Food and Nutrition Sciences
Vol.4 No.8(2013), Article ID:34679,5 pages DOI:10.4236/fns.2013.48099
Heat-Killed Lactobacillus brevis SBC8803 Induces Serotonin Release from Intestinal Cells
![]()
1Frontier Laboratories of Value Creation, Sapporo Breweries Ltd., Shizuoka, Japan; 2Corporate Planning Department, Sapporo Holdings Ltd., Tokyo, Japan.
Email: *yasukazu.nakakita@sapporobeer.co.jp
Copyright © 2013 Yasukazu Nakaita et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received May 29th, 2013; revised June 29th, 2013; accepted July 6th, 2013
Keywords: Serotonin; Lactobacillus Brevis; Mice; Intestinal Cells; 5-HT3 Receptors
ABSTRACT
Previously, we reported that changes induced in autonomic neurotransmission in rats by Lactobacillus brevis SBC8803 may be mediated by serotonin 3 (5-HT3) receptors. In this study, we evaluated the effects of heat-killed L. brevis SBC8803 on serotonin (5-HT) releasing from intestinal cells. In the in vitro study, L. brevis SBC8803 stimulated 5-HT release from cultured rat endocrine RIN-14B cells (SBC8803 vs. sterile water; P < 0.01). For in vivo study, 2 mg of heat-killed L. brevis SBC8803 was administered using a stomach sonde (feeding needle) to C57BL/6J mice. Analysis of plasma by ELISA showed gradually increase in 5-HT concentrations (0 min vs. 60 min; P < 0.05). ELISA of ex vivo cultured intestinal loops composed of duodenum and part of the jejunum, from C3H/HeN and C57BL/6J male mice indicated that L. brevis SBC8803 effectively induced 5-HT release (SBC8803 vs. sterile water; P < 0.01). These experimental results suggest that heat-killed L. brevis SBC8803 may stimulate 5-HT release from mouse intestinal cells such as enterochromaffin cells.
1. Introduction
Neurogastroenterology is a research area of gastroenterology that investigates interactions between the central nervous system (CNS; brain) and the gut [1-3]. The enteric nervous system (ENS) communicates with the CNS through the parasympathetic and sympathetic nervous systems, which comprise nerves and hormones such as enteric hormones.
Lactic acid bacteria (LAB) are one of the most important types of bacteria found in the human and animal gastrointestinal tract. Tanida et al. reported that Lactobacillus strain affects autonomic neurotransmissions and alter physiological phenomena [4,5]. These reports also described that Lactobacillus paracasei ST11 may induce changes in autonomic neurotransmission through the central histaminergic nerves in brain [5], although the molecules that may be involved such as hormones, neuropeptides, and cytokines in the gut have not been identified.
We examined the effects of heat-killed Lactobacillus brevis SBC8803 [6-8] on autonomic neurotransmissions and found that changes induced in autonomic neurotransmissions by this heat-killed strain may be mediated by serotonin 3 (5-HT3) receptors [9]. On the basis of these results, it was suggested that intestinally injected L. brevis SBC8803 stimulates afferent intestinal vagal nerve activity (IVNA) through 5-HT3 receptors; this information is then transmitted to the CNS, which in turn increases efferent gastric vagal nerve activity (GVNA), and GVNA stimulation enhances appetite [9]. To the best of our knowledge, this is the first report that 5-HT3 receptors may be involved in the interactions between LABs and autonomic nerves.
To clarify whether the heat-killed L. brevis SBC8803 stimulates the release of serotonin (5-HT) from intestinal cells, we designed experiments to examine 5-HT release from intestinal cells induced by L. brevis SBC8803 and performed an “in vitro cell-based study”, an “in vivo plasma study”, and an “ex vivo intestinal loop study” experiments.
2. Materials and Methods
2.1. Animals
Male C3H/HeN (Japan SLC Inc.) and C57BL/6J (Charles River Laboratories Japan) mice with a body weight of 20 - 23 g at the time of experiment were used in the in vivo and ex vivo studies. Mice were housed in a cage with free access to chow and water at 23˚C ± 2˚C under a 12 h light-dark cycle (Lights on: 08:00 - 20:00). The animals were allowed at least 1 week to acclimatize to their housing conditions before experiments. The Animal Care and Use Committee of Frontier Laboratories of Value Creation, Sapporo Breweries Ltd. approved all animal care and handling procedures.
2.2. Preparation of Heat-Killed SBC8803
L. brevis SBC8803 were cultured for 24 h at 30˚C in broth containing 2% maltose, 1.4% yeast extract, 0.5% sodium acetate, and 0.005% MnSO4·5H2O. Bacteria were collected by centrifugation at 8000 × g for 10 min at 10˚C (Himac CR21G; Hitachi Koki Co. Ltd.) and washed 3 times with deionized water. After washing, bacteria were heat-killed at 105˚C for 10 min and lyophilized.
2.3. In Vitro Cell Based Study
The rat endocrine RIN-14B cell line (ATCC, CRL-2059) was suspended in RPMI1640 medium supplemented with 10% fetal calf serum (Gibco, Life Technologies) at 1 × 105 cells/0.5 mL, seeded in 24-well plates, and cultured for 3 d at 37˚C in a 5% CO2 incubator (MCO-20 AIC; SANYO Co.). The medium was removed, and the cells were washed with Hank’s balanced salt solution (HBSS) containing Ca and Mg, (Wako Pure Chemical Industries, Ltd.). After washing, HBSS was removed and replaced with 0.5 mL HBSS containing samples (0 - 0.3 mg/mL), and the cells were further incubated for either 30 min or 60 min at 37˚C. The assay solution was collected and centrifuged for 5 min to remove any detached cells. Serotonin concentrations in supernatants were measured using a serotonin-ELISA kit (Enzo Life Science). Results are represented as mean ± SD (n = 4 - 6). Statistical evaluation of the results was performed using one-way analysis of variance (ANOVA).
2.4. In Vivo Plasma Study
The sample was suspended in sterile water and then orally administered using a stomach sonde (feeding needle) to male C57BL/6J mice (2 mg of sample/mouse). At 0, 15, 30, 60, and 90 min after administration, blood samples were collected from mice and treated with heaprin, followed by centrifugation with 5000 rpm for 10 min at 4˚C. Plasma 5-HT concentrations were measured using 5-HT ELISA kits. Results are represented as the mean ± SD (n = 5). Statistical evaluation of the results was performed using one-way ANOVA.
2.5. Ex Vivo Intestinal Loop Study
Male C3H/HeN and C57BL/6J mice were killed. An intestinal loop (approximately 5 cm), composed of duodenum and part of the jejunum, was then removed from the small intestine. Each end of the intestinal loop was ligated with silk sutures and the loops filled with 0.1 ml sterilized water containing 2 mg/mL sample. To measure releasing effects, the loops were incubated for 60 min at 37˚C in a 5% CO2 incubator. The loops were placed into the center section of the organ culture dish (3.5 cm in diameter) in 3 mL of RPMI 1640. RPMI 1640 media in the culture dish were collected at 60 min to determine 5-HT release from the lumen to the medium bathing the loops. The 5-HT level in the medium was measured using the 5-HT-ELISA kit. Results are represented as mean ± SD (n = 5). Statistical evaluation of the results was performed using one-way ANOVA.
3. Results
3.1. 5-HT Release from RIN-14B Cells (in Vitro Studies)
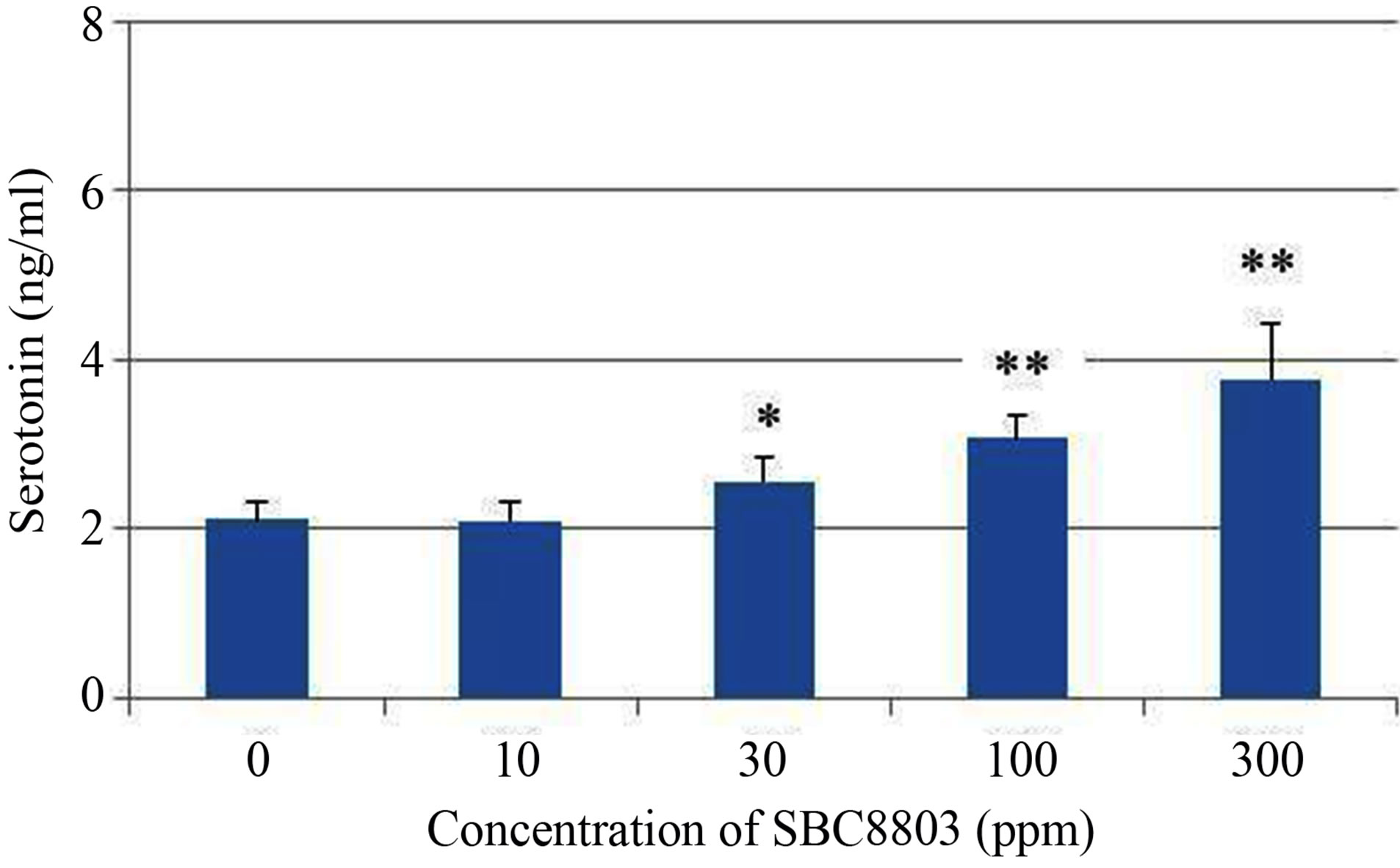
The in vitro experiments were performed to evaluate 5-HT release from the rat endocrine RIN-14B cells, which were used as a tentative model for the investigation of enterochromaffin (EC) cell function [10]. As shown in Figure 1 (a), 5-HT release from RIN-14B cells increased depending on the concentration of SBC8803 (0 - 0.3 mg/mL) after incubation for 30 min (0 ppm vs. 30 ppm; P < 0.05, 0 ppm vs. 100 ppm and 300 ppm; P < 0.01). Moreover, maximum 5-HT release from RIN-14B cells was observed after incubation for 60 min (Figure 1 (b)). Subsequently, we performed an in vitro study using cellulose powder (100 ppm) as a physical stimulus in the 60 min reaction. The 5-HT concentration at 60 min after cellulose powder addition was equal to that obtained after addition of sterile water (Figure 2). Thus, cellulose powder had no effect on 5-HT release from these cells. On the other hand, 5-HT release was observed after addition of heat-killed L. brevis SBC8803 cells (SBC8803 vs. sterile water and cellulose powder; P < 0.01).
3.2. 5-HT Increase in Plasma of Mice (in Vivo Studies)
We determined 5-HT concentrations in blood after administration of heat-killed L. brevis SBC8803. Heatkilled L. brevis SBC8803 was suspended in sterile water and administered using a stomach sonde (feeding needle) to C57BL/6J mice (2 mg of SBC8803/mouse). This dose was equivalent to the daily intake of food containing 0.05% L. brevis SBC8803. At 0, 15, 30, and 60 min after administration, plasma 5-HT concentrations were determined using 5-HT ELISA kits. As shown in Figure 3,
 (a)
(a)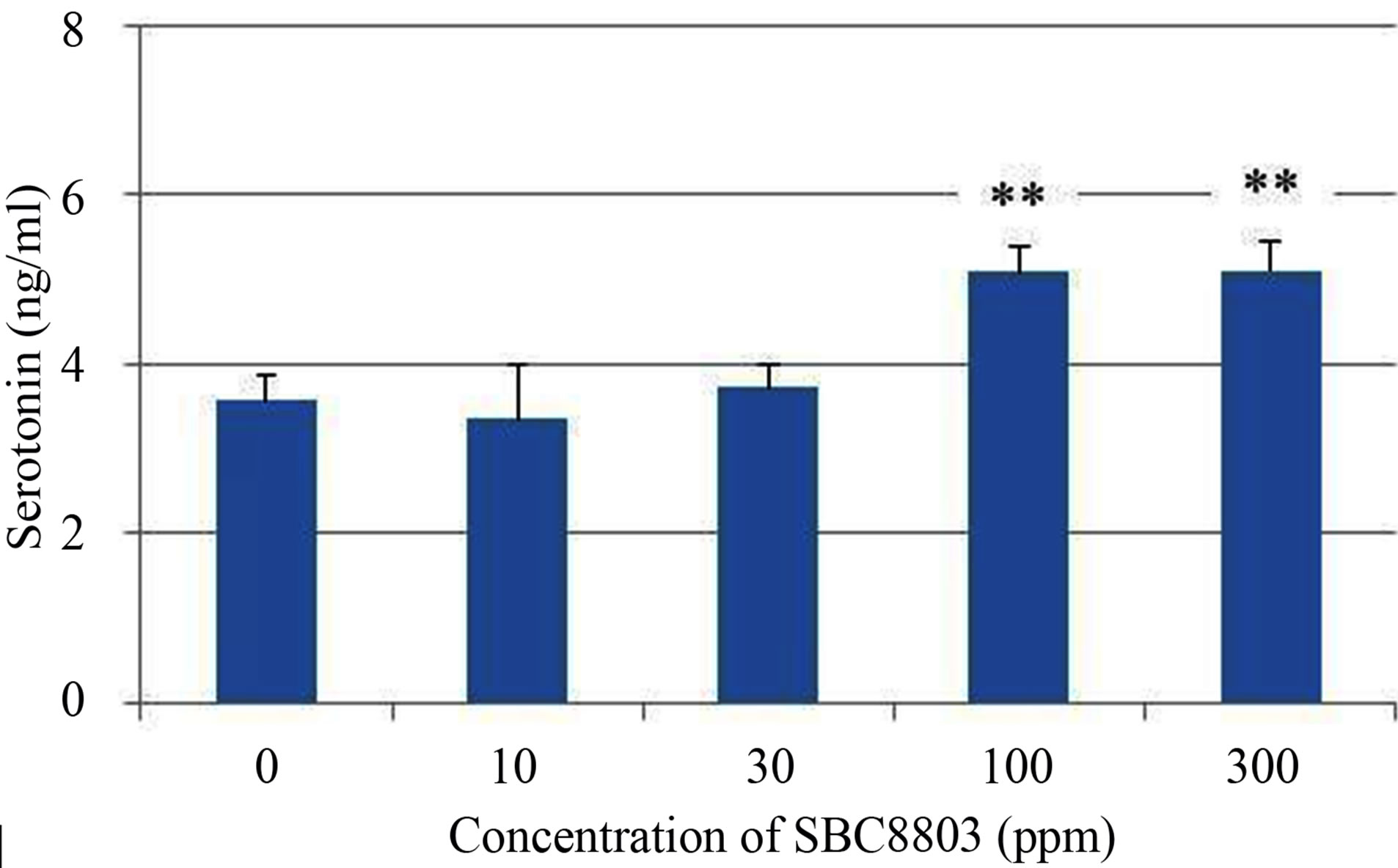 (b)
(b)
Figure 1. Influence of concentration of Lactobacillus brevis SBC8803 on 5-HT release from rat endocrine RIN-14B cells. The results obtained after incubation for 30 min are shown in (a). *P < 0.05 for 0 ppm vs. 30 ppm; **P < 0.01 for 0 ppm vs. 100 ppm and 300 ppm. The results obtained after incubation for 60 min are shown in (b). **P < 0.01 for 0 ppm vs. 100 ppm and 300 ppm. Results are represented as mean ± SD (n = 4).
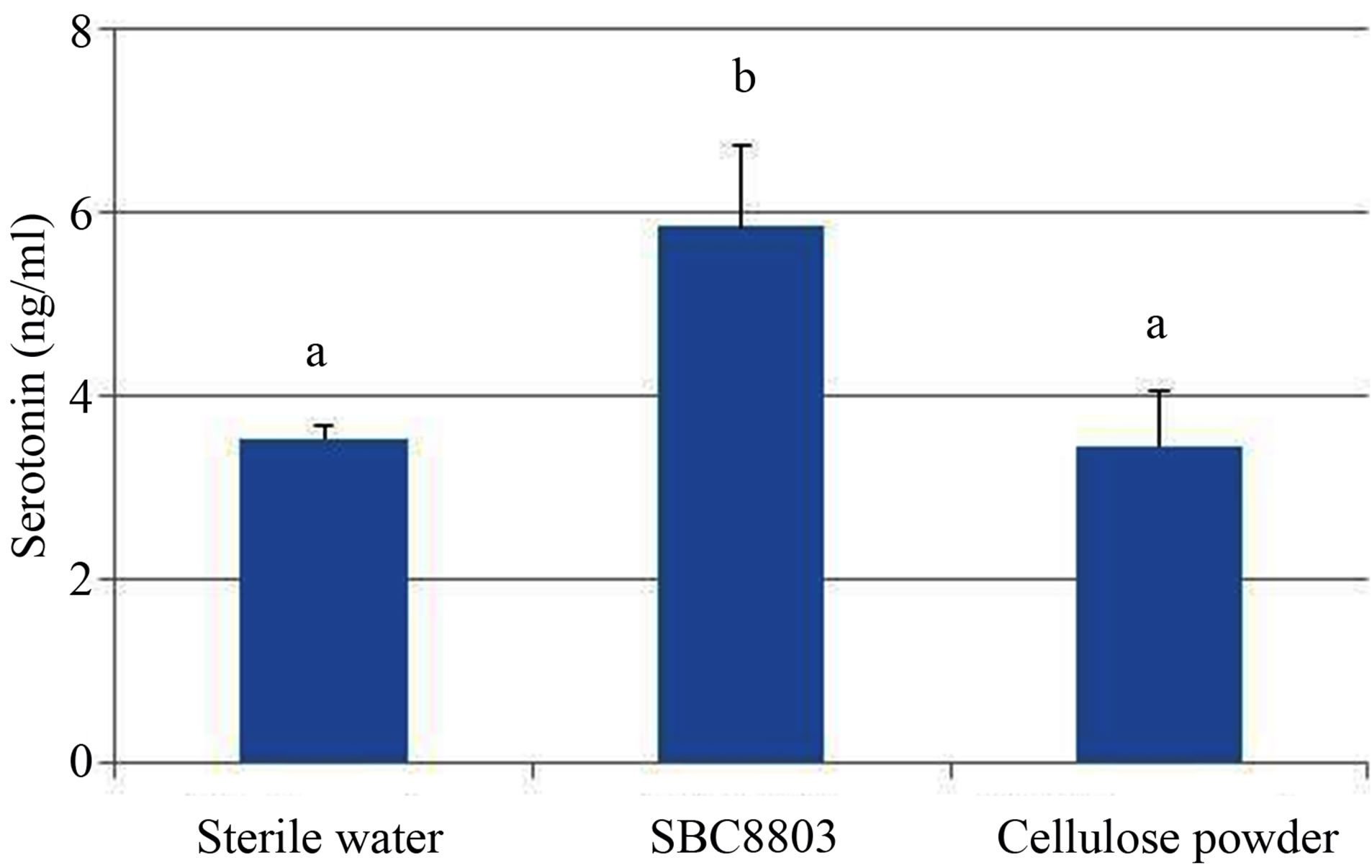
Figure 2. Comparison of the effects of Lactobacillus brevis SBC8803 and cellulose powder on 5-HT release from rat endocrine RIN-14B cells. The ability of heat-killed L. brevis SBC8803 and cellulose powder (both 100 ppm) to stimulate of 5-HT release from RIN-14B cells was evaluated after incubation for 60 min. Results are represented as mean ± SD (n = 6). The different letters indicate statistical differences (P < 0.01).
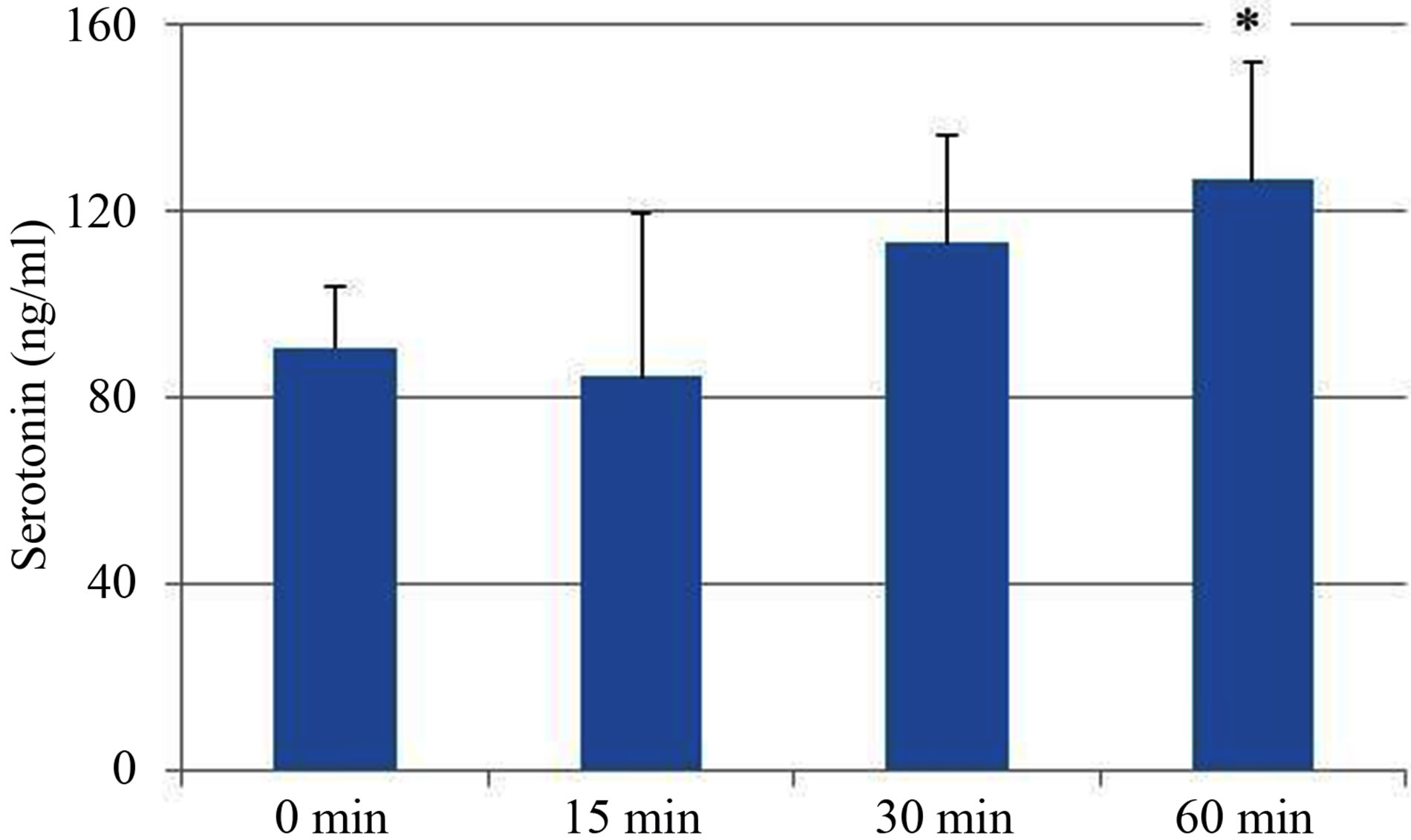
Figure 3. In vivo plasma study of the effects of Lactobacillus brevis SBC8803. Heat-killed L. brevis SBC8803 was administrated using a stomach sonde to C57Bl/6J mice (2 mg/ mouse). At 0, 15, 30, and 60 min after administration, the mice were killed humanely and blood samples were collected. Plasma 5-HT concentrations were evaluated. Results are represented as the mean ± SD (n = 5). *P < 0.05 for 0 min vs. 60 min.
plasma 5-HT concentrations gradually increased after L. brevis SBC8803 administration (0 min vs. 60 min; P < 0.05). Subsequently, we performed an in vivo administration study using cellulose powder (2 mg/mouse) as a physical stimulus. The 5-HT concentration at 90 min after cellulose powder administration was equal to that observed after administration of sterile water (Figure 4), which was similar to that observed in the in vitro study using RIN-14B cells.
On the other hand, administration of heat-killed L. brevis SBC8803 led to higher plasma 5-HT concentrations than did sterile water or cellulose powder (SBC 8803 vs. sterile water; P = 0.08). These in vivo studies results (Figures 3 and 4) showed that oral administration of heat-killed L. brevis SBC8803 in mice increased 5-HT concentrations in the blood.
3.3. 5-HT Release from the Small Intestine of Mice (ex Vivo Studies)
Finally, we carried out ex vivo experiments using the intestinal loop, which is composed of the duodenum and part of the jejunum from male C3H/HeN and C57BL/6J mice. We injected 0.1 mL of sterile water containing heat-killed L. brevis SBC8803 (2 mg/mL) into the intestinal loop. We then evaluated the 5-HT-releasing ability of L. brevis SBC8803 by using the procedure described in the Material and Methods section. In C57BL/6J mice, injection of L. brevis SBC8803 led to 5-HT release from the intestinal cells into the RPMI1640 medium, whereas the amount of 5-HT released did not differ statistically from the amount release after injection of sterile water into intestinal loops, as shown in Figure 5. However, injection of heat-killed L. brevis SBC8803 into the intestinal loop of C3H/HeN mice led to release of significantly
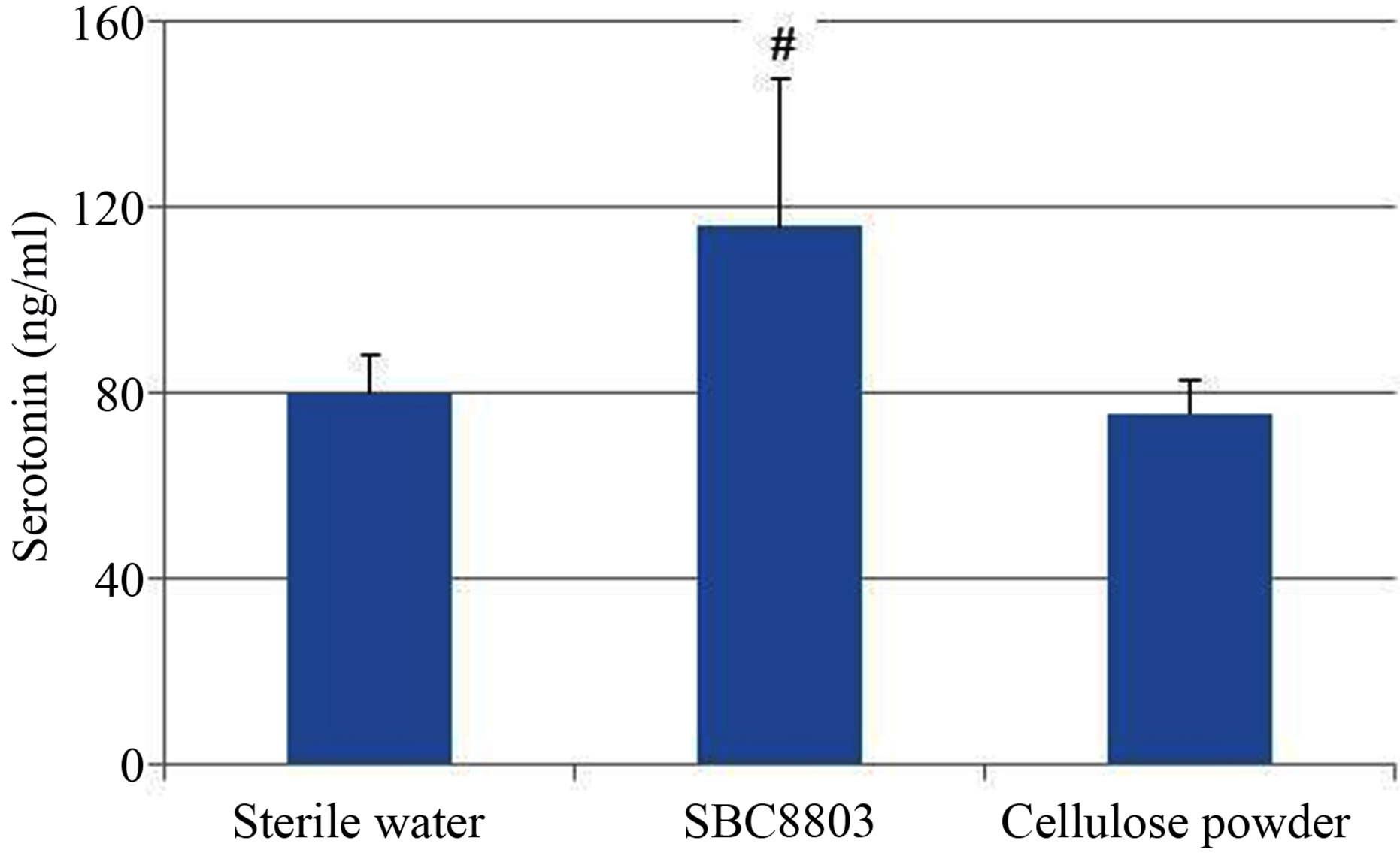
Figure 4. In vivo plasma study for comparing the effects of Lactobacillus brevis SBC8803 and cellulose powder. Samples were administrated using a stomach sonde to C57Bl/6J mice (2 mg/mouse). At 90 min after administration, mice were killed humanely and blood samples were collected. Plasma 5-HT concentrations were determined. Results are represented as mean ± SD (n = 5). #P < 0.1 for SBC8803 vs. sterile water and cellulose powder.
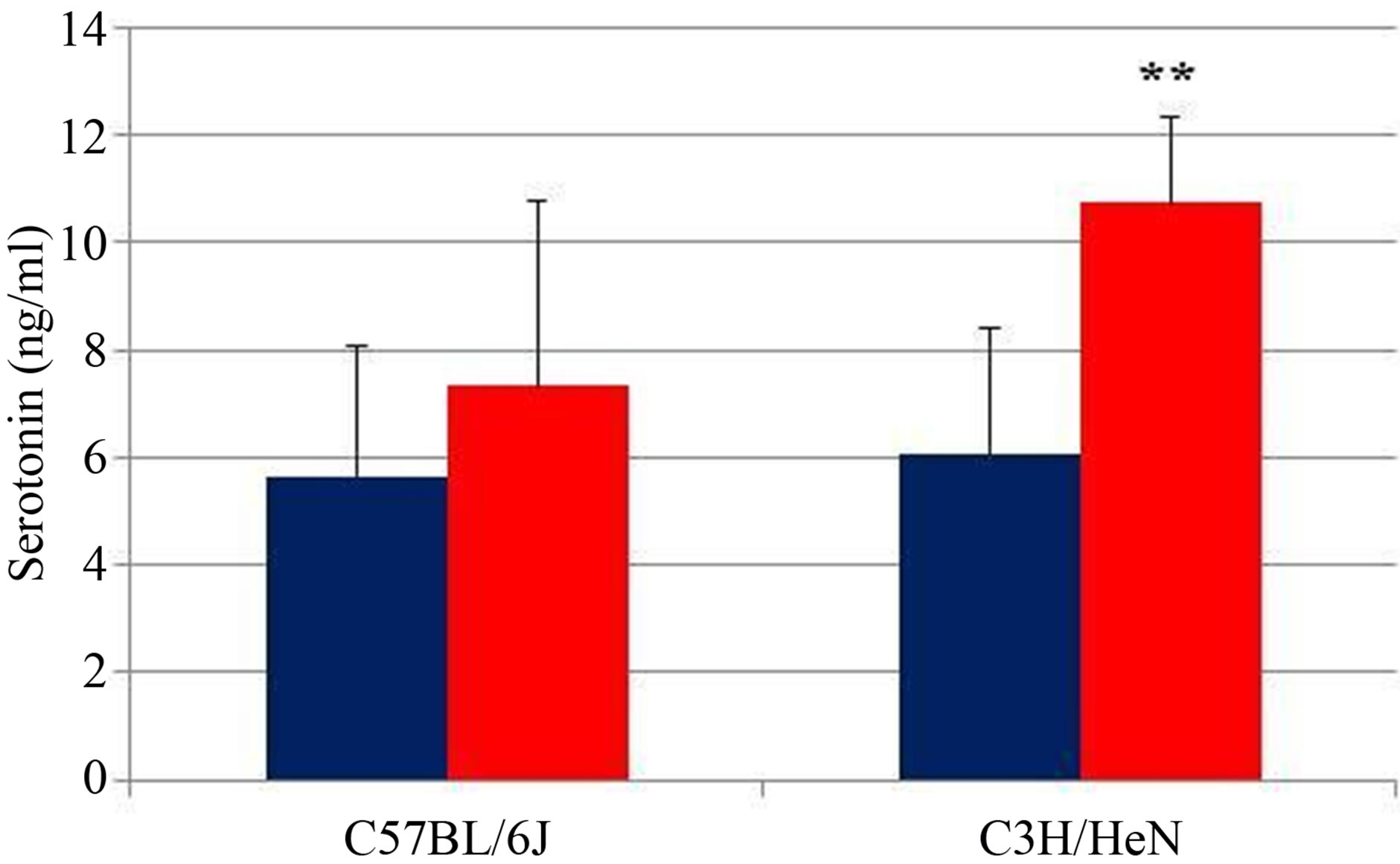
Figure 5. 5-HT release from the small intestine of mice. Effect of heat-killed L. brevis SBC8803 on 5-HT release from the small intestine was evaluated using the intestinal loop, which is composed of the duodenum and part of the jejunum, from male C3H/HeN and C57BL/6 J mice. Results are represented as mean ± SD (n = 5). **P < 0.01 for SBC8803 vs. sterile water.
higher concentrations of 5-HT from the intestinal cells compared to that released after sterile water injection (SBC8803 vs. sterile water; P < 0.01).
4. Discussion
EC cells have been suggested to have important functions in bacterial recognition in afferent neural pathways from the gut to the CNS. For example, short-chain fatty acids, which can be derived from bacteria, stimulate intraluminal 5-HT release from EC cells [11]. Release of 5-HT is known to stimulate 5-HT3 receptors on the vagal sensory fibers, and this sensory information is subsequently transferred to the CNS through the afferent and efferent vagal nerve [12]. In addition, EC cells are known to express functional Toll-like receptors [13], which play important roles in bacterial recognition in the intestine.
The results of our experiments using the intestinal loops, which are composed of duodenum and part of the jejunum, from male C3H/HeN and C57BL/6J mice showed that heat-killed L. brevis SBC8803 significantly stimulates 5-HT release from the loops of C57BL/6J mice and effectively stimulates 5-HT release from loops of C3H/HeN mice (SBC8803 vs. sterile water; P < 0.01). In addition, heat-killed L. brevis SBC8803 stimulates 5-HT release from the rat endocrine RIN-14B cells (SBC8803 vs. sterile water; P < 0.01). From these results, heat-killed L. brevis SBC8803 may be considered to stimulate the bacterial recognition system in intestinal cells such as EC cells. Although short-chain fatty acids were reported to stimulate intraluminal 5-HT release from EC cells [11], acetate, butyrate, and propionate at 10 ppm had no influence on 5-HT release from RIN-14B cells (data not shown).
A schematic diagram depicting the sequence of events in gut mucosal 5-HT signaling has been proposed by Costedio et al. [14]. The sequence is as follows: at rest, 5-HT is stored in secretory vesicles at the basal half of EC cells; following mucosal stimulation, 5-HT is released from EC cells and activates 5-HT receptors on nearby afferent nerve fibers, 5-HT signaling is terminated by removal of 5-HT from the interstitial space, most of the 5-HT is transported into epithelial cells, and the remainder enters the blood stream from where it is taken up by platelets.
Our results for the in vitro cell-based study, in vivo plasma study, and ex vivo intestinal loop study using heat-killed L. brevis SBC8803 seem to agree with this proposed sequence of events in gut mucosal 5-HT signaling.
On the basis of our results, we propose that heat-killed L. brevis SBC8803 induces 5-HT release from intestinal cells, such as EC cells and this released 5-HT stimulates afferent-IVNA through 5-HT3 receptors. This information is subsequently transmitted to the CNS, which then promotes the changes in autonomic nerve activity such as efferent-GVNA.
Studies on the active components of L. brevis SBC8803 and the mode of action involved in SBC8803- induced 5-HT release from intestinal cells are in progress.
5. Acknowledgements
We thank Miss K. Sano for technical support with the serotonin assays and Mr. M. Kawaguchi and Miss K. Shiraiwa of the LA center, Oriental Yeast Co., Ltd. for helpful support with the animal experiments.
REFERENCES
- J. F. Cryan and T. G. Dinan, “Mind-Altering Microorganisms: The Impact of the Gut Microbiota on Brain and Behaviour,” Nature Reviews Neuroscience, Vol. 13, No. 10, 2012, pp. 701-712. doi:10.1038/nrn3346
- P. Forsythe and W. A. Kunze, “Voices from within: Gut Microbes and the CNS,” Cellular Molecular Life Sciences, Vol. 70, No. 1, 2013, pp. 55-69. doi:10.1007/s00018-012-1028-z
- D. M. Saulnier, Y. Ringel, M. B. Heyman, J. A. Foster, P. Berick, R. J. Shulman, J. Versalovic, E. Verdu, T. Dinan, G. Hecht and F. Gurner, “The Intestinal Microbiome, Probiotics and Prebiotics in Neurogastroenterology,” Gut Microbes, Vol. 4, No. 1, 2013, pp. 1-11. doi:10.4161/gmic.22973
- M. Tanida, T. Yamano, K. Maeda, N. Okumura, Y. Fukushima and K. Nagai, “Effects of Intraduodenal Injection of Lactobacillus johnsonii La1 on Renal Sympathetic Nerve Activity and Blood Pressure in Urethane-Anesthetized Rats,” Neuroscience Letters, Vol. 389, No. 2, 2005, pp. 109-114. doi:10.1016/j.neulet.2005.07.036
- M. Tanida, Y. Fukushima, T. Yamano, K. Maeda, Y. Horii, J. Shen and K. Nagai, “Effect of Probiotics Strain Lactobacillus paracasei ST11(NCC2461) on Autonomic Nerve Activities, Blood Pressure and Appetite in Rats,” Current Topics Nutraceutical Research, Vol. 5, No. 4, 2007, pp. 157-164.
- S. Segawa, Y. Nakakita, Y. Takata, Y. Wakita, T. Kaneko, H. Kaneda, J. Watari and H. Yasui, “Effect of Oral Administration of Heat-Killed Lactobacillus brevis SBC 8803 on Total and Ovalbumin-Specific Immunoglobulin E Production through the Improvement of Th1/Th2 Balance,” International Journal of Food Microbiology, Vol. 121, No. 1, 2008, pp. 1-10. doi:10.1016/j.ijfoodmicro.2007.10.004
- S. Segawa, Y. Wakita, H. Hirata and J. Watari, “Oral Administration of Heat-Killed Lactobacillus brevis SBC 8803 Ameliorates Alcoholic Liver Disease in EthanolContaining Diet-Fed C57BL/6N Mice,” International Journal of Food Microbiology, Vol. 128, No. 2, 2008, pp. 371-377. doi:10.1016/j.ijfoodmicro.2008.09.023
- N. Ueno, M. Fujiya, S. Segawa, T. Nata, K. Moriichi, H. Tanabe, Y. Mizukami, N. Kobayashi, K. Ito and Y. Kohgo, “Heat-Killed Body of Lactobacillus brevis SBC8803 Ameliorates Intestinal Injury in a Murine Model of Colitis by Enhancing the Intestinal Barrier Function,” Inflammatory Bowel Disease, Vol. 17, No. 11, 2011, pp. 2235-2250.
- Y. Horii, Y. Nakakita, Y. Fujisaki, S. Yamamoto, N. Itoh, K. Miyazaki, H. Kaneda, K. Oishi, T. Shigyo and K. Nagai, “Effects of Intraduodenal Injection of Lactobacillus brevis SBC8803 on Autonomic Neurotransmission and Appetite in Rodents,” Neuroscience Letters, Vol. 539, 2013, pp. 32-37. doi:10.1016/j.neulet.2013.01.037
- K. Nozawa, E. Kawabata-Shoda, H. Doihara, R. Kojima, H. Okada, S. Mochizuki, Y. Sano, K. Inamura, H. Matsushime, T. Koizumi, T. Yokoyama and H. Ito, “TRPA1 Regulates Gastrointestinal Motility through Serotonin Release from Enterochronaffin Cells,” Proceedings of National Academy Sciences of the USA, Vol. 106, No. 9, 2009, pp. 3408-3413. doi:10.1073/pnas.0805323106
- S. Fukumoto, M. Tatewaki, T. Yamada, M. Fujimiya, C. Mantyh, M. Voss, S. Eubanks, M. Harris, T. N. Pappas and T. Takahashi, “Short-Chain Fatty Acids Stimulate Colonic Transit via Intraluminal 5-HT Release in Rats,” American Journal of Physiology: Regulatory, Integrative and Comparative Physiology, Vol. 284, No. 5, 2003, pp. R1269-R1276.
- H. Uneyama, A. Niijima, A. S. Gabriel and K. Torii, “Luminal Amino Acid Sensing in the Rat Gastric Mucosa,” American Journal of Physiology: Gastrointestinal Liver Physiology, Vol. 291, No. 6, 2006, pp. G1163- G1170. doi:10.1152/ajpgi.00587.2005
- S. H. Bogunovic, J. S. Tilstra, D. T. Chang, N. Harpaz, H. Xiong, L. F. Mayer and S. E. Plevy, “Enteroendocrine Cells Express Functional Toll-Like Receptors,” American Journal of Physiology: Gastrointestinal Liver Physiology, Vol. 292, No. 6, 2007, pp. G1770-G1783. doi:10.1152/ajpgi.00249.2006
- M. M. Costedio, N. Hyman and G. M. Mawe, “Serotonin and Its Role in Colonic Function and in Gastrointestinal Disorders,” Diseases of the Colon and Rectum, Vol. 50, No. 3, 2007, pp. 376-388. doi:10.1007/s10350-006-0763-3
NOTES
*Corresponding author.

