Food and Nutrition Sciences
Vol.4 No.4(2013), Article ID:29734,11 pages DOI:10.4236/fns.2013.44047
Behavioral Pattern of Native Food Isolates of Yersinia enterocolitica and Yersinia intermedia under Simulated Time-Temperature Combinations of the Food Chain
![]()
1Human Resource Development, CSIR-Central Food Technological Research Institute, Mysore, India; 2Food Microbiology, CSIRCentral Food Technological Research Institute, Mysore, India.
Email: *varadaraj@cftri.res.in
Copyright © 2013 Kattathara H. Divya, Mandyam C. Varadaraj. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received January 25th, 2013; revised March 5th, 2013; accepted March 12th, 2013
Keywords: Yersinia enterocolitica; Yersinia intermedia; D-Values; z-Values; Low Temperature Effect; Packaged Drinking Water
ABSTRACT
The public health significance of Yersinia spp. gives a new dimension to the prevailing food chain, wherein the foods do get exposed to heat and cold treatments. In this study, the effect of heat treatment on the native isolates of Yersinia enterocolitica CFR 2301 and Y. intermedia CFR 2303 revealed the D-values ranging from the lowest of 0.08 min at 65˚C in skim milk/beef gravy to the highest of 18.52 min at 50˚C in beef gravy. The heat sensitivity of both these cultures was in the order of Milli-Q water > 0.85% saline > skim milk > beef gravy. The z-values of the test cultures ranged from 7.55˚C for Y. intermedia to 12.08˚C for Y. enterocolitica. The heat sensitivity in Y. enterocolitica appeared to be related with growth incubation temperatures and also fatty acid profile of cell membrane. The effect of low temperature treatments (−20˚C, 0˚C and 4˚C for 20 d) in water, saline and skim milk revealed the ability of Y. enterocolitica to survive more efficiently at −20˚C, while Y. intermedia was more tolerant at 0˚C. In packaged drinking water, Y. enterocolitica could survive and grow at 4˚C and 16˚C, while at 30˚C, inactivation was rapid. The findings did indicate that heat and cold treatments would not always ensure safety from Y. enterocolitica and Y. intermedia in the food chain.
1. Introduction
Microbial safety has become a focal theme in the current scenario of food chain establishment and advanced detection methods have been evolved over the years [1,2]. Among the food borne pathogenic bacteria, Yersinia enterocolitica and related species are of public health concern, in that, being psychrophilic, they also exhibit a varied behavioral pattern to physical treatments, nonthermal processes, biopreservatives, micronutrients and bioactive spice constituents [3-7]. Although considered as a comparatively heat sensitive organism, research studies have indicated that heat sensitivity of Y. enterocolitica in culture systems and food matrices is affected by several factors such as temperature, medium composition, growth phase, conditions of heat treatment and physiology of the organisms [8-11].
The ability of Y. enterocolitica to grow at low temperatures is of considerable concern to food processors.
True to its characteristics of being a psychrophile, strains of this bacterial species has a wide range of growth temperatures from −2˚C to 42˚C [12,13]. Further, the ability of Y. enterocolitica to survive and grow competitively in foods has been amply established in a few of the earlier research studies [12,14-17]. Further, certain relationship has been proposed with the pre-growth temperatures and fatty acid profile of Y. enterocolitca [18- 20]. Along with the strains of Y. enterocolitica, focus has also been on isolates of Y. intermedia, as there is an increased isolation of this species from diseased individuals [21].
In the background of a few research investigations being undertaken to study the individual effects of heat and cold treatments on the behavior of Y. enterocolitica, the objective of this study was to evaluate the potentiality of toxigenic native food isolates of Y. enterocolitica and Y. intermedia to survive the time-temperature combinations of both heat and cold temperatures, which are commonly encountered in a food chain system. Besides, also to evaluate its persistence, if occurs as a chance contaminant in packaged drinking water.
2. Materials and Methods
2.1. Materials
All glasswares, media and other materials used in the present study were either wet sterilized or dry sterilized. Wet sterilization was carried out at 121˚C for 20 min in an autoclave and dry sterilization at 180˚C for 4 h in a Hot Air Oven. All bacteriological media used were those of dehydrated media procured from Hi-Media Lab., Mumbai, India. The media were prepared as per manufacturer’s instructions. The water used in the experimenttal trials was Milli-Q water (A10 Elix 3, Millipore Corporation, Billerica, USA).
2.2. Bacterial Cultures and Inoculum Preparation
These included: 1) native food isolates of Y. enterocolitica CFR 2301 and Y. intermedia CFR 2303 obtained from Indian traditional fast foods and known to harbour toxigenic traits [22]; and 2) a reference culture of Y. enterocolitica MTCC 859 obtained from Microbial Type Culture Collection, Institute of Microbial Technology, Chandigarh, India. The cultures were maintained at 6˚C on brain heart infusion (BHI) agar slants in the Culture Collection Stock of this Department and propagated twice successively in BHI broth for 20 h at 30˚C prior to use in the experimental trials.
A loopful of previously propagated culture broth of the individual test cultures was inoculated in to 10 ml aliquots of BHI broth and incubated for 20 h at 30˚C in an orbital shaker incubator (Alpha Scientific Co., Bangalore, India) at 140 rpm. The culture broth was centrifuged at 8000 rpm for 20 min at 4˚C (Superspin R-V/FM, Plasto Crafts, Mumbai, India). The supernatant was discarded and the resulting cell pellet was washed with saline and resuspended in sterile 10 ml aliquots of 0.85% saline. Prior to use in the experimental trials, the cell titers were enumerated by surface plating of serial dilutions of the cell suspensions on pre-poured nutrient agar plates with incubation for 24 h at 30˚C. Appropriate serial dilutions in sterile saline were used, so as to obtain the final desired individual inoculum levels (4.3, 7.3 and 8.3 log10 CFU/ml) in aliquots of prepared media and substrates.
2.3. Menstra for the Treatments
The menstra used in this study were Milli-Q water, 0.85% saline, skim milk and beef gravy. Skim milk was prepared by reconstituting commercially available skim milk powder at 10% level in water. Beef gravy was prepared in accordance with the procedure described by Juneja and Eblen [23]. All the four menstra were prepared individually, dispensed in requisite quantities in test tubes of 12 × 75 mm and sterilized. Milli-Q water, saline and beef gravy were autoclaved at 121˚C for 15 min, while skim milk was for 10 min.
2.4. Effect of Heat Treatment and Determination of D-Values
Individual pre-sterilized heating menstra (Milli-Q water, 0.85% saline, skim milk and beef gravy) in aliquots of 4 ml were placed initially at pre-selected temperatures in a thermostatically controlled water bath (Julabo SW 22, Labortechnik GMBH, Seelbach, Germany), so that the menstra attains the defined temperature prior to use in the experimental trials. These tempered aliquots of individual heating menstra were inoculated with individual bacterial test cultures of Yersinia spp. at a level of 8.3 log10 CFU/ml. The inoculated tubes were individually subjected to temperatures of 50˚C, 55˚C, 60˚C and 65˚C in a thermostatically controlled water bath for specific durations. After the specific time-temperature periods were completed, tubes were removed and immediately cooled in ice-water bath. The experimental samples were appropriately diluted in 0.85% saline and surface plated on pre-poured plates of nutrient agar and incubated aerobically for 48 - 72 h at 30˚C. Colonies formed in the incubated plates were enumerated and recorded in log10 CFU/ml. The experiments were performed in duplicates and average of these counts was taken for the determination of D-value.
The D-values (time in min) required to reduce the viable cell population by 90% were determined by separately plotting the log10 number of survivors against time at each temperature using Microsoft Excel Software Program 2010 (Microsoft Corporation, Redmond, WA, USA). Only the values in straight portion of the curve were considered for calculation. The line-of-best fit for survivor plots were determined by regression analysis [24]. A regression equation of the type y = a + bx was derived, where b was the slope of the straight line that when inverted and changed from negative to positive, which gives the D-value for a specific temperature (D = −1/slope). The z-values were obtained by plotting the log10 D-values against respective temperatures and calculating the negative inverse of the slope of the curve [9].
2.5. Pre-Growth Temperatures on Heat Resistance and Fatty Acid Profile of Y. enterocolitica CFR 2301
This experimental trial was undertaken with only the native isolate of Y. enterocolitica CFR 2301. The culture was pre-grown in BHI broth at individual temperatures of 5˚C, 10˚C, 15˚C, 30˚C, 37˚C and 43˚C till stationary phase was reached, wherein the time period was temperature dependent. The resultant cells of individual growth temperatures were harvested by centrifugation at 8000 rpm for 20 min at 4˚C in a refrigerated centrifuge as mentioned previously. The resulting cell pellets were washed twice with sterile 0.85% saline and finally suspended in 10 ml of saline in pre-sterilized 25 × 75 mm screw cap tubes and stored at 6˚C till further use. The D-values were determined for the cells of Y. enterocolitica CFR 2301 at these pre-growth temperatures at a level of 8.3 log10 CFU/ml in BHI broth at 55˚C as described previously.
Simultaneously, fatty acid profile of the cells of Y. enterocolitica obtained at the above-mentioned pregrowth temperatures was performed. Aliquots of the resultant cells of individual growth temperatures suspended in sterile saline were lyophilized in a Laboratory Model Lyophilizer (Heto Dry Winner, Jouan Nordic, Allerod, Denmark). The dried bacterial cells (approx. one gram) were methanolyzed by the procedure of Marr and Ingraham [25]. The fatty acids were converted to fatty acid methyl esters (FAME) by the method of Morrison and Smith [26]. The fatty acid analysis was carried out in a GC-14B Gas Liquid Chromatograph (Shimadzu, Kyoto, Japan) fitted with 30 m × 0.3 mm fused silica capillary column (BP21) with a flame ionization detector (FID) connected with a Clarity 420 Integrator. The analysis was carried out using isothermal conditions. The column temperature was set at 220˚C, injector temperature 230˚C and the detector temperature of 240˚C. Nitrogen was used as carrier gas with a flow rate of 1ml/min. Individual fatty acids were identified by comparison with retention time of authentic fatty acid standards and quantified by Clarity Integrator.
2.6. Effect of Cold Treatment
This was undertaken with individual isolates of Y. enterocolitica CFR 2301 and Y. intermedia CFR 2303. The temperatures included for cold treatment were −20˚C, 0˚C and 4˚C, respectively. Individual pre-sterilized media (Milli-Q water, 0.85% saline and skim milk) were inoculated with individual bacterial test cultures of Yersinia spp. at a level of 4.3 log10 CFU/ml and transferred to pre-sterilized tubes in 4 ml aliquots. The inoculated tubes were placed at −20˚C, 0˚C and 4˚C, respectively, for 20 d. After the specific time-temperature periods were completed, tubes were removed, appropriately diluted in 0.85% saline and surface plated on prepoured plates of nutrient agar and incubated aerobically for 48 - 72 h at 30˚C. Colonies formed in the incubated plates were enumerated and recorded in log10 CFU/ml.
2.7. Fate of Y. enterocolitica in Packaged Drinking Water
This experimental trial was performed with Y. enterocolitica CFR 2301. Membrane filtered packaged drinking water of a commercial brand in 100 ml quantities (in duplicates) taken in pre-sterilized screw cap bottles were tempered to 4˚C, 16˚C and 30˚C, respectively, in a BOD Incubator (Sub-Zero, Industrial and Laboratory Tools Corporation, Chennai, India), prior to inoculation with the test culture. The tempered water samples were inoculated with cells of Y. enterocolitica to obtain initial inoculum levels of 7.3 and 4.3 log10 CFU/ml, individually. Inoculated samples were placed at the respective temperatures of 4˚C, 16˚C and 30˚C for a period of 90 d. Samples were drawn at frequent time (d) intervals, serially diluted in saline (if required) and surface plated on pre-poured plates of nutrient agar. Inoculated plates were incubated for 24 - 72 h at 30˚C. Colonies of Y. enterocolitica appearing in the incubated plates were counted and average counts in log10 CFU/ml from the duplicate samples were recorded and survivor plots were prepared with log CFU/ml of survivors against time (d).
2.8. Statistical Analysis
All the experimental trials were carried out independently, in triplicates and the average values with standard errors (SE) are presented at requisite and appropriate places, either in Tables or Figures. All calculations and statistical analyses were performed in Microsoft Excel Programme, 2010 (Microsoft Corporation, Redmond, WA, USA).
3. Results and Discussion
3.1. Heat Treatment on Yersinia spp.
Thermal death time curves were plotted for the 3 cultures at selected temperatures and heating menstra and decimal reduction times are presented in Figure 1. The survival curves plotted for high temperature inactivation were found to be more or less linear with correlation coefficients (R2) ranging from 0.94 to 0.99 (data not shown). The D-values for the 3 cultures across different menstra ranged from the lowest of 0.08 min at 65˚C to the highest of 18.52 min at 50˚C, both in beef gravy (Figure 1(d)). The sensitivity of the test cultures was in the order of Milli-Q water > 0.85% saline > skim milk > beef gravy.
Figure 1. Decimal reduction time (D-values) at 50˚C (●), 55˚C (□), 60˚C (■) and 65˚C (▲) in cultures of Yersinia spp. in Milli-Q water (a), normal saline (b), skim milk (c) and beef gravy (d). Cultures of Yersinia spp. are 1: Y. enterocolitica CFR 2301; 2: Y. intermedia CFR 2303; and 3: Y. enterocolitica MTCC 859.
The thermal resistance was greatly influenced by the heating menstruum. Although, D-values could not be determined for Y. intermedia CFR 2303 at 60˚C with Milli-Q water, saline and skim milk, it appears that at lower heat treatments, the culture had higher D-values with beef gravy and skim milk (Figures 1(c) and (d)). The z-values of the test cultures (Table 1) ranged from 7.55˚C for Y. intermedia CFR 2303 to 12.08˚C for Y. enterocolitica MTCC 859, both in beef gravy. The zvalues for the heating menstra of water, saline and skim milk tested for these cultures were not significantly different (p < 0.05), which may indicate that heating medium could not be considered as the only singular effect in bringing about heat inactivation. Nevertheless, the z-values obtained with beef gravy were quite different, with the likely possibility of this specific medium influencing heat resistance.
Table 1. z-values of Y. enterocolitica CFR 2301 and Y. intermedia CFR 2303 in different heating menstra.
Figure 2. Thermal reduction curves of Y. enterocolitica CFR 2301 obtained at individual pre-incubation temperatures of 5˚C (■), 10˚C (□), 15˚C (●), 37˚C (♦), and 43˚C (◊) and heated at 55˚C in BHI broth.
In an attempt to study the effect of pre-growth temperatures on heat sensitivity, it was observed that the D-values of Y. enterocolitica CFR 2301 pre-grown at five incubation temperatures and heated at 55˚C in BHI broth was found to be highly dependent on the growth temperature (Figure 2). The D-value was found to be the highest for cells grown at 43˚C and lowest for cells grown at 5˚C. The D-values were found to show a gradual increase with values of 1.35, 1.42, 1.78, 2.5 and 4.02 min at growth temperatures of 5˚C, 10˚C, 15˚C, 37˚C and 43˚C, respectively. The profile of fatty acids elaborated by Y. enterocolitica CFR 2301 pre-grown at incubation temperatures of 5˚C, 10˚C, 15˚C, 37˚C and 43˚C are presented in Table 2 and representative chromatograms of selected incubation temperatures are shown in Figure 3. The fatty acids of C16:1 and C18:1 were found in high proportions at growth temperatures of 5˚C, 10˚C and 15˚C, whereas C12:0, C13:0 and C14:0 were found in lesser quantities at most of the growth temperatures. C15:0 was present only at 5˚C and 43˚C at almost same levels. C16:0 and C17:0 were found to gradually increase in their proportion with an increase in temperature and C18:0 was found only at 43˚C. C18:2 was detected only at 5˚C, while C18:3 was
Table 2. Fatty acid profile of Y. enterocolitica CFR 2301 pre-grown at different incubation temperatures.
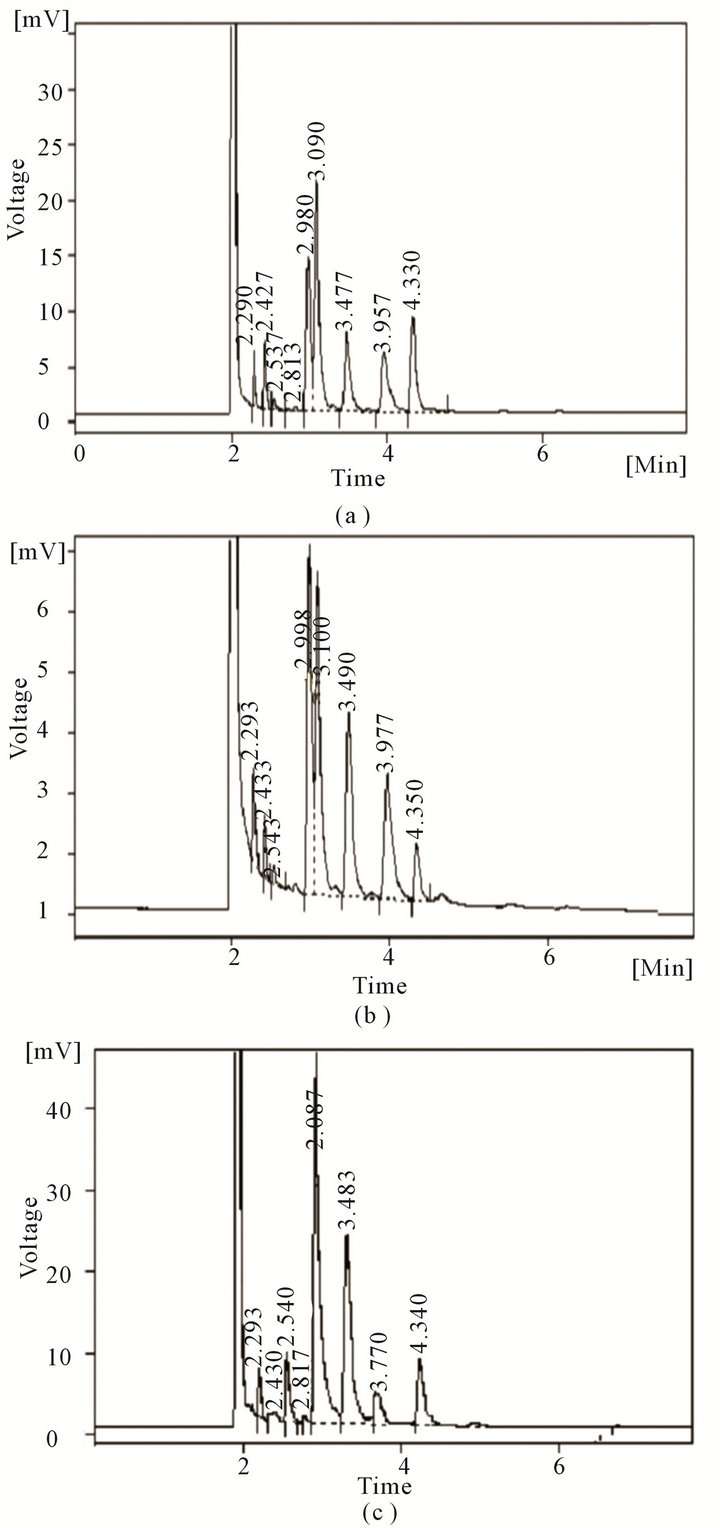
Figure 3. GC profile of fatty acids obtained with Y. enterocolitica CFR 2301 grown at 5˚C (a), 15˚C (b) and 43˚C (c).
present at all temperatures, except 5˚C. Moreover, at 37˚C and 43˚C, C18:3 was the only unsaturated fatty acid detected. The ratio of unsaturated to saturated fatty acids was also related to the growth temperature in that, it gradually decreased with an increase in growth temperature. The ratio of unsaturated to saturated fatty acids at 5˚C, 10˚C, 15˚C, 37˚C and 43˚C were 1.46, 1.29, 0.98, 0.26 and 0.1, respectively.
In general, strains of Y. enterocolitica are known to be sensitive to pasteurization temperatures. In one of the research studies undertaken to present a comparative picture of the available reports on the heat resistance of selected organisms, it was found that as compared to other organisms, the number of Y. enterocolitica strains investigated for heat resistance was low. It was also reported that the findings of different investigators indicated that the heat resistance of this organism varied greatly among different strains. When compared to few other foodborne pathogenic bacteria, Y. enterocolitica was found to have a higher average D-value compared to that of Salmonella species and Campylobacter jejuni and lower heat resistance than Enterococcus faecium, E. faecalis, Listeria monocytogenes, L. innocua and Escherichia coli [3].
Cultures of Y. enterocolitica isolated from pasteurized milk and cream from two dairy processing plants in Australia did suggest that complete destruction of Y. enterocolitica may not occur during pasteurization, if the initial contamination level in milk was very high [27]. Similarly, it was also suggested that some Y. enterocolitica cells may survive the cooking process in the preparation of cut meats under conditions of in-effective temperature, when the internal temperature was only 51˚C instead of 55˚C - 60˚C [28]. However, in another study, unusual heat resistance of three strains of Y. enterocolitica, having D62.8 values in the range of 0.24 - 0.96 min in milk medium was reported [29].
In the present study, the values obtained for skim milk was comparable with those reported in the literature. Francis et al. [30] found that the D62.8 values of 21 strains of enterotoxigenic and non-enterotoxigenic Y. enterocolitica were in the range of 0.7 to 17 sec. Interpolated to 62.8˚C, in the present study, the D62.8 values for Y. enterocolitica CFR 2301 and MTCC 859, respectively, were 9.15 and 9.2 sec. These values were also comparable to the D62.8 values obtained in an earlier study with milk medium, wherein D62.8 values were 10.53 and 10.35 sec in skim milk and whole milk, respectively [16]. At the same time, it was observed that cultures of Y. enterocolitica and Y. intermedia were found to have higher D-values in beef gravy medium, which may be due to the protection afforded by the complex nutrients. The Dvalues recorded in this study for Y. enterocolitica in beef gravy was comparable to that of non-heat shocked cells of Y. enterocolitica in ground pork [31]. The D55 value of 6.5 min obtained in their study was slightly higher than that obtained in the present study (3.11 - 4.0 min), but the D60 value of 1.7 min was quite similar to the values obtained in the present study (1.4 - 1.7 min).
In another research study, the D-values of Y. enterocolitica in minced beef were 17.4, 1.96 and 0.97 min when heated in vacutainer and 21.2, 1.06 and 0.055 when heated in vacuum pack at 50˚C, 55˚C and 60˚C, respecttively. The D55 and D60 values obtained in beef gravy medium in the present study were slightly higher than that obtained in minced beef. The difference in D-values may also be accounted to the variations in pH of the heating media, wherein the pH of minced beef was 5.8 [9]. In the present study, beef gravy medium had a pH of 6.7. A lower pH appears to result in increased thermal sensitivity and, therefore lower D-values when the cells are grown at normal temperature (37˚C) and the reverse occurs for cells grown at lower temperature (4˚C). In the case of Y. enterocolitica and Y. intermedia, saline was found to have slightly higher values as against that of water. Salts such as sodium and potassium chlorides have been shown to have a pronounced effect on the hydration of proteins and thereby influence the stability of enzymes and other proteins. They can also decrease the water activity and hence, increase heat resistance. The protective effect of sodium chloride towards thermal inactivation has been shown in few of the studies with L. monocytogenes and Salmonella [23,32,33].
There appears to be a relationship between the pregrowth temperatures and fatty acid profile of the cultures of Y. enterocolitica. In the present study, the effect of growth temperatures on the heat sensitivity of Y. enterocolitica was evident, wherein increasing D-values were obtained with an increase in incubation temperatures (5˚C, 10˚C, 15˚C, 37˚C, 43˚C). The analysis of fatty acids from cells grown at these temperatures showed an increase in the proportion of unsaturated fatty acids with a decrease in incubation temperature. In comparison to the present study, earlier studies revealed a slightly higher ratio of unsaturated to saturated fatty acids, wherein it was 2.2, 1.1 and 0.4 at temperatures of 5˚C, 22˚C and 37˚C, respectively. Further, unsaturated fatty acids mainly C16:1 and C18:1 were found in higher levels at low temperatures of 5˚C, 10˚C and 15˚C [18-20,34].
Since fatty acids are mainly located in membrane phospholipids and unsaturated fatty acids contribute to the membrane fluidity [35], the findings of present study suggest that the membrane fluidity was different for the cells grown at different incubation temperatures. Besides, alterations in fatty acid profiles were accompanied by an increase in the heat resistance of cells grown at higher temperature conditions. Thermal sensitivity of bacteria depends upon the physico-chemical state of the membrane as well as the heating environment. Earlier studies have shown correlation between bacterial membrane fatty acid composition and heat resistance in E. coli, Vibrio parahaemolyticus and L. monocytogenes [36,37]. These studies suggest that reduced heat resistance of cells grown at low temperatures may be due to an increase in the concentration of unsaturated fatty acids in the cytoplasmic membrane which increases membrane fluidity, reduces viscosity and thus decreases thermotolerance. Although, these fatty acids enable the cells to maintain membrane fluidity at lower temperatures, at the same time makes the cells more heat sensitive when subjected to high temperature treatments [37].
Figure 4. Survival/resistance pattern of Y. enterocolitica CFR 2301 during storage at 4˚C (a), 0˚C (b) and −20˚C (c) in water (●), saline (■) and skim milk (□).
Figure 5. Survival/resistance pattern of Y. intermedia CFR 2303 during storage at 4˚C (a), 0˚C (b) and −20˚C (c) in water (●), saline (■) and skim milk (□).
3.2. Low Temperature on Yersinia spp.
The survival/resistance pattern of Y. enterocolitica and Y. intermedia at 3 lower temperatures in different media are presented in Figures 4 and 5. Among the three media (Milli-Q water, 0.85% saline and skim milk), the survival of Y. enterocolitica and Y. intermedia was greater in skim milk, followed by saline. The organism was more susceptible in Milli-Q water at the low temperatures used in the experimental trial. At 4˚C, Y. enterocolitica was found to increase by 1 log in skim milk in a period of 20 d, while at 0˚C and −20˚C, the organism survived at the initial inoculum level with only a slight decrease. The culture of Y. enterocolitica was found to survive at −20˚C more efficiently than at 0˚C in all the three media tested. The inactivation of Y. enterocolitica was found to be more rapid in water at 0˚C with the organism level decreasing to undetectable levels in 15 d of storage. However, at −20˚C and 4˚C, the organisms could be isolated till the end of the storage period of 20 d. In comparison to Y. enterocolitica, the native isolate of Y. intermedia was found to be more tolerant at 0˚C in water and saline. In skim milk, the effect was similar. However, at −20˚C Y. enterocolitica survived to a greater extent than Y. intermedia in saline and skim milk.
Yersinia enterocolitica being a psychrophile can grow or at least tolerate different low temperature conditions for extended periods [12,13]. A good number of studies on survival of Y. enterocolitica have been carried out in meat samples and a few of them in milk. In the present study, the growth/survival of Y. enterocolitica and Y. intermedia in 3 media at 3 temperatures was compared. The viable numbers of Y. enterocolitica increased in milk samples stored at 4˚C, with the extent of growth being low as compared to the earlier reports on the growth of Y. enterocolitica in milk at temperatures ≤4˚C. Even though Y. enterocolitica was found to be a poor competitor among the more common psychrophilic spoilage bacteria, in the absence of competing microflora, an initial inoculum of 2 log CFU/ml of Y. enterocolitica increased to more than 7 logs in milk in less than 21 d at 3˚C [38]. In a subsequent study, the ability of Y. enterocolitica to grow competitively at 4˚C in pasteurized milk with an initial inoculum of 1 - 3 log CFU/ml of milk and recovering 5 - 7 log CFU/ml after 7 d was demonstrated [14].
As in the case of thermal sensitivity, the extent of survival and growth at low temperatures also may be strain dependent. A few of the earlier studies have shown altered behavior of two strains of Y. enterocolitica out of five strains assessed for their ability to grow in raw and cooked beef and pork samples at various low temperature conditions [39]. Similarly, the ability of Y. enterocolitica to survive in soil and water at low temperature conditions were dependent on serotypes of the strains [40]. In the present study, in Milli-Q water and 0.85% saline, the inactivation was faster at 0˚C than at −20˚C, while in skim milk, no significant difference was observed. The most rapid inactivation was observed in Milli-Q water at 0˚C, in which case the organism declined to undetectable levels within 15 d of storage. In all other conditions, the organism was present in detectable levels till the end of the storage period. Almost comparable results were obtained in earlier studies under conditions of freezethawing and constant freezing at −20˚C. The inactivation of Y. enterocolitica was found to be more in water as against that of milk. Besides, constant freezing at −20˚C was found to have a negligible effect on survival of Y. enterocolitica in milk [16].
3.3. Survival/Growth of Y. enterocolitica in Packaged Drinking Water
The behavioral pattern of Y. enterocolitica CFR 2301 in packaged drinking water introduced at 2 levels of initial inoculum is presented in Figure 6. At an initial inoculum level of 7.3 log10 CFU/ml, it was observed that a slow inactivation of Y. enterocolitica occurred in 90 d storage at 4˚C, wherein slight reduced levels were recorded.

Figure 6. Behavioral pattern of Y. enterocolitica CFR 2301 introduced at initial levles of 7.3 log10 CFU/ml (a) and 4.3 log10 CFU/ml (b) in packaged drinking water and stored at 4˚C (●), 16˚C (■) and 30˚C (□).
However, storage at 16˚C revealed an increase in cell population in the first 7 d, after which the numbers declined to the initial inoculum level. Subsequently, the same number continued till the end of storage period with a slight decrease. At 30˚C, the initial inoculum level remained constant during the first 7 d of storage, followed by the phase of inactivation and finally the cell numbers decreased to almost 1 log CFU/ml by the end of 90 d storage (Figure 6(a)).
In the case of a lower initial inoculum of 4.3 log10 CFU/ml, the behavior at 16˚C storage was almost same as that observed with 7.3 log10 CFU/ml inoculum level, wherein there was an increase in cell numbers in the first 7 - 9 d of storage, followed by a steady decline during the storage period. The observation of interest was at 4˚C storage, wherein there was a gradual increase in viable cell numbers during the storage period resulting in approximately 2 log increase by the end of 90 d storage (Figure 6(b)). However, at 30˚C storage, the inactivation was quite rapid with the numbers reaching undetectable levels in 40 d of storage.
The microbiological quality of packaged drinking water has gained a lot of public health significance in view of its consumption pattern, world-wide. Even though, it is considered to be microbiologically safe, cross-contamination with pathogenic bacteria can also occur. Such a contamination was implicated in an outbreak of cholera associated with the consumption of bottled natural mineral water in Portugal in 1974 [41]. Since bottled water of 5 liters and above capacity is available, it increases the probability of contamination as these bottles may be in use for extended period of consumption. Under such conditions, the water may be stored under refrigeration, chilling or at ambient temperatures and the contamination level also may be different depending upon the type of cross-contaminants. Considering the global increase in the consumption of bottled water, the present study attempted to assess the behavior of Y. enterocolitica occurring as a cross-contaminant during the usage of packaged drinking water and subsequent storage till the same gets completed or is disposed off. This is more significant as the isolate of Y. enterocolitica CFR 2301 had revealed the presence of major virulence related traits [22].
There have been very few reports on the survival of Y. enterocolitica in bottled mineral water, though few studies have focused on its survival in mere distilled, ground and surface waters [42-46]. In one of the documented studies on the survival of Y. enterocolitica in bottled mineral water, the effect of various factors at 21˚C under dark conditions showed 1 log reduction of Y. enterocolitica over a period of 17 d [47]. These results were comparable with the findings of present study at 30˚C. In the present study, even though no reduction was observed for the first 14 d, a reduction of 1 log occurred within 20 d. At the same time, the results of this study were also comparable with those obtained for Y. enterocolitica in sterile spring water at 4˚C [45]. They also observed an increase in 3 logs at 4˚C within 21 d of incubation, whereas in the present study, a 2 log increase was observed in 90 d of storage at this temperature.
Terzieva and McFeters [46] observed that the survival of Y. enterocolitica in stream water was higher at 6˚C as against that of 16˚C, wherein with an initial inoculum of 106 and 108, the cell numbers increased slightly for a period of 2 - 3 d and thereafter the counts lowered to almost 4 logs within 15 d. In contrast to this observation, in the present study, in 90 d storage, there was no change from the initial inoculum level of 4.3 log CFU/ml or less than one log reduction occurred. The variations in findings may be attributed to water quality (bottled mineral water, stream water, spring water), experimental procedures (incubation at dark, use of membrane diffusion chambers and use of closed systems, plating onto selective or non-selective agar, pre-growth conditions) and physiological status of cultures being studied.
In this experimental study, the increase in cell numbers observed at 4˚C with an initial inoculum of 4.3 log CFU/ml may be due to lower number of competing cells and slower multiplication rate which might have allowed the survival of more number of cells for longer periods. On the other hand, when the inoculum level was high, the internal competition might have occurred which might have prevented the growth of the organism, or even if growth occurred, it might not have become evident due to simultaneous death as a result of competition. This can be observed at 16˚C also, wherein the increase in cell number was comparatively greater with a lower initial inoculum level. At 30˚C, certain metabolic activeties will be faster, with the result cell death might have occurred at a faster rate due to low availability of nutriaents.
A varied behavioral pattern of Yersinia spp. under heat and cold treatments was evident, which may be related with the type of isolates prevailing in different habitats. The use of heat and cold treatments does not always ensure safety from Y. enterocolitica and Y. intermedia in a food chain, as the native isolate of Y. enterocolitica used in this study was shown to harbour several of the potential toxigenic traits.
4. Acknowledgements
The authors are thankful to Director, CSIR-CFTRI, Mysore, India for providing the facilities. The first author is grateful to Council of Scientific and Industrial Research, New Delhi for awarding Research Fellowship.
REFERENCES
- M. Mor-Mur and J. Yuste, “Emerging Bacterial Pathogens in Meat and Poultry: An Overview,” Food and Bioprocess Technology, Vol. 3, No. 1, 2010, pp. 24-35. doi:10.1007/s11947-009-0189-8
- M. Severgnini, P. Cremonesi, C. Consolandi, G. DeBellis and B. Castiglioni, “Advances in DNA Microarray Technology for the Detection of Foodborne Pathogens,” Food and Bioprocess Technology, Vol. 4, No. 6, 2011, pp. 936- 953. doi:10.1007/s11947-010-0430-5
- S. Sorqvist, “Heat Resistance in Liquids of Enterococcus spp., Listeria spp., Escherichia coli, Yersinia enterocolitica, Salmonella spp. and Campylobacter spp.,” Acta Veterinarian Scandinavian, Vol. 44, 2003, pp. 1-19. doi:10.1186/1751-0147-44-1
- T. Koutchma, “Advances in Ultraviolet Light Technology for Non-Thermal Processing of Liquid Foods,” Food and Bioprocess Technology, Vol. 2, No. 2, 2009, pp. 138-155. doi:10.1007/s11947-008-0178-3
- L. Acuña, R. D. Morero and A. Bellomio, “Development of Wide Spectrum Hybrid Bacteriocins for Food Biopreservation,” Food and Bioprocess Technology, Vol. 4, No. 6, 2011, pp. 1029-1049. doi:10.1007/s11947-010-0465-7
- J. M. Miranda, F. Jorge, L. Dominguez, A. Cepeda and C. M. Franco, “In Vitro Growth Inhibition of Foodborne Pathogens and Food Spoilage Microorganisms by Vitamin K5,” Food and Bioprocess Technology, Vol. 4, No. 6, 2011, pp. 1060-1065. doi:10.1007/s11947-010-0413-6
- K. H. Divya and M. C. Varadaraj, “Response Surface Plots for the Behavioral Pattern of Yersinia enterocolitica in Chocolate Milk as Affected by Trans-Cinnamaldehyde, a Spice Essential Oil Constituent,” Food and Bioprocess Technology, Vol. 5, No. 2, 2012, pp. 498-507. doi:10.1007/s11947-009-0297-5
- R. Pagan, P. Manas, J. Raso and F. J. S. Trepat, “Heat Resistance of Yersinia enterocolitica Grown at Different Temperatures and Heated in Different Media,” International Journal of Food Microbiology, Vol. 47, No. 1-2, 1999, pp. 59-66. doi:10.1016/S0168-1605(99)00008-2
- D. J. Bolton, C. M. McMahon, A. M. Doherty, J. J. Sheridan, D. A. McDowell, I. S. Blair and D. Harrington, “Thermal Inactivation of Listeria monocytogenes and Yersinia enterocolitica in Minced Beef under Laboratory Conditions and in Sous-Vide Prepared Minced and Solid Beef Cooked in a Commercial Retort,” Journal of Applied Microbiology, Vol. 88, 2000, pp. 626-632. doi:10.1046/j.1365-2672.2000.01001.x
- H. Hayashidani, Y. Hara-Kudo, S. Kinoshita, K. Saeki, A. T. Okatani, Y. Nomura and S. Kumagai, “Differences in Heat Resistance among Pathogenic Yersinia enterocolitica Depended on Growth Temperature and Serotype,” Journal of Food Protection, Vol. 68, No. 5, 2005, pp. 1081-1082.
- G. I. Favier, M. E. Escudero and A. M. S. de Guzman, “Thermal Inactivation of Yersinia enterocolitica in Liquid Egg Products,” Journal of Food Safety, Vol. 28, No. 2, 2008, pp. 157-169. doi:10.1111/j.1745-4565.2008.00103.x
- C. O. Gill and M. P. Reichel, “Growth of the Cold-Tolerant Pathogens Yersinia enterocolitica, Aeromonas hydrophila and Listeria monocytogenes on High pH Beef Packaged under Vacuum or Carbon Dioxide,” Food Microbiology, Vol. 6, No. 4, 1989, pp. 223-230. doi:10.1016/S0740-0020(89)80003-6
- J. A. Hudson, S. J. Mott and N. Penny, “Growth of Listeria monocytogenes, Aeromonas hydrophila and Yersinia enterocolitica on Vacuum and Saturated Carbon Dioxide Controlled Atmosphere Packaged Sliced Roast Beef,” Journal of Food Protection, Vol. 57, No. 3, 1994, pp. 204- 208.
- M. K. Amin and F. A. Draughon, “Growth Characteristics of Yersinia enterocolitica in Pasteurised Skim Milk,” Journal of Food Protection, Vol. 50, No. 10, 1987, pp. 849- 852.
- J. P. Erickson and P. Jenkins, “Behavior of Psychrotropic Pathogens Listeria monocytogenes, Yersinia enterocolitica, and Aeromonas hydrophila in Commercially Pasteurized Eggs Held at 2, 6.7 and 12.8˚C,” Journal of Food Protection, Vol. 55, No. 1, 1992, pp. 8-12.
- S. Toora, E. Badu-Amoako, R. F. Ablett and J. Smith, “Effect of High-Temperature Short-Time Pasteurization, Freezing and Thawing and Constant Freezing, on the Survival of Yersinia enterocolitica in Milk,” Journal of Food Protection, Vol. 55, No. 10, 1992, pp. 803-805.
- C. L. Little and S. Knochel, “Growth and Survival of Yersinia enterocolitica, Salmonella and Bacillus cereus in Brie Stored at 4, 8 and 20˚C,” International Journal of Food Microbiology, Vol. 24, No. 1-2, 1994, pp. 137-145. doi:10.1016/0168-1605(94)90113-9
- H. Tsuchiya, M. Sato, N. Kanematsu, M. Kato, Y. Hosnino, N. Takagi and I. Namikawa, “Temperature-Dependent Changes in Phospholipid and Fatty Acid Composition and Membrane Lipid Fluidity of Yersinia enterocolitica,” Letters in Applied Microbiology, Vol. 5, No. 1, 1987, pp. 15-18. doi:10.1111/j.1472-765X.1987.tb01634.x
- E. Nagamachi, S. Shibuya, Y. Hirai, O. Matsushita, K. Tomochika and Y. Kanemasa, “Adaptational Changes of Fatty Acid Composition and the Physical State of Membrane Lipids Following the Change of Growth Temperature in Yersinia enterocolitica,” Microbiology and Immunology, Vol. 35, No. 12, 1991, pp. 1085-1093.
- P. W. Bodnaruk and D. A. Golden, “Influence of pH and Incubation Temperature on Fatty Acid Composition and Virulence Factors of Yersinia enterocolitica,” Food Microbiology, Vol. 13, No. 1, 1996, pp. 17-22. doi:10.1006/fmic.1996.0002
- A. Sulakvelidze, “Yersiniae Other than Yersinia enterocolitica, Yersinia pseudotuberculosis and Yersinia pestis: The Ignored Species,” Microbes and Infection, Vol. 2, No. 5, 2000, pp. 497-513. doi:10.1016/S1286-4579(00)00311-7
- K. H. Divya and M. C. Varadaraj, “Prevalence of Very Low Numbers of Potential Pathogenic Isolates of Yersinia enterocolitica and Yersinia intermedia in Traditional Fast Foods of India,” Indian Journal of Microbiology, Vol. 51, No. 4, 2011, pp. 461-468. doi:10.1007/s12088-011-0181-7
- V. K. Juneja and B. S. Eblen, “Predictive Thermal Inactivation Model for Listeria monocytogenes with Temperature, pH, NaCl, and Sodium Pyrophosphate as Controlling Factors,” Journal of Food Protection, Vol. 62, No. 9, 1999, pp. 986-993.
- B. Ostle and L. C. Malone, “Statistics in Research: Basic Concepts and Techniques for Research Workers,” 4th Edition, Iowa State Press, Iowa, 1988.
- A. G. Marr and J. L. Ingraham, “Effect of Temperature on the Composition of Fatty Acids in Escherichia coli,” Journal of Bacteriology, Vol. 84, No. 6, 1962, pp. 1260-1267.
- W. R. Morrison and L. M. Smith, “Preparation of Fatty Acid Methyl Esters and Dimethylacetals from Lipids with Boron Fluoride-Methanol,” Journal of Lipid Research, Vol. 5, 1964, pp. 600-608.
- D. Hughes, “Isolation of Yersinia enterocolitica from Milk and a Dairy Farm in Australia,” Journal of Applied Bacteriology, Vol. 46, No. 1, 1979, pp. 125-130. doi:10.1111/j.1365-2672.1979.tb02589.x
- M. O. Hanna, J. C. Stewart, Z. L. Carpenter and C. Vanderzant, “Effect of Heating, Freezing, and pH on Yersinia enterocolitica-Like Organisms from Meat,” Journal of Food Protection, Vol. 40, No. 10, 1977, pp. 689-692.
- J. Lovett, J. G. Bradshaw and J. T. Peeler, “Thermal Inactivation of Yersinia enterocolitica in Milk,” Applied and Environmental Microbiology, Vol. 44, No. 2, 1982, pp. 517-519.
- D. W. Francis, P. L. Spaulding and J. Lovett, “Enterotoxin Production and Thermal Resistance of Yersinia enterocolitica in Milk,” Applied and Environmental Microbiology, Vol. 40, No. 1, 1980, pp. 174-176.
- K. Shenoy and E. A. Murano, “Effect of Heat Shock on the Thermotolerance and Protein Composition of Yersinia enterocolitica in Brain Heart Infusion Broth and Ground Pork,” Journal of Food Protection, Vol. 59, No. 4, 1996, pp. 360-364.
- F. M. Bartlett and A. E. Hawke, “Heat Resistance of Listeria monocytogenes Scott A and HAL 957E1 in Various Liquid Egg Products,” Journal of Food Protection, Vol. 58, No. 11, 1995, pp. 1211-1214.
- V. K. Juneja, H. M. Marks and T. Mohr, “Predictive Thermal Inactivation Model for Effects of Temperature, Sodium Lactate, NaCl, and Sodium Pyrophosphate on Salmonella Serotypes in Ground Beef,” Applied and Environmental Microbiology, Vol. 69, No. 9, 2003, pp. 5138- 5156. doi:10.1128/AEM.69.9.5138-5156.2003
- C. A. Abbas and G. L. Card, “The Relationships between Growth Temperature, Fatty Acid Composition and the Physical State and Fluidity of Membrane Lipids in Yersinia enterocolitica,” Biochemistry Biophysics Acta, Vol. 602, No. 3, 1980, pp. 469-476. doi:10.1016/0005-2736(80)90326-0
- J. E. Cronan and E. Gelmann, “Physical Properties of Membrane Lipids: Biological Relevance and Regulation,” Bacteriological Reviews, Vol. 39, No. 3, 1975, pp. 232-256.
- N. Katsui, T. Tsuchido, M. Takano and I. Shibasaki, “Effect of Pre-Incubation Temperature on the Heat Resistance of Escherichia coli Having Different Fatty Acid Compositions,” Journal of General Microbiology, Vol. 122, No. 2, 1981, pp. 357-361.
- V. K. Juneja, T. A. Foglia and B. S. Marmer, “Heat Resistance and Fatty Acid Composition of Listeria monocytogenes: Effect of pH, Acidulant, and Growth Temperature,” Journal of Food Protection, Vol. 61, No. 6, 1998, pp. 683-687.
- N. J. Stern, M. D. Pierson and A. W. Kotula, “Growth and Competitive Nature of Yersinia enterocolitica in Whole Milk,” Journal of Food Science, Vol. 45, 1980, pp. 972-974. doi:10.1111/j.1365-2621.1980.tb07490.x
- M. O. Hanna, J. C. Stewart, D. L. Zink, Z. L. Carpenter and C. Vanderzant, “Development of Yersinia enterocolitica on Raw and Cooked Beef and Pork at Different Temperatures,” Journal of Food Science, Vol. 42, No. 5, 1977, pp. 1180-1184. doi:10.1111/j.1365-2621.1977.tb14455.x
- K. Tashiro, Y. Kubokura, Y. Kato, K. Kaneko and M. Ogawa, “Survival of Yersinia enterocolitica in Soil and Water,” Journal of Veterinary Medical Science, Vol. 53, 1991, pp. 23-27. doi:10.1292/jvms.53.23
- P. A. Blake, M. L. Rosenberg, J. Florencia, J. B. Costa and E. J. Gangarosa, “Cholera in Portugal, 1974. II. Transmission by Bottled Mineral Water,” American Journal of Epidemiology, Vol. 105, No. 4, 1977, pp. 344-348.
- A. K. Highsmith, J. C. Feeley, P. Skaliy, J. G. Wells and B. T. Wood, “Isolation of Yersinia enterocolitica from Well Water and Growth in Distilled Water,” Applied and Environmental Microbiology, Vol. 34, No. 6, 1977, pp. 745-750.
- L. M. Evison, “Comparative Studies on the Survival of Indicator Organisms and Pathogens in Fresh and Sea Water,” Water Science and Technology, Vol. 20, No. 11- 12, 1988, pp. 309-315.
- Z. Filip, D. Kaddumulindwa and G. Milde, “Survival of Some Pathogenic and Facultative Pathogenic Bacteria in Ground Water,” Water Science and Technology, Vol. 20, No. 3, 1988, pp. 227-231.
- M. Karapinar and S. A. Gonul, “Survival of Yersinia enterocolitica and Escherichia coli in Spring Water,” International Journal of Food Microbiology, Vol. 13, No. 4, 1991, pp. 315-320.
- S. I. Terzieva and G. A. McFeters, “Survival and Injury of Escherichia coli, Campylobacter jejuni and Yersinia enterocolitica in Stream Water,” Canadian Journal of Microbiology, Vol. 37, 1991, pp. 785-790. doi:10.1139/m91-135
- R. Ramalho, A. Afonso, C. Joaquim, P. Teixeira and P. A. Gibbs, “Survival Characteristics of Pathogens Inoculated into Bottled Mineral Water,” Food Control, Vol. 12, No. 5, 2001, pp. 311-316. doi:10.1016/S0956-7135(01)00010-X
NOTES
*Corresponding author.

