Case Reports in Clinical Medicine
Vol.04 No.10(2015), Article ID:60293,6 pages
10.4236/crcm.2015.410065
Safety and Efficacy of PTCTS Cosmetic Gel: Study on Human Radiodermatitis Lesions
Silvia Regina Graziani1, Brigitte M. Van Eyll1, Carlos Elias Fristachi1, Maria de Lourdes Higuchi2, Renata Ikegami2, Jose Antonio Ramires2
1Arnaldo Vieira de Carvalho Cancer Institute, Sao Paulo, Brazil
2Heart Institute (InCor) of Clinical Hospital, Medical School, University of São Paulo, São Paulo, Brazil
Email: anplourdes@incor.usp.br
Copyright © 2015 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 26 August 2015; accepted 12 October 2015; published 15 October 2015
ABSTRACT
Radiodermatitis is a constant complication after radiotherapy with no efficient drug for prevention and treatment. Its physiopathology is complex, possibly related to injury of epidermis and endothelial cells from the basal layer by radiolysis, overproduction of free radicals, pro-inflam- matory cytokines, and inflammation. PTCTS gel is an after sun cosmetic gel composed by anti- oxidative plant extract rich in natural nanoparticles and thermal water (Complex A) and recombinant protein of Trypanosoma cruzi transialidase (Complex B), with high anti-oxidative and anti- apoptotic effects, presenting anti-aging and healing cutaneous properties. Objective: To study the safety and efficacy of PTCTS gel for topic use in volunteer subjects with healthy skin, and in patients with radiodermatitis, in different concentrations of Complexes A and B. Material and Methods: The project was approved by the Ethical Committee of Arnaldo Vieira de Carvalho Institute, Sao Paulo, Brazil. The individuals were submitted to laboratorial toxicity study through tests for liver and kidney function, levels of blood glucose and blood count before and after 7 days of PTCTS application. We studied two groups: GI-22 volunteers with healthy skin, presenting articular or skeletal muscle pain and GII-38 patients in radiotherapy for breast or head and neck cancer, with radiodermatitis grade 2 or 3. The patients were evaluated before and after 1.0 ml/cm2 PTCTS gel application, twice a day. We tested Complex B in four different concentrations, and Complex A concentrations varied inversely. Results: No irritation signs were observed in GI nor GII group, 64% of GI had important decrease in the intensity of the muscular or articular pain, and 99% of GII showed regression of radiodermatitis, all exhibiting relief of pain. No patient presented hematologic, renal or hepatic toxicity. Conclusion: PTCTS cosmetic gel is a safe option for radiodermatitis treatment, with no side effects or laboratorial toxicity in different concentrations. This product presented excellent clinical results even if used diluted 4 times more than the original product and may be a good option for treatment of radiodermatitis. Further studies may show if it is also indicated in the prevention of the lesion, and if it is better than products containing corticoids.
Keywords:
Radiodermatitis, Nanoparticles, Thermal Water, Transialidase, Toxicity, PTCTS Cosmetic Gel

1. Introduction
Radiodermatitis or acute radiation skin reactions (ARSR) is a constant complication after radiotherapy with no efficient drug for prevention and treatment [1] [2] , characterized by erythema, dry desquamation or moist desquamation. This wet desquamation is a very painful condition for the patient and often leads to interruption of radiotherapy.
Its physiopathology is complex, possibly related to injury of epidermis and endothelial cells from the basal layer by radiolysis, overproduction of free radicals, pro-inflammatory cytokines, and inflammation [3] . The presence of elements called inflammasomes, innate immune system receptors and sensors that regulate the activation of caspase-1 may induce inflammation in response to infectious microbes and molecules derived from host proteins [4] [5] or stress such as exposition of queratinocytes to radiation [6] , releasing pro-inflammatory cytokines and exacerbated immune response. Thus, anti-oxidant drugs neutralizing inflammosomes are good candidates to protection against radiodermatitis.
PTCTS gel (trade INCI name, protected by US patents US7335638B2, US7674832B2 and US7906114B2) is an after sun cosmetic gel, non-commercialized yet. It is composed by anti-oxidative plant extract rich in natural nanoparticles and thermal water (Complex A) and recombinant protein of Trypanosoma cruzi transialidase (Complex B), with high anti-oxidative and anti-apoptotic effects, presenting anti-aging and healing cutaneous properties. The proposed mechanism of action is that radiation induces release of inflammasomes, free radicals, pro-inflammatory cytokines and inflammation. Anti-oxidative nanoparticles may neutralize free radicals, and consequently decrease the inflammation. On the other hand, transialidase releases sialic acid, a ligand from siglecs (sialic-acid-binding immunoglobulin-like-lectins), family of immune system cell receptors with decreasing inflammation activity [7] and increasing the capacity for basal layer of epidermis healing the injured skin.
Objective: To study the safety and efficacy (Phase I/II) of PTCTS cosmetic gel for topic use in different concentrations of Complexes A and B in two groups: patients with radiodermatitis and volunteer subjects with healthy skin and articular or muscle pain.
2. Material and Methods
The project was approved by the Ethical Committee of Arnaldo Vieira de Carvalho Institute, Sao Paulo, Brazil.
The volunteers were submitted to laboratorial toxicity study through tests for liver and kidney function, levels of blood glucose and blood cell count before and after 7 days of PTCTS application.
The individuals were divided in two groups:
GI: Volunteer subjects with healthy skin, presenting articular or skeletal muscle pain (myofascial syndrome).
GII: Patients with radiodermatitis lesion, grade 2 or 3.
The gel was applied in the radiodermatitis lesion or in healthy skin at a place with muscle or articular pain, in doses of 1.0 ml of gel/cm2, twice a day. We tested Complex B in four different concentrations (25%, 50%, 100% and 200%), and Complex A concentrations varied inversely (200%, 100%, 50% and 25%).
The primary endpoint for evaluating efficacy was the follow-up of ARSR before and after application of thin layer of PTCTS gel on the lesion during one week, assessed with the Radiation Therapy Oncology Group/The Organization for Research and Treatment of Cancer Acute Radiation Morbidity Scoring Criteria (RTOG/ EORTC scale). We studied patients with ARSR grade 2 and 3.
The muscular pain were measured by Visual Analog Scale (VAS), a unidimensional measure of pain intensity [8] , which has been widely used in diverse adult populations, including those with rheumatic diseases.
A decrease pain was considered positive when the VAS changed to a low category (for example from severe pain to moderate or mild pain).
The paired Student T test was used for comparing values of laboratory tests before and after PTCTS gel application and p < 0.05 was considered significant different.
3. Results
In GI we did not observe any cutaneous alteration by visual scoring of the skin reactions such as edema, hyperemia or other abnormality on the healthy skin. 14 of the 22 individuals (64%) had important decrease in the intensity of the muscular or articular pain.
In GII, occurred regression of grade 2 or 3 radiodermatitis in 37 of 38 patients (99%). Only one patient presented mild hyperemia at the lesion. We considered efficacy of treatment when after one week of PTCTS administration the grade decreased at least one level. All patients exhibited relief of pain.
Laboratorial evaluation:
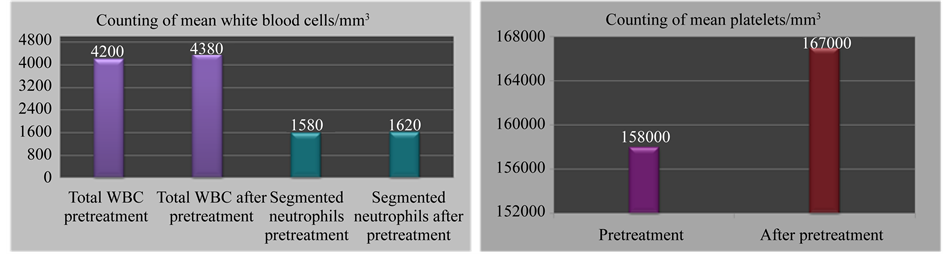
Hemoglobin levels: There was no difference in the hemoglobin levels before and after treatment.
Pretreatment―mean of 12.5 g/dL.
After treatment―mean of 11.8 g/dL.
White blood cells, segmented neutrophils levels and counting of platelets are demonstrated in Figure 1.
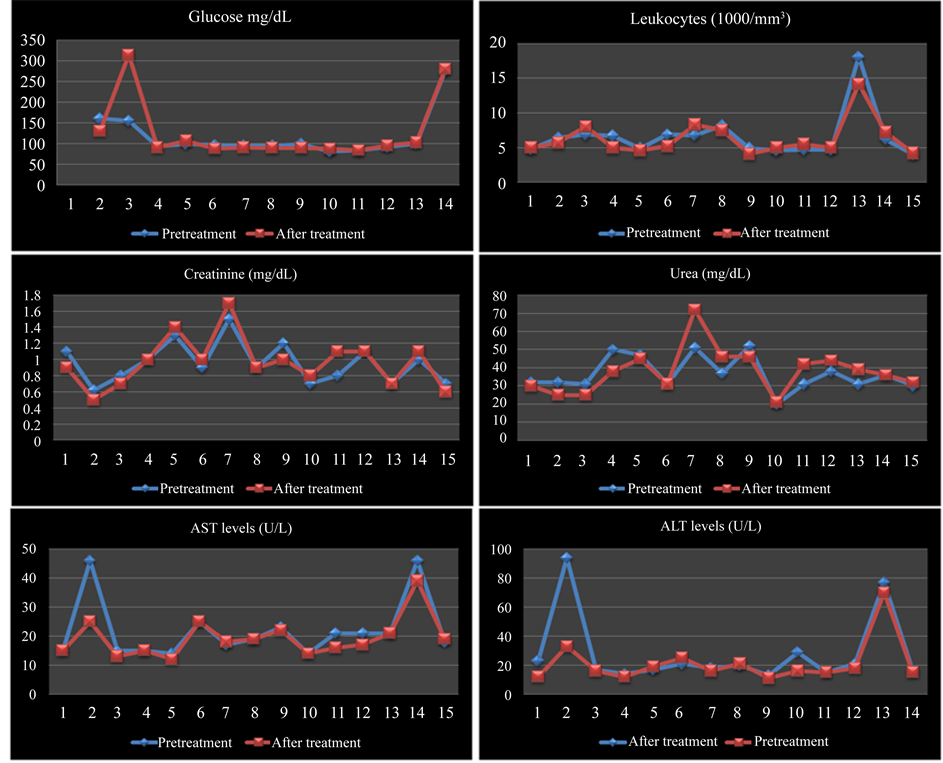
Levels of glycemia, leukocytes, creatinine, urea, serum alanine transaminase (ALT) and serum aspartate transaminase (AST) remained normal before and after treatment. There was no significant difference in paired student T test (p > 0.05) in all variables analyzed. Figure 2 shows 14 healthy skin patients who had important decrease in the intensity of the muscular or articular pain.
We did not perform other tests, since no side effect was seen with 4 times higher concentration and excellent results were obtained even in the most diluted concentration of PTCTS (Figure 3).
4. Discussion
Acute radiation skin reactions (ARSR) are a common side effect, occurring in the majority of patients undergoing curative radiotherapy. Skin reactions can range from mild erythema, through dry desquamation (dry, flaky or scaly skin) to confluent moist desquamation, where blistering, peeling and sloughing of the skin occur. These lesions are classified in grades 1, 2, 3 and 4, the last being the most severe stage with necrosis, which is rarely seen nowadays. In our study we included patients with grade 2 (tender, bright erythema; patchy, moist desquamation or moderate edema) or 3 (confluent, moist desquamation other than skin folds; pitting edema) assessed with the Radiation Therapy Oncology Group/The Organization for Research and Treatment of Cancer Acute Radiation Morbidity Scoring Criteria (RTOG/EORTC scale) at follow-up. We used this scale to determine efficacy of the present new cosmetic gel, PTCTS.
Although radiation skin reactions are not very well understood, it is important to consider the way in which healthy skin regenerates. The skin is composed of two main layers: the epidermis (superficial layer) and the dermis (deep layer). The process of skin homeostasis is normally substitution of superficial cells by shed
Figure 1. Counting of white blood cells, segmented neutrophils levels and of platelets (mean) before and after treatment.
Figure 2. Levels of glycemia, leukocytes, creatinine, urea, serum ALT and AST.
Figure 3. Two patients with grade 3 radiodermatitis after resection of breast cancer, which healed using PTCTS, Complex A 100% and Complex B diluted 4× (25%).
through normal desquamation, new cells are formed in the basal layer of the epidermis, and these continually replace those that are lost. The dermis, which contains blood vessels, glands, nerves and hair follicles, provides the supportive structure required for the epidermis to renew. Repopulation of the entire epidermis takes approximately 4 weeks, although this process can be shorter during times of healing [9] . In general, the basal layer of the epidermis proliferates rapidly, so it is particularly sensitive to radiotherapy. Radiation essentially damages the mitotic ability of stem cells within the basal layer, thus preventing the process of repopulation and weakening the integrity of the skin. Radical radiotherapy repeatedly impairs cell division within the basal layer, and so the degree to which a skin reaction develops is dependent on the survival of actively proliferating basal cells in the epidermis. Moist desquamation occurs when clonogenic cells in the basal layer are sterilized, thus rendering cells unable to repopulate in time to replace the damaged tissue. Consequently, the epidermis becomes broken [10] [11] . Small areas of moist desquamation tend to heal from the basal layer, whereas large areas of broken epidermis require cells to migrate from the surrounding epidermis [11] . Healing becomes visible as islands of epidermal cells expand and reform in central and peripheral regions of the desquamation [12] .
The PTCTS, a new cosmetic gel, has characteristics that favors the healing of radiodermititis as prevents apoptosis and inflammation was able to ameliorate the grade of lesion in one week, much faster than the usual 4 weeks.
Many studies have investigated the efficacy of topical agents on the prevention of ARSR. An initial study showed that the use of topical corticosteroids in the prevention of ARSR among patients undergoing radiotherapy compared with placebo, other topical medication or no treatment appears to significantly reduce the incidence of ARSR, specifically moist desquamation [13] . Skin washing, including gentle washing with water alone with or without mild soap, should be permitted in patients receiving radiation therapy to prevent acute skin reaction. But there is insufficient evidence to support or refute specific topical or oral agents for the prevention or management of acute skin reaction. Some experts recommend that the use of a plain, nonscented, lanolin-free hydrophilic cream may be helpful in preventing radiation skin reactions. In addition, a low dose (i.e., 1%) corticosteroid cream may be beneficial in the reduction of itching and irritation [14] .
However, a more extensive review was completed but did not provide evidence to inform clinical practice guidelines. The British Columbia Cancer Agency’s (BCCA) guidelines initially written in 2000 were revised in 2002 and again in March 2006. A BCCA working group actively monitors the literature for relevant new evidence or skin care management guidelines. There remains a scarcity of available research demonstrating that particular products can prevent, delay, or improve radiation skin reactions, and there is still little consensus on a sensitive grading scale or assessment tool for radiation skin reactions [15] . A systematic review published in 2006 by the Cancer Care Ontario Supportive Care Guidelines Group concluded there was insufficient evidence to support the use of any topical agent. A systematic review published in 2010 reported that topical corticosteroids and hyaluronic acid might be of some benefit, which was validated for corticosteroids, but the evidence was inconsistent for hyaluronic acid and trolamine [16] .
These data show the importance to find a really efficient product for prevention and treatment of radiodermatitis complication in the treatment of cancer.
In this study, we evaluated volunteer with healthy skin to determine side effects (toxicity) as a primary objective. As a secondary objective we also evaluated if the PTCTS decreased the articular or muscular pain that these volunteers presented, using the Visual Analog Scale (VAS).
The pain VAS is a unidimensional measure of pain intensity [8] , which has been widely used in diverse adult populations, including those with rheumatic diseases.
For pain intensity, the scale is most commonly anchored by “no pain” (score of 0) and “pain as bad as it could be” or “worst imaginable pain” (score of 100). Using a ruler, the score is determined by measuring the distance (mm) on the 10-cm line between the “no pain” anchor and the patient’s mark, providing a range of scores from 0 - 100. Based on the distribution of pain VAS scores, the following cut points on the pain VAS have been recommended: no pain (0 - 4 mm), mild pain (5 - 44 mm), moderate pain (45 - 74 mm), and severe pain (75 - 100 mm) [17] .
The present study was conducted in order to show safety and efficacy of PTCTS gel cosmetic in treatment of the established radiodermatitis. The results based on visual scoring of the skin reactions, and relief of the symptoms reported by the patients, indicated that this product is safe and efficient in treating the lesion and pain symptoms.
Particulated transialidase (PTCTS) is a compound containing transialidase from Trypanosoma cruzi from recombinant bacteria diluted 1,000,000× in thermal water and extract of orchid, that presents anti-aging and anti- oxidative activities [18] produced by Higuchi & Santos Ciência e Biotecnologia Ltda. Inflammatory response is a normal physiological reaction of the body to restore and maintain homeostasis [19] . Innate immune system mechanisms of defense are activated to kill and remove most of the harmful substances. The innate and the adaptive immune response, although often viewed as separated systems, are intertwined and affect each other [20] .
PTCTS organic nanoparticles would work as exogenous anti-oxidant benign exosomes and the transialidase would remove sialic acid from their main sites, releasing them to act as ligand of the siglecs components family, which reduce inflammation. In many inflammatory processes, there was a release of microparticles called inflammasomes, activating innate immune system receptors and sensors that regulate the activation of caspase-1. The inflammation may occur in response to infectious microbes and molecules derived from host proteins [4] [5] or stress such as exposition of queratinocytes to radiation [6] , releasing pro-inflammatory cytokines and exacerbated immune response. Thus, anti-oxidant drugs neutralizing inflammosomes are good candidates to protection against radiodermatitis. PTCTS acts on inflammasomes, decreasing the exacerbated inflammation, apoptosis and pain, favoring the healing of cutaneous injury caused by the radiation.
Future studies focusing on methods to prevent ARSR (occurring within the first 6 months of irradiation) are necessary to determine if PTCTS would be a good product to prevent acute skin reactions related to radiation therapy.
5. Conclusion
PTCTS cosmetic gel is a safe option for radiodermatitis treatment, with no side effects or laboratorial toxicity in different concentrations. This product presented excellent clinical results even if used diluted 4 times more than the original product and may be a good option for treatment of radiodermatitis. Further studies may show if it is also indicated in the prevention of the lesion, and if it is better than products containing corticoids.
Cite this paper
Silvia ReginaGraziani,Brigitte M.Van Eyll,Carlos EliasFristachi,Maria deLourdes Higuchi,RenataIkegami,Jose AntonioRamires, (2015) Safety and Efficacy of PTCTS Cosmetic Gel: Study on Human Radiodermatitis Lesions. Case Reports in Clinical Medicine,04,327-333. doi: 10.4236/crcm.2015.410065
References
- 1. Gosselin, T.K., Schneider, S.M., Plambeck, M.A. and Rowe, K. (2010) A Prospective Randomized, Placebo-Controlled Skin Care Study in Women Diagnosed with Breast Cancer Undergoing Radiation Therapy. Oncology Nursing Forum, 37, 619-626.
http://dx.doi.org/10.1188/10.ONF.619-626 - 2. Maddocks-Jennings, W., Wilkinson, J.M. and Shillington, D. (2005) Novel Approaches to Radiotherapy-Induced Skin Reactions: A Literature Review. Complementary Therapies in Clinical Practice, 11, 224-231.
http://dx.doi.org/10.1016/j.ctcp.2005.02.001 - 3. McQuestion, M. (2011) Evidence-Based Skin Care Management in Radiation Therapy: Clinical Update. Seminars in Oncology Nursing, 27, e1-e17.
http://dx.doi.org/10.1016/j.soncn.2011.02.009 - 4. Guo, H., Callaway, J.B. and Ting, J.P. (2015) Inflammasomes: Mechanism of Action, Role in Disease, and Therapeutics. Nature Medicine, 21, 677-687.
http://dx.doi.org/10.1038/nm.3893 - 5. Franchi, L., Eigenbrod, T., Munoz-Planillo, R. and Nunez, G. (2009) The Inflammasome: A Caspase-1-Activation Platform That Regulates Immune Responses and Disease Pathogenesis. Nature Immunology, 10, 241-247.
http://dx.doi.org/10.1038/ni.1703 - 6. Stoecklein, V.M., Osuka, A., Ishikawa, S., Lederer, M.R., Wanke-Jellinek, L. and Lederer, J.A. (2015) Radiation Exposure Induces Inflammasome Pathway Activation in Immune Cells. Immunology, 194, 1178-1189.
http://dx.doi.org/10.4049/jimmunol.1303051 - 7. Crocker, P.R. and Redelinghuys, P. (2008) Siglecs as Positive and Negative Regulators of the Immune System. Biochemical Society Transactions, 36, 1467-1471.
http://dx.doi.org/10.1042/BST0361467 - 8. McCormack, H.M., Horne, D.J. and Sheather, S. (1988) Clinical Applications of Visual Analogue Scales: A Critical Review. Psychological Medicine, 18, 1007-1019.
http://dx.doi.org/10.1017/S0033291700009934 - 9. Sitton, E. (1992) Early and Late Radiation-Induced Skin Alterations. Part 1: Mechanisms of Skin Changes. Oncology Nursing Forum, 19, 801-807.
- 10. Glean, E., Edwards, S., Faithfull, S., Meredith, C., Richards, C., Smith, M. and Colyer, H. (2001) Intervention for Acute Radiotherapy Induced Skin Reactions in Cancer Patients: The Development of a Clinical Guideline Recommended for Use by the College of Radiographers. Journal of Radiotherapy in Practice, 2, 75-84.
http://dx.doi.org/10.1017/S1460396900000133 - 11. Hopewell, J.W. (1990) The Skin: Its Structure and Response to Ionizing Radiation. International Journal of Radiation Biology, 57, 751-773.
http://dx.doi.org/10.1080/09553009014550911 - 12. Cox, J.D., Byhardt, R.W., Wilson, F., Haas, J.S., Komaki, R. and Olson, L.E. (1986) Complications of Radiation Therapy and Factors in Their Prevention. World Journal of Surgery, 10, 171-188.
http://dx.doi.org/10.1007/BF01658134 - 13. Meghrajani, C.F., Co, H.C., Ang-Tiu, C.M. and Roa, F.C. (2013) Topical Corticosteroid Therapy for the Prevention of Acute Radiation Dermatitis: A Systematic Review of Randomized Controlled Trials. Expert Review of Clinical Pharmacology, 6, 641-649.
http://dx.doi.org/10.1586/17512433.2013.841079 - 14. Bolderston, A., Lloyd, N.S., Wong, R.K., Holden, L. and Robb-Blenderman, L., the Supportive Care Guidelines Group of Cancer Care Ontario Program in Evidence-Based Care (2006) The Prevention and Management of Acute Skin Reactions Related to Radiation Therapy: A Systematic Review and Practice Guideline. Supportive Care in Cancer, 14, 802-817.
http://dx.doi.org/10.1007/s00520-006-0063-4 - 15. Nystedt, K. (2007) In Response to the Article “The Prevention and Management of Acute Skin Reactions Related to Radiation Therapy: A Systematic Review and Practice Guideline” (Bolderston et al. 2006). Supportive Care in Cancer, 15, 1219.
http://dx.doi.org/10.1007/s00520-007-0249-4 - 16. Herst, P.M., Bennett, N.C., Sutherland, A.E., Peszynski, R.I., Paterson, D.B. and Jasperse, M.L. (2014) Prophylactic use of Mepitel Film Prevents Radiation-Induced Moist Desquamation in an Intra-Patient Randomised Controlled Clinical Trial of 78 Breast Cancer Patients. Radiotherapy and Oncology, 110, 137-143.
http://dx.doi.org/10.1016/j.radonc.2014.01.005 - 17. Hawker, G.A., Mian, S., Kendzerska, T. and French, M. (2011) Measures of Adult Pain: Visual Analog Scale for Pain (VAS Pain), Numeric Rating Scale for Pain (NRS Pain), McGill Pain Questionnaire (MPQ), Short-Form McGill Pain Questionnaire (SF-MPQ), Chronic Pain Grade Scale (CPGS), Short Form-36 Bodily Pain Scale (SF-36 BPS), and Measure of Intermittent and Constant Osteoarthritis Pain (ICOAP). Arthritis Care & Research, 63, S240-S252.
http://dx.doi.org/10.1002/acr.20543 - 18. Higuchi, M.L., Santos, M.H.H., Fagundes, R.Q., Palomino, S.A.P. and Reis, M.M. (2011) Trans-Sialidase from Trypanosoma cruzi: An Anti-Atherosclerotic Drug. Keystone Symposia on Molecular and Cellular Biology, Snowbird, 3-7 April 2011, 66.
- 19. Greenberg, S. and Grinstein, S. (2002) Phagocytosis and Innate Immunity. Current Opinion in Immunology, 14, 136-145.
http://dx.doi.org/10.1016/S0952-7915(01)00309-0 - 20. Hansson, G.K., Libby, P., Schonbeck, U. and Yan, Z.Q. (2002) Innate and Adaptive Immunity in the Pathogenesis of Atherosclerosis. Circulation Research, 91, 281-291.
http://dx.doi.org/10.1161/01.RES.0000029784.15893.10