Journal of Minerals and Materials Characterization and Engineering
Vol.03 No.06(2015), Article ID:60946,14 pages
10.4236/jmmce.2015.36047
Electroslag Remelting of High Technological Steels
Hossam Halfa1,2, A. M. Reda2,3
1Steel Technology Department, Central Metallurgical R&D Institute (CMRDI), Helwan, Egypt
2Department of Physics, College of Science & Humanities, Shaqra University, Al-Dawadme, Saudi Arabia
3Physics Department, Faculty of Science, Zagazig University, Zagazig, Egypt

Copyright © 2015 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).


Received 23 September 2015; accepted 6 November 2015; published 9 November 2015

ABSTRACT
This work aims at the suitability of electroslag process on production of high technological steels such as Maraging steel, modified high speed tool steel (niobium, high nitrogen, and free nitrogen) has been investigated. The experimental results show that high recovery of alloying elements during electroslag remelting of such steels especially high nitrogen tool steel. The previous results are attributed to the slag used in electroslag protect the molten metal from atmospheric oxygen. Also higher recovery of alloying elements during remelting high nitrogen high speed tool steel are due to partial dissolution of nitrides during remelting of such steel which increase nitrogen content above the molten slag which decrease the partial pressure of oxygen leads to protection of molten metal from further oxidation. Also, the results show that, produced ingots are free from internal pipes, porosity and other surface defects. Microstructure obtained for remelted steels is very fine and well distributed for all steel under investigation. In the case of electroslag remelted Maraging steels lower non-metallic inclusions with very fine inclusions and redistribution retained austenite with very fine structure leads to increasing all tensile properties of investigated steels. In the case of high speed tool steels, also the structure is very fine, well distributed, densely and short carbides with lower non-metallic inclusions contents. High cooling rate accompanying with electroslag process has a great effect on type, morphology and content of carbides precipitated in both nitrogen and niobium modified tool steels.
Keywords:
Electroslag, Maraging, Cobalt Free, Tool-Steel, Nitrogen, Niobium

1. Introduction
Maraging steels were originally developed at the Inco R and D Center [1] . They were produced by American Steel Industry and finally by production on a worldwide basis. These alloys are iron nickel martensites that are hardened by precipitation of Mo and Ti containing inter-metallic compounds. The absence of carbon in the alloys confers significantly better harden-ability, toughness and weld-ability compared to conventional high strength steels. Notable developments in recent years have been cobalt free grades of Maraging steels, and number of other specialized compositions [2] .
A combination of Co and Mo was found to produce much greater hardening during Maraging than the sum of the individual Co and Mo hardening contributions [3] . Work with the Co-Mo hardening system led to the development of three new Maraging steels compositions, the so-called 18 Ni (200), 18 Ni (250) and 18 Ni (300) alloys. The numbers in parenthesis refer to the nominal recovery strengths of the alloys in Ksi units after aging. Titanium was also added to these alloys as a supplemental hardener [4] . The properties of these alloys were considerably better than the earlier 25 Ni and 20 Ni steels.
High speed steels are high alloyed carbon steels with a complex pattern of carbide. They are employed in cutting tools operating at high speeds. Further, nitrogen and niobium have been used in the recent years as alloying elements in many steel grades [5] [6] . AISI M41 high speed tool steels may be considered as high speed AISI M2 tool steel but with addition of cobalt (5% - 10%). The increased cobalt aids the crystallization of primary carbonitride phase colonies. Size and quantity of such colonies increasing with the higher cobalt alloying because cobalt removes carbon and nitrogen from the solid solution resulting in increasing the carbonitrides quantity [7] .
It seems very attractive to examine the possibility of alloying the high speed steel with niobium and nitrogen and study its effect on the refining and mechanical properties of one of the most important high speed steel (AISI M41).
These quality steel grades require sophisticated production technology. The properties of Maraging steel and high speed tool steels containing niobium and nitrogen depend to a large extent on the production and refining technology which affect the recovery and homogeneity of alloying elements as well as the cleanness of the produced steel [8] .
The contamination of these steels with non-metallic inclusion (NMI), the homogeneity of matrix composition and zone segregation of alloying elements has great influence of their properties.
Among the different refining processes such as vacuum arc remelting, electron beam remelting, plasma arc remelting and electroslag remelting, electroslag remelting (ESR) process is considered as the most distinguished secondary refining process due to its reality, economical production cost, low needed investments, system with non-complicated upgrading and the competitive and high efficiency in refining different and complicated steel grades. ESR of Maraging, high speed tool steels containing niobium and nitrogen have many advantages that could be concluded in the improvement of quality structure, chemistry, processing, application and properties [8] - [10] .
The improvement in the quality of steel ingots produced by ESR process arises from obtaining sound ingots with complete absence of pipes and porosity, clean smooth surface and high product yield. The structure of ESR ingots may be improved through the uniformity, elimination of banding and zone segregation, control over the direction and rate of solidification, control of grain size and control of carbides size [9] [10] .
ESR process produces much cleaner ingots with smaller and uniform non-metallic inclusions (NMI), uniformly chemical composition, higher recovery of alloying elements, controlled reduction of undesirable elements, ability to correct out the electrode chemistry by proper slag chemistry and protection of molten metal from atmospheric oxidation. ESR process improves the processing of steel ingots as it improves the weld-ability and the workability and needs fewer critical conditions in electrode castings [9] - [11] .
Refining of steels by ESR process improves their properties through improvement of ductility, impact transition characteristics, transverse properties, elevated temperature properties and corrosion resistance [9] - [11] .
This work aims at study the suitability of electroslag process on production different advanced material such as Maraging steel, niobium containing, high nitrogen and free nitrogen high speed tool steels.
2. Experimental Work
2.1. Melting
With the objective of this study, cobalt free low nickel newly developed Maraging steel and a new grade of modified super hard high speed tool steel (niobium containing, nitrogen free and nitrogen containing) AISI M41 have been produced. The investigated steels were produced by double melting routine [8] [10] [11] . A medium frequency induction furnace (IF) was used to produce the investigated steels (Maraging and modified high speed tool steels). The molten metal was casted in form of rods with 75 mm diameter and 120 mm height, where these rods were forged and used as consumable electrodes in ESR process. Such electrodes were electro-slag remelted under pre-fused calcium fluoride (CaF2) based slag with different additions of alumina (Al2O3), calcium oxide (CaO) and titanium oxide (TiO2). These slags have approximately the same density and different viscosity and interfacial tension, Table 1.
2.2. Evaluation
2.2.1. Non Destructive Test and Chemical Analysis
The produced ingots were first examined by radiographic tests to make sure they are free from solidification defects. Then the produced ingot was cut in the longitudinal and transverse directions to physically examine the presence of any cavity or holes. To evaluate the efficiency of ESR process, effect of slag chemical and physical properties and the behavior of alloying elements during the refining process, samples from consumable electrode produced by air melting induction furnace, IF and ingots produced by electroslag remelting, ESR at the top and the bottom of the ingots were taken both at the center, half radius and the edge then chemically analyzed by using spectrographic analysis (SPGA).
2.2.2. Maraging Steel
Maraging steel obtained from air melted induction furnace, IF and electroslag remelting, ESR were cut into 10 mm × 10 mm square specimen pieces for observation under optical microscope. Careful machining and grinding first flattens the specimen surface. The flat surface was then polished using emery paper (80 - 1200 grit). Final polishing of the sample surface has been carried out on a polishing cloth smeared with paste of fine particles of alumina to ensure scratch-free polished surface. In order to reveal the surface details, the polished surface of the samples are finally etched with an etchant containing 1 gm of CuCl2 + 30 ml HCl + 50 ml HNO3 + 100 ml H2O. Subsequently, the metallographically polished and etched samples are observed in an optical microscope with reflected light at different magnification.
A quantitative determination of the volume fraction of the existing phases in bulk specimens, especially the retained austenite, is essential for the evaluation of the Maraging steel properties. The amount of retained austenite in investigated Maraging steel was evaluated by X-ray measurements. The X-ray diffraction (XRD) was carried out in BRUKER AXS D8-advance diffractometer with the Cu?Kα radiation (λ = 1.5406 Å) in the Bragg-Brentano configuration operated at 40 KeV and 50 mA. XRD patterns were recorded with step size of 0.02˚ and step durations of 4 s at each step. The volume fraction of austenite, VA, was calculated by the following equation:
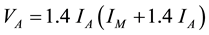 (1)
(1)
where IA is the average of the integrated intensity from the (111)A and (200)A planes and IM is the intergraded intensity from the (110)M planes. The correction factor 1.4 was determined experimentally by many investigator [13] [14] .
The investigated Maraging steel was solution-treated at 820˚C for 1 h and then air-cooled to room temperature. The aging treatments were performed at optimum condition [15] . To investigate the mechanical properties, the plane strain tension Maraging steels test specimens were made according to ASTM specification E-8. The data reported in this investigation are average values of three tests for each investigated steels.
Table 1. Chemical composition and physical properties [12] of synthetic slags at 1600˚C.
2.2.3. High Speed Tool Steel
Produced high speed tool steels were heat treated where they are packed annealed at 870˚C for 1 hour then left to cool slowly in the furnace. The steel is then hardened by austenitization at 1220˚C for 20 minutes. During the heating cycle, samples are held at 400˚C for about 5 minutes and then held at 810˚C for 5 minutes for equalizing the specimen temperature [16] . Samples were quenched from the austenitizing temperature by means of forced air cooling. Quenched samples were tempered twice at optimum conditions [8] and then air cooled. Quenched and full heat treatment samples (quenched and Double tempered at optimum tempering temperature for 1 hour and then air cooled) were polished and finally lapped by diamond paste of 1 µm size prior to etching with 2% natal solution and digital micrographs were recorded using optical (Axiovert 40 MAT, Carl Zeiss, Switzerland).
Identification of the exact nature of the carbides was difficult by XRD analysis of the bulk specimens due to their small amount. Therefore, carbide particles were electrolytically extracted from investigated steel specimens following the report of Nykiel and Hryniewicz [17] . XRD analysis of the extracted carbide particles were done in an identical manner to that for bulk specimens. The phases in the extracted carbide particles and bulk specimens were identified from the XRD profiles with the help of PHILIPS X’Pert software.
Samples used for optical and scanning electron microscopy (SEM) were polished and finally lapped by diamond paste of 1 µm size prior to etching with 2% natal solution and digital micrographs were recorded using optical (Axiovert 40 MAT, Carl Zeiss, Switzerland). Precipitates were analyzed using the connected energy- dispersive X-ray spectroscopy (EDS) unit.
3. Results and Discussion
Radiographic tests for produced steel ingots after ESR process show that the produced ingots are free from internal and external solidification defects.
3.1. Maraging Steels
3.1.1. Recovery of Alloying Elements
Table 2 shows the chemical composition of consumable electrodes produced by induction furnace and ingots electro-slag remelted under different investigated slags. Samples were cut and subjected to evaluate chemical analysis, and hence the recovery of alloying elements was evaluated. The recovery of alloying elements in all ingots produced by electroslag remelting (ESR) process using any of the three calcium fluoride slags are given in Table 3. The recovery can be defined as metal out wt% divided by metal in wt%.
As it is clear from Table 3, high recovery values for all elements were obtained by electroslag remelting under all investigated slags. However, remelting under either slag 1 or slag 3 produced steel ingots with much
Table 2. Chemical composition of the consumable electrodes and the produced ingots by ESR processes of investigated steel.
Table 3. Recovery* of different elements during ESR process.
*Recovery = Metal output/Metal input * 100.
higher elements recovery comparing with that obtained by remelting under slag 2.
The small deviation in chemical composition in produced ingot than consumable electrode may be attributed to transfer of oxygen from atmosphere into metal through slag enhancing oxidation process. Thus, most alloying elements (expect carbon) are slightly lower in ingot than consumable electrode. On the contrary to the other alloying elements, carbon content in the ingot is slightly higher than consumable electrode. This behavior may be attributed to the effect of carbon content in slag at the start of operation. This slag was pre-fused in an arc furnace. Carbon is picked up by slags to some extent. The greatest degree of carbon pick up is with slag containing free lime, the lime combining with the graphite to form carbide
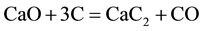 (2)
(2)
XRD showed CaC2 in investigated slags after fusion. As it is usual for refining under such slags, the carbon, C in the slag enters the metal being refined, and carbon (C) is expected to be increased during ESR operation and this explains the slight higher carbon content in the ingot comparing with the consumable electrode. In the process of remelting under slags containing metals of variable valency, metal such as Al, Fe, Mn, Ti anions of these metals (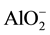 ,
,
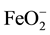 ,
,
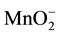 ,
,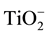 ) diffuse through the slag to the metal. Their passage to the metal is accelerated substantially by convective current in the metal. At the slag-metal interface, anions of trivalent metals are reduced to bivalent ones, as shown in Equation (3):
) diffuse through the slag to the metal. Their passage to the metal is accelerated substantially by convective current in the metal. At the slag-metal interface, anions of trivalent metals are reduced to bivalent ones, as shown in Equation (3):
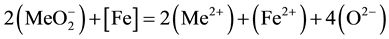 (3)
(3)
Since oxygen passes from the slag to the metal by the reaction:
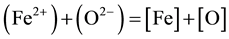 (4)
(4)
Equation (3) shows that high oxygen dissolved in the molten metal which lead to high loss of alloying elements that have high affinity to oxygen Ti, Al, Cr, Mo, Ni in the molten metal pool. Table 3 illustrates the variation of different alloying elements content in the produced steels remelted under the different investigated slag compositions comparing with the initial alloying elements content in consumable electrode. It is clear from Table 2 that the degree of oxidation of alloying elements is depending on the slag composition and residual deoxidation elements. The variation in alloying elements content depends on the affinity of such elements towards oxygen. Element which has a low affinity towards oxygen such as Ni and Cr, its content has a little change. On the other hand, titanium with its higher affinity towards oxygen suffers from a high change in its content.
It is known that [18] , there are two valances of chromium (Cr2+ and Cr3+) dissolved in their slag. The ratio Cr2+/Cr3+ increases with increasing temperature, decreasing oxygen potential and decreasing slag basicity. Under ESR condition, i.e. in the basic slags and at low oxygen potential (at slag-metal interface), the bivalent chromium predominates in the slag. As the chromic oxide particles in the slag react with de-oxidant elements Ti, Al in molten metal droplets and slag metal interfaces, resulting in some recovery back into the steel. The temperature and molten metal composition have a significant effect on the direction of the reaction. Chromium is recovered from slag by reducing its oxide with Ti, Al, Si. Under this reducing condition (molten metal/slag interface and molten metal droplet/slag interface) the reaction to be considered is as formulated below
 (5)
(5)
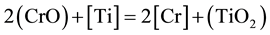 (6)
(6)
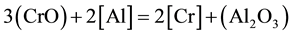 (7)
(7)
As it is seen from experimental data, the higher slag basicity (high CaO) and the higher de-oxidant elements content of steel, the lower is the change of chromium, i.e. the greater the chromium recovery from the slag.
Due to the high affinity of titanium towards oxygen to form TiO2 and its affinity to nickel to form Ni-Ti intermetallic compound, it could be expected that for high titanium steels content, the losses of titanium and Ni content either by forming titanium oxide or Ni-Ti intermetallic compound is associated with increment of chromium in the produced ingot. The decreasing of Ni content in different steels may be explained by formation Ni-Ti intermetallic compound with rather big volume which picked in slag layer during ESR process.
Unfortunately, due to the lack of published data on the nature of alloying elements during ESR process, one could expect the oxidation behavior of alloying elements during the electroslag process in the following:
・ As the temperature of slag bath rises above the melting point of the metal, droplets melt off the tip of the electrode fall through the slag.
・ As the temperature of fallen droplet is fairly high, the oxidation of constituent elements is taken place depending on its affinity towards oxygen.
・ Increasing the wetting of fallen droplet with slag, i.e., decreasing the interfacial tension represents a protective layer against diffusion of oxygen towards metal droplet with the result of decreasing the oxidation rate and hence increasing the recovery of alloying elements.
・ Inhibition of the diffusion of oxygen towards metal droplet by increasing the slag viscosity results in increasing the recovery of alloying elements. So one could expect that among the three used slags, the slag with the highest viscosity and the lowest interfacial tension will lead to the highest recovery of alloying elements Ni, Cr and Ti.
The recovery of alloying elements in ESR can be carried with the physical properties of slags, Table 2 and Table 3. Table 2 and Table 3 show clearly the effect of slag viscosity and slag/metal interfacial tension on the behavior of different alloying elements during ESR process. Among the three investigated slags, slag 1 has the lowest viscosity and highest interfacial tension. As the temperature of slag bath rises above the melting point of the metal, droplets melt off the tip of electrode fall through the slag. Inhibition of the diffusion of oxygen through slag towards metal droplet by increasing the slag viscosity results in decreasing the oxidation of alloying elements and consequently increasing of the recovery of these elements, Table 3. Furthermore, increasing the wetting of fallen droplet with slag, i.e. decreasing the interfacial tension, represents a protective layer against diffusion of oxygen towards metal droplet with the result of decreasing the oxidation rate and hence increasing the recovery of alloying elements, Table 3. This explains the higher recovery of alloying elements by remelting under either slag 2 or 3 comparing with that obtained by remelting under slag 1.
The role of slag viscosity is clearly shown in steels with high titanium content. Increasing the slag viscosity from 0.25 poise (slag 1) to 3 poise (slag 3) through 0.8 poise (slag 2) is accompanied by increasing the recovery of Cr, Ni, Mo and Ti alloying elements.
Generally, we can conclude that, there is a trend to increase the recovery of alloying element, i.e. decreasing the losses during ESR process by increasing the viscosity of used slag and decreasing the slag/metal interfacial tension of used slag.
Furthermore, increasing the TiO2 content in the used slag increases the tendency to equilibrium situation (Ti)/[Ti], which in turn decreases the oxidation of titanium element at metal droplet/slag interface. This phenomenon leads to decreasing the percentage of Ti losses. So, it is expected that the highest recovery of titanium will be obtained by increasing TiO2 in slag 3% to 30%.
3.1.2. Metallography
Figure 1 shows the microstructure of different heats of Maraging steel under investigation produced by induction furnace (IF) and electroslag remelting (ESR) after optimum aging conditions. Microstructure of steel produced by both IF and ESR comprises martensite + retained austenite. By comparing between microstructure of investigated steels we found that, the structure of ESR are very finer, well distributed and free from segregation or band structure than IF steels.
Typical optical micrographs of the material produced by IF and aged under optimum condition are shown in first column in Figure 1. The microstructure, in general appeared lamellar in morphology. The prior-austenite grain boundaries could not be resolved easily. The bright patchy regions, shown by arrows in the micrograph, correspond to regions having considerable volume fraction of reverted austenite. The presence of inter lath austenite, though not fully resolved, is also indicated in the microstructure.
The optical micrographs of the material produced by ESR in the peak-aged conditions are shown in Figure 1. The microstructure in the peak-aged condition essentially consisted of packets of martensite, within prior-aus- tenite grains. The austenite grains, which had transformed into packets of martensite, could still be recognized due to the preferential etching along their boundaries and also due to the fact that the martensite packets within an austenite grain did not extend beyond the respective prior-austenite grain boundary. The martensite substructure could not be observed because of the narrowness of the martensite laths. During aging of the steels under investigation the well-known precipitation reactions occur which leading to hardening. It is generally believed

Figure 1. Metallographical microscope observation of investigated steel before and after ESR.
that initial precipitation in cobalt free molybdenum containing Maraging steel at 480˚C occurs as Ni3Mo, which on prolonged aging is replaced by either Fe2Mo or the σ phase [19] . Since the alloy additionally contains titanium as a supplemental hardener, the precipitation of Ni3Ti has also been reported; alternatively, it has been suggested that part of the titanium may be present in the molybdenum precipitate, i.e. as Ni3(Mo, Ti) [2] - [20] .
3.1.3. Effect of ESR Process on Austenite Reversion
The percentage of retained austenite influences significantly the mechanical properties of Maraging steels: limiting its usefulness as a high strength material (i.e. dual phase). Further deterioration in the mechanical properties of Maraging steels is obtained by micro-segregation of retained austenite in localized area i.e. the solute segregation to the existing dislocations that causes dislocation locking.
It is clear from Figure 1 that ESR process under any of the investigated slags leads to uniform distribution of austenite grain. It is expected that this redistribution of austenite grains may improve the mechanical properties, Table 4.
The amount of retained austenite formed after solution treatment and after aged at optimum condition for consumable electrode produced by IF and ingots produced from different heats of ESR were studied using X-ray diffraction, Figure 2. For IF steel, after solid solution annealing treatment at 820˚C for 1 h (air cooled), with no refrigeration treatment, about 1% ± 0.5% retained austenite was detected X-ray diffraction results confirmed that there is a complete martensite transformation after solution-treatment for investigated steels as shown in Figure 2.
3.1.4. Mechanical Properties
The effect of ESR process on the mechanical properties of investigated steels is summarized in Table 4. Table 4, represents the average of four results at room temperature.
Examination of these results reveals generally the strong positive influence on the mechanical properties i.e., ultimate tensile strength (UTS), yield tensile strength (YTS), and reduction of area. Table 4 illustrates the influence of ESR slages on the yield strength and elongation comparing with that of steel melted in induction furnace. It was noticed that ESR process has a rather smaller effect on the recovery strength of steel with lowest content of Ti.
As mention above the actual factors which control the mechanical properties of these steels are:
・ solid solution strengthening.
・ precipitation strengthening.
・ retained austenite in martensite matrix.
By studying Table 4, the effect of Mo and Cr on the mechanical properties of investigated steels was clarified. The contribution of Mo and Cr and Mo in ultimate tensile strength indicates that the positive effect of Cr and Mo may be attributed to solid solution strengthening mechanism of each element. However, the strength increment of some investigated steels is accompanied by deterioration of steel ductility (Elongation). This deterioration could not be attributed to the aggregate of austenite grains only but also to initial chemical composition of steels and its contamination of NMI. The negative effect of NMI, Ti and Mo content on steel ductility is compensating by the positive effect of both Cr and austenite content.
It well known that [21] - [24] , a portion of titanium in solution is precipitated during solution treatment as Ni3Ti, which has been confirmed by dilatometry studies, causing intermetallic precipitation strengthening.
In high titanium steels, titanium in excess of stoichiometric with nitrogen and carbon and did not precipitate


Figure 2. (a) X-Ray pattern of M3 Maraging steel before ESR. a) Solid solution annealing at 820˚C, b) Solid solution annealing at 820˚C and Aging; (b) X-ray diffraction pattern for steel M3 after ESR. a) Under slag 1 (70/15/15), b) Under slag 2 (52.5/22.5/25), c) Under slag 3 (70/30).
Table 4. Mechanical properties of investigated Maraging steel.
All reading is average of three measurements.
as intermetallic compound could be in solution causing solid solution strengthening. Consequently, the strengthening effect of titanium solution treated Maraging steel is combined effects of solid solution, microstructure refinement, non-metallic precipitation and intermetallic precipitation strengthening.
3.2. High Speed Tool Steel
3.2.1. Recovery of Alloying Elements
Table 5 and Table 6 show the chemical composition of consumable electrodes produced by induction furnace and ingots remelted under different investigated slags. Samples were cut from top to bottom at surface and center positions to evaluate the recovery of alloying elements. The recovery of alloying elements in all ingots produced by electroslag remelting (ESR) process using any of the three calcium fluoride slags are given in Table 7 and Table 8. Recovery can be defined as “metal-out Wt% divided by metal-in Wt%”.
Table 5. Chemicalcomposition of the high nitrogen and free nitrogen steels (wt%).
Note: IF = air melted induction furnace heat (free nitrogen). ESR1 = free electroslag remelting (free nitrogen) under slag no. 1. ESR2 = electroslag remelting (free nitrogen) under slag no. 2. ESR3 = electroslag remelting (free nitrogen) under slag no. 3. IFN = air melted induction furnace heat (nitrogen containing). ESRN1 = electroslag remelting (nitrogen containing) under slag no. 1. ESRN2 = electroslag remelting (nitrogen containing) under slag no. 2. ESRN3 = electroslag remelting (nitrogen containing) under slag no. 3.
Table 6. Chemical composition of the niobium containing high speed tool steels (wt%).
Table 7. Recovery* of different elements during ESR process.
*Recovery = Metal output/Metal input * 100.
Table 8. Recovery* of different elements during ESR process niobium containing high speed tool steels.
*Recovery = Metal output/Metal input * 100.
As it is clear from Table 7 and Table 8, high recovery values for all elements were obtained by electroslag remelting under all investigated slags. However, remelting under either slag 1 or slag 3 produced steel ingots with much higher elements recovery comparing with that obtained by remelting under slag 2.
It is noticeable that recoveries of alloying elements in ESR are high. This result could be attributed to the physical properties of slags, Table 7 and Table 8. Among the three investigated slags, slag 2 has the lowest viscosity and highest interfacial tension. As the temperature of slag bath rises above the melting point of the metal, droplets melt off the tip of electrode fall through the slag. Inhibition of the diffusion of oxygen through slag towards metal droplet by increasing the slag viscosity results in decreasing the oxidation of alloying elements and consequently increasing the recovery of these elements. Furthermore, increasing the wetting of fallen droplet with slag, i.e. decreasing the interfacial tension, represents a protective layer against diffusion of oxygen towards metal droplet with the result of decreasing the oxidation rate and hence increasing the recovery of alloying elements. This explains the higher recoveries of alloying elements by remelting under either slag 1 or 3 comparing with that obtained by remelting under slag 2.
It is also noticeable that recoveries of alloying elements are higher for steel M41N comparing with that of steel M41. This could be explained by the behavior of nitrogen during remelting process. A part of nitrogen transfers to slag and surrounding atmosphere from the consumable electrode. This is expected to reduce the partial pressure of oxygen and retard oxidation process with the consequence of increasing the recovery of alloying elements.
As it is clear from Table 8, high recovery values for niobium was obtained by electroslag remelting under slag no. 1 and slag no. 3 with lower viscosity. This result can be also attributed to the same reasons of nitrogen containing and nitrogen free high speed tool steel.
3.2.2. Metallography High Speed Tool Steel
One of many advantages claimed for electroslag remelting (ESR) process is that of lowering local solidification time which affects the type of carbide formed during solidification process and helps in forming fine carbides with higher alloying element and higher carbon content.
For high nitrogen containing steels, the nitrogen alloying leads to form broken eutectic lattice and reduce eutectic colonies. This effect is very noticeable when the carbon is also reduced and the reduced size of the eutectic colonies is proportional to N/C ratio which has strong effect on steel properties.
Table 9 and Table 10 summarize the chemical composition and structure of the observed precipitates. The obtained results show that in the traditional grade with normal nitrogen content, which arose from the break down of atmospheric nitrogen, contains carbides and some carbonitrides rich in chromium (Cr), tungsten (W), and molybdenum (Mo) while the nitrogen alloyed grade contains the carbonitrides precipitates with large content of nitrogen. Vanadium and iron form the major alloying elements constituents in the precipitates while Cr, W, and Mo form the minority. One of the most important observations is that cobalt (Co) forms trace constituents in the traditional grade while it is found with larger amount in the nitrogen alloyed grade.
Matrix of high speed steels consists of well-tempered martensite and the carbides causing secondary hardness [25] - [28] . Microstructure of correctly heat treated high speed steel should consist of a hard and homogeneous matrix with a high volume fraction of fine and uniformly distributed carbides both the un-dissolved during austenitizing and being formed during tempering.
In the matrix, carbide of almost the same grain size is precipitated evenly and densely in ESR ingots as shown in different microstructure photos, Figure 3. The above mentioned improvement of the shape, size and distribution of carbides precipitated in ESR ingot could be attributed to shorter holding time of molten steel
Table 9. EDS analysis of NMI of investigated nitrogen free high tool speed steel, AISIM41.
Table 10. EDS analysis of NMI of investigated nitrogen high tool speed steel, AISIM41.

Figure 3. Metallographical observation for investigated high speed tool steel.
and directional solidification, which do not allow elements, above all carbon to segregate. Therefore, carbides grow longer and thicker and make their distribution more uniform.
X-ray diffraction pattern confirms the presence of the phases recognized from the metallographic observations. The presence of MC, M6C, M2C and M23C6 carbides is ascertained depends on the chemical compositions of steels. The chemical composition of the carbides was determined using EDS microanalysis in the SEM.
Typical composition of carbides in wt%, as determined by EDS microanalysis are presented in Tables 9-11. For all steel under investigation, the major chemical elements in M6C carbide are tungsten, molybdenum and iron. For standard high speed tool steel, AISI M41 M2C carbide contains nearly four times as much-chromium and three times as much-vanadium as M6C carbide. Molybdenum in M6C carbides is three times greater than M2C carbides while tungsten in M2C is two times greater than M6C.
On the other hand, for niobium containing steel, molybdenum and in particular tungsten concentrations in M2C are generally less than in M6C. The considerable amount of iron in the M2C carbide is presumably due to the high cooling rate during solidification, as high cooling rates are prevailing in the ESR process. As well as MC carbides are vanadium rich carbide in standard steel while in niobium containing steel, this carbide mainly consists of niobium which dissolve considerable amount of vanadium, tungsten and molybdenum. EDS micro-
Table 11. EDS analysis of NMI of investigated Nb-containing high speed tool steels.
chemical analysis of carbide precipitated during cooling/solidification of investigated steel show that the M6C carbides in AISI M41 contain two times more tungsten than niobium containing steels, Table 11. From the results summarized in Table 11, the M2C and M4C3 type carbides may precipitate also in certain solidification/cooling conditions. The fine (dispersive) carbides of the MC, M2C and M4C3 types precipitate during solidification in electroslag remelting process. The considerable amount of the M2C carbide is presumably due to the high cooling rate during solidification/cooling, as high cooling rates are prevailing in the ESR process. From the result of X-ray diffraction and micro-chemical analysis, carbides precipitate from standard steel specimens has been identified mainly as MC, M2C, M6C and M23C6 on the other hand; decrease of M23C6 carbides for niobium containing steels was presumably.
4. Conclusions
4.1. Recovery and Homogeneity of Alloying Elements
・ Recovery of all alloying elements in ESR is very high.
・ Higher recovery of alloying elements is obtained by ESR under slag of high viscosity and low interfacial tension (slags 1 and 3).
・ The new production technique “air induction melting followed by ESR” is suitable technique for producing this kind of steels.
4.2. Microstructure
・ In the case of Maraging steels, the structure of induction heats are lath martensite + austenite and NMI. On the other hand microstructure of ESR ingots was very fine and well distributed and austenite grain are impeding between lath martensite.
・ For high speed tool steels, solidification parameters in electroslag remelting process not only change the amount of carbides precipitated but also the morphology and the chemical composition of this carbides.
・ Nb-alloying improves the shape, size and distribution of carbides precipitated in the produced ingot.
・ For high speed tool steels, added niobium causes a strong stabilization of the MC carbide and the MC stability strongly increases with increasing niobium content.
・ Extracted carbides precipitated from investigated electroslag remelted steel specimens have been identified mainly as MC, M2C, M6C and M23C6. But niobium carbides (NbC) precipitated from niobium containing steels restricted the formation of M23C6. Quantity of M23C6 carbides precipitated from niobium containing steel is low than that precipitated from standard steel.
・ The main carbides in the free nitrogen and nitrogen contained high speed steels are M6C and M7C3.
・ The nitrogen contained high speed steel has the highest precipitates content among the investigated steels.
4.3. Mechanical Properties
・ ESR process has a positive effect on tensile strength properties of Maraging steel. This is due to its effect on reducing the NMI content, improving the homogeneity of matrix composition, minimizing the zone segregation of alloying elements and improving the NMI distribution.
・ Retained austenite inter-lathe film can increase the strength by transforming to martensite during tensile test similar to behavior of trip steel. For this steel, retained austenite has a positive effect on all tensile ductility properties.
Acknowledgements
The steel samples used in this study have been produced and refined at the pilot plant of Steel and Ferroalloys Department, Central Metallurgical Research and Development Institute “CMRDI”, Egypt. May ALLAH shower his mercy and forgiveness on the soul of my teacher and supervisor Prof. Dr. Kamal Mohamed Abd-Rabu. I am indebted to Prof. Dr. Mamdouh Mahmoud Eissa, for his supervision and continuous guidance through all my work. I would like to express my thanks and gratitude to Prof. Dr. Taha Mohamed Taha Mattar, Head of Metal division, CMRDI, and Prof. Hoda Salama El-Faramawy, Head of Steel Technology Department. I gratefully acknowledge the pleasant atmosphere and support from all of my colleagues at the metal Division, Steel Technology Department of CMRDI.
Cite this paper
HossamHalfa,A. M.Reda,11, (2015) Electroslag Remelting of High Technological Steels. Journal of Minerals and Materials Characterization and Engineering,03,444-457. doi: 10.4236/jmmce.2015.36047
References
- 1. Vasudevan, V.K., Kim, S.J. and Wayman, C.M. (1990) Precipitation Reactions and Strengthening Behavior in 18 Wt Pct. Maraging Steels. Metallurgical Transaction A, 21, 2655-2668. http://dx.doi.org/10.1007/BF02646061
- 2. Sha, W., Cerezo, A. and Smith, G.D.W. (1993) Phase Chemistry and Precipitation Reactions in Maraging Steels: Part II. Co-Free T-300 Steel. Metallurgical Transaction A, 24, 1221-1239. http://dx.doi.org/10.1007/BF02668190
- 3. Urzinger, P.W., Rabitsch, R. and Meyer, W. (2004) Production of Maraging Steel Grades and the Influence of Specified and Non-Specified Elements for Special Applications. Proceedings of the 2003 International Symposium on Liquid Metals, Journal of Materials Science, 39, 7295-7302.
- 4. Decker, R.F., Eash, J.T. and Goldman, A.J. (1962) 18% Nickel-Maraging Steel. ASM Transactions Quarterly, 55, 58-76.
- 5. Halfa, H., Mattar, T. and Eissa, M. (2012) Precipitation Behavior of Modified as Cast High Nitrogen Super Hard High-Speed Tool Steel. Steel Research International, 83, 1071-1078. http://dx.doi.org/10.1002/srin.201100228
- 6. Halfa, H. (2013) Characterization of Electroslag Remelted Super Hard High Speed Tool Steel Containing Niobium. Steel Research International, 84, 495-510. http://dx.doi.org/10.1002/srin.201200332
- 7. Sinha, A.K. (2003) Physical Metallurgy Handbook. The McGraw-Hill Companies, Inc., New York.
- 8. Halfa, H. (2003) Improvement of High Speed Tool Steel by Using Electro-Slag Remelting Technology. MSc. Thesis, Faculty of Engineering, Cairo University, Cairo.
- 9. Kato, M. (1985) Study on Electro-Slag Remelting. Nagoya International Training Centre, Naggoya.
- 10. Biebricher, U. and Scholz, H. (1998) Electro Slag Remelting Technologies in the Past and in the Future. Metallurgical Plant and Technology International (Germany), 22, 36-38.
- 11. Kelin, H.J. (1970) The Effect of Variation Melt Parameters on the Electroslag Remelting of a Nickle-Base Alloy. Proceedings of Electric Furnace Conference, 28, 21-27.
- 12. Mills, K.C. and Keeme, B.J. (1981) Physicochemical Properties of Molten CaF2-Based Slags. International Metals Reviews, 1, 21-69.
- 13. Wang, Z.C., Kim, S.J., Lee, C.G. and Lee, T.H. (2004) Bake-Hardening Behaviour of Cold-Rolled CMnSi and CMnSiCu TRIP-Aided Steel Sheets. Journal of Materials Processing Technology, 151, 141-145. http://dx.doi.org/10.1016/j.jmatprotec.2004.04.029
- 14. Li, Z. and Wu, D. (2006) Effects of Hot Deformation and Subsequent Austempering on the Mechanical Properties of Si-Mn TRIP Steels. ISIJ International, 46, 121-128. http://dx.doi.org/10.2355/isijinternational.46.121
- 15. Halfa, H. (2007) Improvement of Maraging Steels Using Electro-Slag Remelting Technology. PhD Thesis, Faculty of Engineering Cairo University, Cairo.
- 16. Chandler, H. (1995) Heat Treater’s Guide. ASM International, Geauga County, 661-663.
- 17. Nykiel, T. and Hryniewicz, T. (2000) Quantitative Approach to Coagulation, Coalescence, and Polygonization of Carbides in the NCWV/D3 Tool Steel. Metallurigical and Material Transaction A, 31, 2661-2665. http://dx.doi.org/10.1007/s11661-000-0211-2
- 18. Grigoryan, V., Belyanchikov, L. and Stomakhin, A. (1983) Theortical Principle of Electric Steelmaking. Mir Publisher, Moscow.
- 19. Chen, H.C., Era, H. and Shimizu, M. (1989) Effect of Phosphorus on the Formation of Retained Austenite and Mechanical Properties in Si-Containing Low-Carbon Steel Sheet. Metallurgical Transaction A, 20, 437-445. http://dx.doi.org/10.1007/BF02653923
- 20. Fathy, A., Mattar, T., El-Faramawy, H. and Bleck, W. (2002) Mechanical Properties of New Low-Nickel Cobalt-Free Maraging Steels. Steel Research, 73, 549-556.
- 21. Decker, R.F., Floreen, S. and Wilson, R.K. (1988) Maraging Steel: Recent Developments and Applications. Proceedings of the Symposium of TMS Annual Meeting, Phoenix, 25-29 January 1988, 1-38.
- 22. Vanderwalker, D.M. (1987) The Precipitation Sequence of Ni3Ti in Co-Free Maraging Steel. Metallurgical Transaction A, 18, 1191-1194. http://dx.doi.org/10.1007/BF02647188
- 23. Ayub, H., Ahmed, M., Husain, S.W. and Khan, A.Q. (1997) Phase Transformation and Magnetic Properties in Cobalt Free and Low Cobalt Maraging Steels. Materials Science and Technology, 13, 110-116. http://dx.doi.org/10.1179/mst.1997.13.2.110
- 24. Rajkumar, K.V., Anish Kumar, T., Jayakumar, Raj, B. and Ray, K.K. (2007) Characterization of Aging Behavior in M250 Grade Maraging Steel Using Ultrasonic Measurements. Metallurigical and Material Transaction A, 38, 236-243. http://dx.doi.org/10.1007/s11661-006-9060-y
- 25. Ding, P., Shi, G. and Zhon, S. (1993) As Cast Carbides in High Speed Steels. Metallurgical Transaction A, 24, 1265-1272. http://dx.doi.org/10.1007/BF02668195
- 26. Hoyle, C. (1988) High Speed Steels. Butterworth Co. Ltd., London.
- 27. Roberts, G.A. and Cary, A.R. (1992) Tool Steels. American Society for Metals, Geauga County.
- 28. Rong, W., Andren, H.-O., Wisell, H. and Dunlop, G.L. (1992) The Role of Alloy Composition in the Precipitation Behaviour of High Speed Steels. Acta Metallurgica et Materialia, 40, 1727-1738. http://dx.doi.org/10.1016/0956-7151(92)90116-V


