protease production (61.3, 56.6, 54.6 U/ml respectively) as depicted in Figure 2. This is quite remarkable observation as one of major bottleneck for bulk production and application of enzymes in different industries is their high cost of production. In the present study the organism not only utilized successfully the complex agricultural residues but induced considerably high enzyme production. Lazim et al. [19] reported that mixture of wheat bran and chopped dates serve good
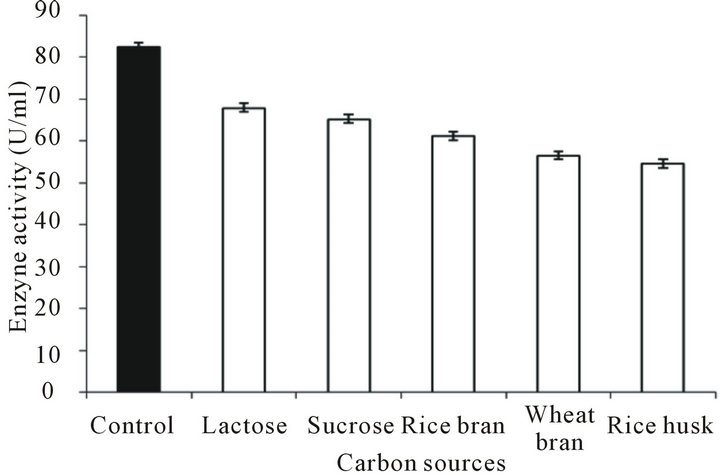
Figure 2. Protease production by Bacillus pumilus D-6 using various crude or refined materials as sole carbon source. Control consisted of glucose as carbon source.
substrate for alkaline protease production from Streptomyces sp. CN902. Although Streptomyces sp. DP2 produced maximum protease when fructose was used as carbon source, however, wheat bran also induced substantial protease production [4]. Vishalakhi et al. [20] reported alkaline protease production from Streptomyces gulbargensis using wheat bran as substrate. B. subtilis MTCC9102 and B. cereus SV1 showed substantial protease production when cultivated on shrimp wastes powder or horn meal as sole carbon sources, respectively [18, 21].
3.3. Protease Production Using Different Nitrogen Sources
Nitrogen is one of the most essential nutrients as it is the ultimate precursor for protein synthesis, besides it also plays important role in regulating the pH of the medium, and hence may be crucial in maintaining the activity and stability of the enzyme. Synthesis of alkaline proteases by microorganisms is strongly stimulated by the presence of proteins or peptides in culture medium [22]. Utilization of low price nitrogen source must be an efficient criterion for economic production of industrial enzymes. Each of the nitrogen sources employed in the PPM lead to substantially enhanced protease production as compared to that of control (Figure 3). Urea was found to be the best and showed 80% more protease activity as compared to that in control, while ammonium sulphate enhanced enzyme production by 40%, and gelatin and soybean meal did so to the tune of 26 and 24%, respectively. We have previously reported [4] that mustard cake and soybean meal are the most-effective nitrogen sources for protease production by Streptomyces ambofaciens DP2. In most of the studies of protease or other enzyme production, soybean meal has been found to be the best nitrogen source [22]. However, a combination of yeast extract and casein [23] has been reported to
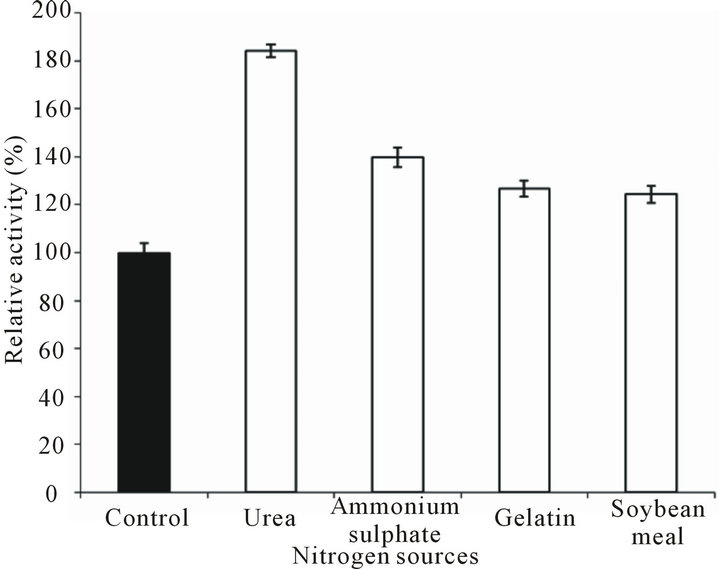
Figure 3. Effect of various nitrogen sources on protease production by Bacillus pumilus D-6. Peptone and yeast extract of production medium were replaced with either of nitrogen source @1%.
be good nitrogen source. Yeast extract as a nitrogen source has been reported to increase the protease production from Streptomyces sp. CN902 [19]. Peptone was found to be optimum nitrogen source for protease production from a Bacillus subtilis MTCC 9102 [18]. Pillai et al. [16] got enhanced protease production from B. subtilis P13 by supplementation of LB medium with various crude nitrogen sources (skimmed milk, green gram powder, bengal gram powder, milled chicken feathers and soybean meal).
3.4. Partial Purification and Characterization of Protease for a Few Properties
Ammonium sulphate precipitation of the enzyme at different saturation levels showed that maximum activity was present in 40% - 60% salt precipitated fraction. Specific activity of ammonium sulphate precipitated fraction was 19.1 while that of crude preparation was 1.3 indicating that protease is purified by 14.69-fold.
Activity assay of partially purified protease at different temperatures showed that enzyme exerted maximum activity at 50˚C. Substantially high activity was observed at 60˚C (75.9%). But activity-reduction occurred at 70˚C (69%), 80˚C (76%), 90˚C (81.1%) and at 100˚C (83%) as shown in Figure 4. Nevertheless the most remarkable observation about B. pumilus D-6 protease was that enzyme showed certain basal level of activity even at very high temperature (90˚C - 100˚C). Thermostability analysis of B. pumilus D-6 protease at various temperatures for 30 min showed that enzyme was thoroughly stable at 30˚C - 40˚C (residual activity 100%) with slight to moderate decrease at 50˚C (9.1%) and 60˚C (31%). However, at still higher temperatures (70˚C - 100˚C) enzyme lost about half of its activity but still it retained 54% - 55% of
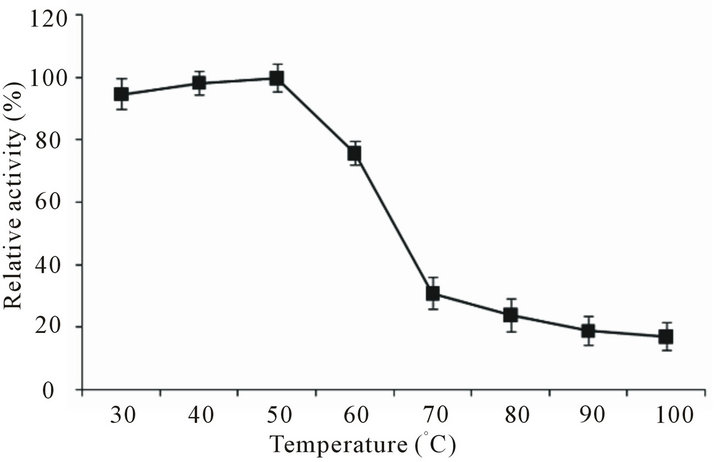
Figure 4. Effect of temperature on the activity of Bacillus pumilus D-6 protease. Enzyme assay was executed at different temperatures (30˚C - 100˚C).
the activity at such high temperatures (Figure 5).
Optimum temperature for different bacterial proteases has been reported to be 50˚C - 75˚C [22]. Thermophilic Geobacillus sp. YMTC 1049 extracellular protease had an optimum temperature of 85˚C and retained activity for 10.0 h at 65˚C [24]. Alkaline protease from Pyrococcus sp. had highest activity at 110˚C [25]. Calcium chloride has been reported to have role in enhancing thermostability of protease [23]. B. cereus SV1 protease showed optimum activity at 60˚C and thermal stability at <55˚C [21]. Alkaline protease from Bacillus lehensis showed activity over 30˚C - 60˚C with optimum at 50˚C [26]. Rai et al. [2] purified alkaline protease from Paenibacillus tezpurensis sp. nov. AS-S24-II by 1.7-fold. The purified protease displayed optimum activity at pH 9.5 and 45 - 50˚C temperature range and exhibited a significant stability and compatibility with surfactants and most of the tested commercial laundry detergents at room temperature. In contrast, Bacillus sp. 17N-1 protease exhibited activity at 14˚C - 33˚C with optimum at 25˚C [7].
For studying the effect of pH on the activity of protease the assay was conducted at varying pH using appropriate buffers. It was observed that the enzyme has astonishingly high ability to work under extremely alkaline pH, and showed 91% - 100% activity over pH range of 7 - 11 (Figure 6) and around 80% of the activity at even higher pH 12 - 13. Results are amazing as the enzyme is capable of working at extremely high pH. Stabilityanalysis of the protease at different alkaline pH (Figure 7) showed that enzyme was thoroughly stable at pH 7 - 9 (residual activity, 94% - 100%), with slight decrease at pH 10 (residual activity 86%). However, further increase in pH caused reduction in the enzyme activity. The residual activity was 64.3%, 54% and 45.6% at pH 10, 11 and 12, respectively. B. cereus SV1 protease showed optimum activity at pH 8.0, pH stability in the range of 6 - 9.5 [21]. Protease from halo-alkaliphilic Bacillus sp. 17N-1 showed activity at pH 6.5 to 8.5 with an optimum at pH 7 [7].
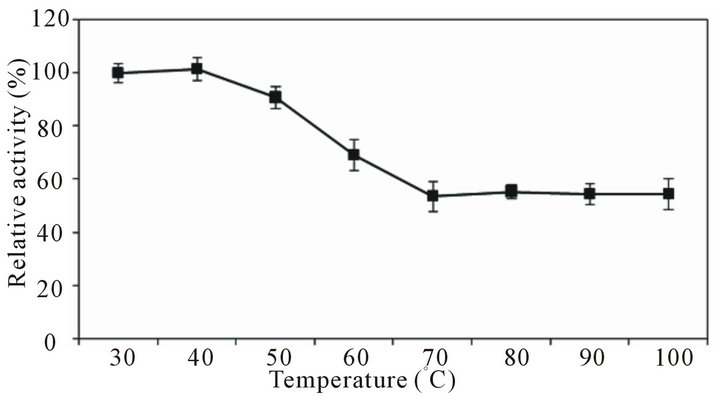
Figure 5. Thermostability-analysis of Bacillus pumilus D-6 protease. Enzyme was pre-incubated at different temperatures (30˚C - 100˚C) for 30 min and then assayed for residual activity.
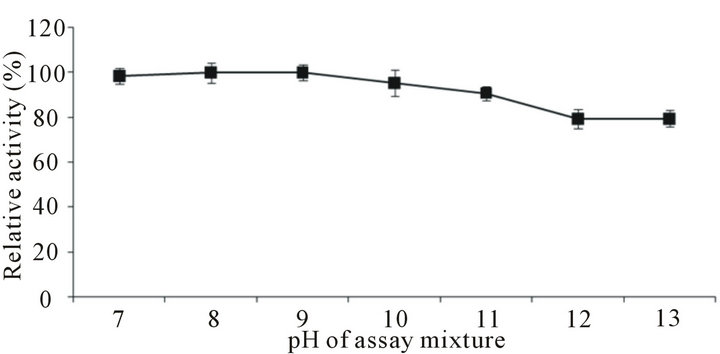
Figure 6. Effect of pH on the activity of alkaline protease from Bacillus pumilus D-6. Activity assay was executed using different buffers: phosphate buffer pH 7 - 8; Tris-HCI buffer pH 8 - 9; carbonate-bicarbonate buffer pH 10 - 11; and KCI-NaOH buffer pH 12 - 13.
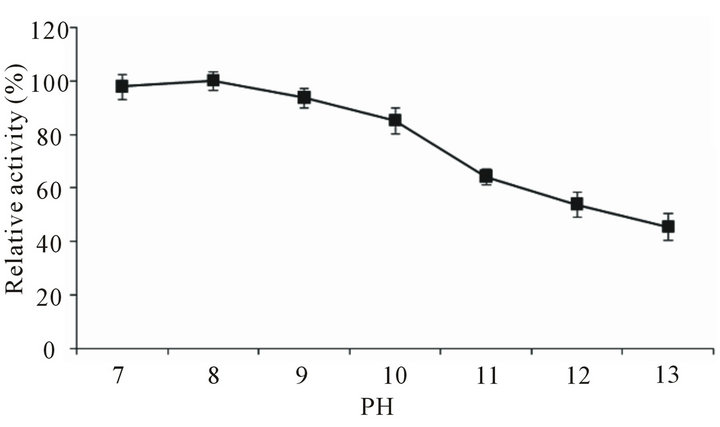
Figure 7. Stability-analysis of Bacillus pumilus D-6 alkaline protease at different pH. Enzyme was pre-incubated with buffer of appropriate pH for 30 min and then assayed for residual activity.
Recombinant Bacillus lehensis protease expressed activity over broad range of pH 8 - 12 [26]. Alkaline protease of Aspergillus clavatus showed activity over a pH range of 6 - 11 with maximum at pH 9.5 [10].
3.5. Effect of Divalent Ions on the Protease Activity
Various ions were included in the enzyme assay reaction mixture at final concentration of 10mM. Ca2+ enhanced the activity maximally (82.35%) followed by Co2+ and Zn2+ (41.17% each) and Cu2+ (17%). Mg2+ did not cause any effect on the activity (Figure 8). The most striking feature was that enzyme activity remained unfazed even in presence of inhibitors like Pb2+ and Hg2+ which are considered universal inhibitors of enzyme activity. More than a quarter of all known enzymes require the presence of metal atoms for full catalytic activity [27]. Metal ions can influence the activity of enzymes by multiple ways: they may accept or donate electrons to activate electrophiles or nucleophiles; they themselves act as electrophiles; they may mask effect of nucleophiles to prevent unwanted side reactions; they may bring together enzyme and substrate by means of co-ordinate bonds and may hold the reacting groups in the required three dimensional orientation; they may simply stabilize the catalytically active conformation of the enzyme [27]. Protease activity of Streptomyces sp. DP2 largely remained uninfluenced by the presence of CuSO4; however, MnCl2 and PMSF did cause a slight reduction (5% - 6%) in activity [4]. Similar to our results, Bacillus sp. PCSIR EA-3 protease was strongly activated by Ca2+ [17]. Ca2+ enhanced thermostability of alkaline protease from B. pumilus although other ions like Mg2+, Mn2+, Co2+ , Cu2+, Fe2+ and Al3+ did not show any affect [5].
4. CONCLUSION
It may be concluded that thermostable alkaline protease from Bacillus pumilus D-6 has the potential for applications in various biotechnological processes. Besides, the enzyme may serve as a model system and may provide new insights about the molecular basis of thermo and alkalistability, and may pave the way for engineering of novel enzymes for industrial applications. In addition, due to organism’s ability to utilize crude agriculture based substrates as carbon and nitrogen source for enzyme production, there is potential for developing economic process for cost-effective bulk production of enzyme. Further work on bulk production of enzyme in Laboratory/pilot scale fermenter must be initiated, and
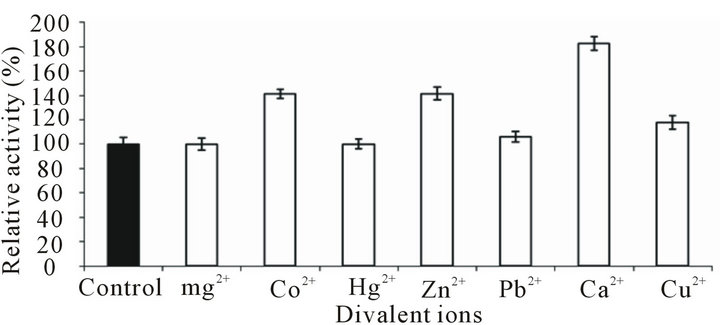
Figure 6. Effect of divalent ions on the activity of Bacillus pumilus D-6 protease. Either of the ion was included in the enzyme assay mixture at final concentration of 10 mm.
the molecular basis of high thermostability and acid/ alkalistability of the proteases must be investigated in depth.
5. ACKNOWLEDGEMENTS
Dr. Bijender Kumar Bajaj acknowledges University Grants Commission, Govt. of India for supporting this research as Major Research Project Ref. F. No. 42-226/2013 (SR); Authors thank the Director, School of Biotechnology, University of Jammu, Jammu, for providing all the necessary laboratory facilities for accomplishment of this work.
REFERENCES
- Jaouadi, B., Ellouz-Chaabouni, S., Ali, M.B., Messaoud, E.B., Naili, B., Dhouib, A. and Bejar, S. (2009) Excellent laundry detergent compatibility and high dehairing ability of the Bacillus pumilus CBS alkaline proteinase (SAPB). Biotechnology and Bioprocess Engineering, 14, 503-512. doi:10.1007/s12257-008-0244-8
- Rai, S.K., Roy, J.K. and Mukherjee, A.K. (2010) Characrization of a detergent-stable alkaline protease from a novel thermophilic strain Paenibacillus tezpurensis sp. nov. AS-S24-II. Applied Microbiology and Biotechnology, 85, 1437-1450. doi:10.1007/s00253-009-2145-y
- Kazan, D., Denizci, A.A., Oner, M.N. and Erarslan, A. (2005) Purification and characterization of a serine alkane protease from Bacillus clausii GMBAE 42. Journal of Industrial Microbiology and Biotechnology, 32, 335-344. doi:10.1007/s10295-005-0260-z
- Bajaj, B.K. and Sharma, P. (2011) An alkali-thermotolerant extracellular protease from a newly isolated Strepmyces sp. DP2. New Biotechnology, 28, 725-732. doi:10.1016/j.nbt.2011.01.001
- Wan, M.Y., Wang, H.Y., Zhang, Y.Z. and Feng, H. (2009) Substrate specificity and thermostability of the dehairing alkaline protease from Bacillus pumilus. Applied Bioemistry Biotechnology, 159, 394-403. doi:10.1007/s12010-008-8497-4
- Rahman, R.N., Mahamad, S., Salleh, A.B. and Basri, M.A. (2007) A new organic solvent tolerant protease from Bacillus pumilus 115b. Journal of Industrial Microbiology and Biotechnology, 34, 509-517. doi:10.1007/s10295-007-0222-8
- Papamichael, E.M., Theodorou, L.G., Perisynakis, A. and Drainas, C. (2010) Purification and characterization of a novel extracellular protease from a halo-alkaliphilic Bacillus sp. 17N-1, active in polar organic solvents. Environmental Technology, 31, 1073-1082. doi:10.1080/09593331003664136
- Bajaj, B.K. and Singh, N.P. (2010) Production of xylanase from an alkalitolerant Streptomyces sp. 7b under solid-state fermentation, its purification and characterization. Applied Biochemistry Biotechnology, 162, 1804-1818. doi:10.1007/s12010-010-8960-x
- Bajaj, B.K. and Abbass, M. (2011) Studies on an alkalithermostable xylanase from Aspergillus fumigatus MA28. 3Biotech, 1, 161-171.
- Tremacoldi, C.R., Monti, R., Selistre-De-Araujo, H.S. and Carmona, E.C. (2007) Purification and properties of an alkaline protease of Aspergillus clavatus. World Journal of Microbiology and Biotechnology, 23, 295-299. doi:10.1007/s11274-006-9211-8
- Haddar, A., Hmidet, N., Ghorbel-Bellaaj, O., FakhfakhZouari, N., Sellami-Kamoun, A. and Nasri, M. (2011) Alkaline proteases produced by Bacillus licheniformis RP1 grown on shrimp wastes: Application in chitin extraction, chicken feather degradation and as a dehairing agent. Biotechnology and Bioprocess Engineering, 16, 669-678. doi:10.1007/s12257-010-0410-7
- Patel, R.K., Dodia, M.S., Joshi, R.H. and Singh, S.P. (2006) Production of extracellular halo-alkaline protease from a newly isolated haloalkaliphilic Bacillus sp. Isolated from seawater in western India. World Journal of Microbiology and Biotechnology, 22, 375-382. doi:10.1007/s11274-005-9044-x
- Moradian, F., Khajeh, K., Naderi-Manesh, H. and Sadeghizadeh, M. (2009) Isolation, purification and characterization of a surfactants, laundry detergents and organic solvents resistant alkaline protease from Bacillus sp. HR- 08. Applied Biochemistry Biotechnology, 159, 33-45. doi:10.1007/s12010-008-8402-1
- Lowry, O.H., Rosenbrough, N.J., Farr, A.L. and Randall, R.J. (1951) Protein measurement with the folin-phenol reagent. Journal of Biological Chemistry, 193, 265-275.
- Hmidet, N., El Hadj Ali, N., Zouari-Fakhfakh, N., Haddar, A., Nasri, M. and Sellemi-Kamoun, A. (2010) Chicken feathers: A complex substrate for the co-production of α-amylase and proteases by B. licheniformis NH1. Journal of Industrial Microbiology and Biotechnology, 37, 983- 990. doi:10.1007/s10295-010-0792-8
- Pillai, P., Mandge, S. and Archana, G. (2011) Statistical optimization of production and tannery applications of a keratinolytic serine protease from Bacillus subtilis P13. Process Biochemistry, 46, 1110-1117. doi:10.1016/j.procbio.2011.01.030
- Quadar, S.A., Shireen, E., Iqbal, S. and Anwar, A. (2009) Optimization of protease production from newly isolated strain of Bacillus sp. PCSIR EA-3. Indian Journal Biotechnology, 8, 286-290.
- Kumar, R., Balaji, S., Uma, T.S., Mandal, A.B. and Shegal, P.K. (2010) Optimization of influential parameters for extracellular keratinase production by Bacillus subtilis (MTCC9102) in solid state fermentation using horn Meal—A biowaste management. Applied Biochemistry Biotechnology, 160, 30-39. doi:10.1007/s12010-008-8452-4
- Lazim, H., Mankai, H., Slama, N., Barkallah, I. and Limam, F. (2009) Production and optimization of thermophilic alkaline protease in solid-state fermentation by Streptomyces sp. CN902. Journal of Industrial Microbiology and Biotechnology, 36, 531-537. doi:10.1007/s10295-008-0523-6
- Vishalakshi, N., Lingappa, K., Amena, S., Prabakar, M. and Dayanad, A. (2009) Production of alkaline protease from Streptomyces gulbargensis and its application in removal of blood strains. Indian Journal Biotechnology, 8, 280-285.
- Manni, L., Jellouli, K., Ghorbel-Bellaaj, O., Agrebi, R., Haddar, A., Sellami-Kamoun, A. and Nasri, M. (2010) An oxidantand solvent-stable protease produced by Bacillus cereus SV1: Application in the deproteinization of shrimp wastes and as a laundry detergent additive. Applied Biochemistry Biotechnology, 160, 2308-2321. doi:10.1007/s12010-009-8703-z
- Chu, W-H. (2007) Optimization of extracellular alkaline protease production from species of Bacillus. Journal of Industrial Microbiology and Biotechnology, 34, 241-245. doi:10.1007/s10295-006-0192-2
- Hadj-Ali, N.E., Agrebi, R., Ghorbel-Frikha, B., SellamiKamoun, A., Kanoun, S. and Nasri, M. (2007) Biochemical and molecular characterization of a detergent stable alkaline serine-protease from a newly isolated Bacillus licheniformis NH1. Enzyme and Microbial Technology, 40, 515-523. doi:10.1016/j.enzmictec.2006.05.007
- Zhu, W., Cha, D., Cheng, G., Peng, Q. and Shen, P. (2007) Purification and characterization of a thermostable protease from a newly isolated Geobacillus sp. YMTC 1049. Enzyme and Microbial Technology, 40, 1592-1597. doi:10.1016/j.enzmictec.2006.11.007
- Morikawa, M., Iwaza, Y., Rashid, N., Hoaki, T. and Imanaka, T. (1994) Purification and characterization of a thermostable thiol protease from a newly isolated hyper-thermophilic Pyrococcus sp. Applied and Environmental Microbiology, 60, 4559-4566.
- Joshi, S. and Satyanarayana T. (2013) Characteristics and applications of a recombinant alkaline serine protease from a novel bacterium Bacillus lehensis. Bioresource Technology, 131, 76-85. doi:10.1016/j.biortech.2012.12.124
- Palmer, T. (2001) The chemical nature of enzyme catalysis, enzymes: Biochemistry, biotechnology and clinical chemistry. Horwood Publishing, Westergate.

