Food and Nutrition Sciences
Vol. 3 No. 9 (2012) , Article ID: 22294 , 5 pages DOI:10.4236/fns.2012.39157
Selenium Content in Blood of Donors from the North of Rio de Janeiro State, Brazil
![]()
1Laboratorio de Tecnologia de Alimentos, Universidade Estadual do Norte Fluminense Darcy Ribeiro (UENF), Campos dos Goytacazes, Brazil; 2Hemocentro Regional de Campos, Hospital Ferreira Machado, Campos dos Goytacazes, Brazil.
Email: karlasilvaferreira@gmail.br
Received July 6th, 2012; revised August 6th, 2012; accepted August 13th, 2012
Keywords: Selenium; Blood; Blood Donors; Nutritional Condition
ABSTRACT
Selenium is an element which participates in antioxidant enzymes. A medium and long term lack of such element is associated, mainly, with heart disease, joint and bone structure problems and thyroid activity. Selenium contents in blood reflect its ingestion and food content variation. It depends on soil characteristics, such as pH and selenium presence. There are few studies concerning selenium levels in food and blood in Brazil. Therefore, the objective of this research was to determine selenium content in a blood donor group in Rio de Janeiro state, conducted from December 2008 to March 2009. From the donated blood to Regional Blood Center of Campos dos Goytacazes, 4.0 mL were submitted to selenium analysis through atomic absorption spectrometry of hydride generation and organic matter oxidation wet basis with nitric and perchloric acid. Selenium content varied from 75 ± 16 ng·g–1 for females and 70 ± 21 ng·g–1 for males. There was no significant difference among selenium contents in blood from donors of different gender, age and residence. Seventy four percent of donors had selenium content in blood below 70 ng·mL–1, the lowest level for maximum selenoproteins production, according to the Nutritional Prevention Cancer.
1. Introduction
In human beings selenium deficiency is associated with heart diseases, joint and bone structure problems. Two endemic diseases associated with selenium deficiency were described in different regions in China where soils lack this element: Keshan disease, cardiomyopathy which affects women and children; and Kashin Bech, osteoarthritis which affects teenagers. Apart from those, selenium deficiency has been related to losses in anti-inflammatory, immunologic system and antioxidant activities [1], besides coronary diseases in adults, the main cause of death in the world. One study conducted in New Zealand with patients with Myocardial Infarction presented low Selenium levels: 82.8 and 87.9 ng·mL–1 in men, and 82.1 and 88.5 ng·mL–1 in women, respectively. This study proved that low Selenium content in blood is a risk factor for Myocardial Infarction, as smoking habits [2].
Selenium also plays an important role in controlling thyroid metabolism. Iodothyronine deiodinases promotes conversion of Thyroxine (T4) to active Thyronine (T3), a selenoprotein. Therefore, people with lower Selenium levels are more vulnerable to diseases such as hypothyroidism [3].
One benefit of selenium is related to selenoproteins antioxidant activities, marked by glutathione peroxidase. This enzyme tranfers a hydrogen atom from a protonade sulphydryl group (-SH) to the peroxide to be deactivated, removing oxygen reactive substances (toxic peroxides) which, under aerobic conditions, is formed during growth and in metabolism [4] Oxidative stress and increase in oxygen reactive substances are strongly associated with central nervous system pathologies, such as cerebral vascular accident, Alzheimer, Parkinson and epilepsy. Due to the high lipid content and less antioxidant enzymes in the brain, it is the most vulnerable tissue to deleterious effects from oxidative stress [5].
Selenium levels in blood correlates to its ingestion. Its dosage can be made in the entire or parts of the blood. Serum levels reflect a recent daily ingestion while in erythrocytes reflect accumulate levels over 120 days before [6]. The ingestion of a balanced diet meets daily needs. Food Agricultural Organization and World Health Organization established daily selenium ingestion equivalent to 26 μg/day for women and 34 μg/day for men, both adults. According to studies conducted in a few countries [7] selenium content found in blood ranged from 39.5 ng·mL–1 to 197.5 ng·mL–1. Based on the searches by Hill et al. [8] and Duffield et al. [9], the Nutritional Prevention of Cancer Study Group recommends that the selenium content in blood is between 70 and 90 ng·mL–1 for optimal activity of selenoproteins [10].
Selenium content in food varies according to soil characteristics. There are soils with less and more selenium. In base soils, selenium is more available to plants. In Brazil, previous studies concerning selenium content in food indicated low content [11]. Therefore, it is important to detect eventual selenium deficiencies in the population.
Blood donation was done by individuals who had passed through a strict investigation concerning transmitted diseases, anemia, blood pressure etc., so as to form a population with no contagious or failing diseases. Based on the fact that these donors spontaneously donate blood, selenium blood analysis can be a complementary test and its result may reflect deficiency of the population which the donor is part of.
The Reginal Blood Center in Campos dos Goytacazes is located in the north of Rio de Janeiro State, Southeast of Brazil, and receives blood donations from residents of 14 cities in the north and northwest of Rio de Janeiro State (Campos dos Goytacazes, São Fidélis, São Francisco do Itabapoana, Cambuci, Bom Jesus do Itabapoan, Itaperuna, Italva, São João da Barra, Aperibé, Itaocara, Porciúncula, Cardoso Moreira, Miracema and Natividade), and it is the only hemocentro in the region. The objective of this research was to quantify selenium content in a group of blood donors in the Regional Blood Center in Campos dos Goytacazes, Rio de Janeiro, southeast region of Brazil.
2. Materials and Methods
Blood sampling was conducted in the Regional Blood Center in Campos dos Goytacazes, RJ, in November and December 2008, from 200 donors. The Blood Center conducted 20 blood samplings a day. Donated blood was transferred to two 4 mL vials with anticoagulant and transported to the analysis laboratory in thermal boxes with ice.
Each donor was aware of the objective of our research and signed an awareness and agreement contract allowing blood sample to be analyzed based on selenium content.
The materials used for selenium determination (becker, pipette, test tube, among others) passed through the cleaning stage with immersion in 5% v/v Nitric solution for 24 hours, finalizing with deionized water rising.
Blood aliquots, in duplicate, were transferred to a 125 mL Erlenmeyer and weighed. The weigh was done based on blood viscosity, because volumetric transference would result in high error. Results were expressed in blood volume converting mass to volume using blood density of 1.051 g·mL–1. Analytical standard reagents were added as follows: 15 mL of concentrated Nitric acid, 2 mL of 30% Hydrogen Peroxide and 1 mL of concentrated Perchloric acid for organic matter oxidation and temperature increase until 150˚C. Perchloric acid was added in the of digestion process and digestion was considered to be done once white vapor was emitted and volume reached 1.0 mL. After this stage, samples were taken to lighter heating, 70˚C for 30 minutes, adding 1.0 mL of 37% HCl for Se+6 reduction to Se+4, the latter being the state in which atomic absorption spectrometry with hydride generation is conducted. Digested samples were diluted in deionized water and brought to final volume of 15 mL [11].
For Selenium detection an atomic absorption spectrophotometer—AAS (GBC Avanta, model 3000) with air flame—acetylene connect to hydride generator—was used. AAS conditions were as follow: hollow cathode light bulb of 10 mA, wave length of 196 nm, gap of 2.0 nm and adjusted flame of acetylene flow of 2 L·min–1 and air flow of 6 L·min–1. Standard curve was prepared from a 1000 mg·L–1 store solution from 1 until 50 µg·L–1 of Selenium.
The method efficiency was verified by intern standardization, with sample digestion without addition and addition of increasing and known quantities of Selenium, obtaining 90% and 103% restoration.
Statistical analysis encompassed GLM (Generalized Linear Mode) and average comparison by LSMEANS procedures, at 5% probability, using SAS—Statistical Analysis System. Selenium content in donor’s blood of the complete group was correlated to variables such as age, gender and residence.
3. Results
Selenium content in donor’s blood in Regional Blood Center in Campos dos Goytacazes, Rio de Janeiro, Brazil ranged from 31 ng·mL–1 to 126 ng·mL–1, average ± standard deviation 75 ± 16 ng·mL–1, for females, and from 11 ng·mL–1 to 158 ng·mL–1, average ± standard deviation 70 ± 21 ng·mL–1, for males, as shown in Table 1. There was no significant difference among Selenium blood content among sex, age and residence, in which 80% of donors were male.
4. Discussion
Although there seems to be differences between the levels of selenium in the blood of donors from groups of different sexes and ages, between 18 and 25 years old and between 56 - 65 years old, these differences were not significant (Table 1). Probably it happened due to the presence, in each of these groups, of an individual with very high and very low selenium levels in the blood, contributing to the increase the average in a group and decreasing on the other.
This result agrees with a research conducted in Belgium [6] which revealed selenium blood concentration equal to 79.7 ng·mL–1, ranging from 55 to 117.4 ng·mL–1, with no significant difference between gender. However, in a research conducted in Canada [12] there was difference
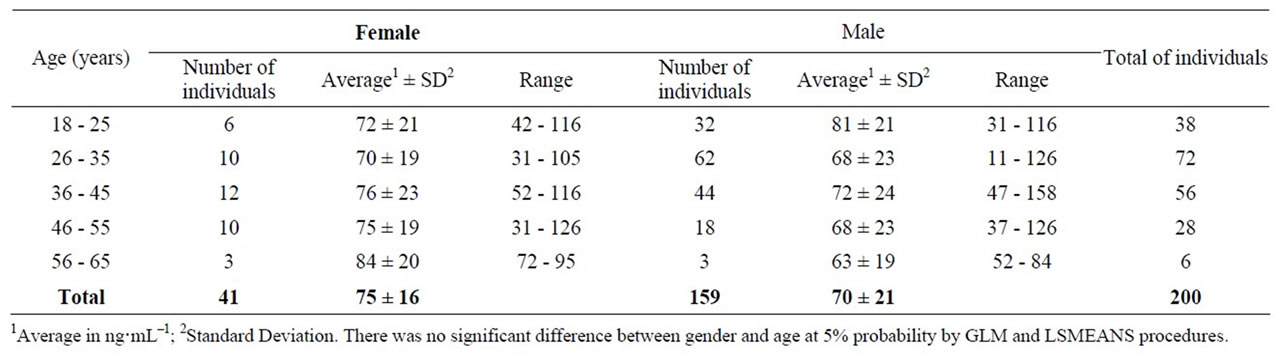
Table 1. Selenium content detected in donors’ blood (ng·mL–1) at the blood center in Campos dos Goytacazes, RJ, Brazil.
between Selenium levels in men and women blood, 137 ng·mL–1 and 127.2 ng·mL–1, respectively.
According to the Nutritional Prevention of Cancer Study Group, plasma concentration of 70 - 90 ng·mL–1 guarantees appropriate selenoproteins performance, such as glutathione peroxidase and selenoprotein P [10]. However, there are authors who suggest higher values, in accordance to studies conducted with blood plasma to investigate selenoprotein performance in antioxidant activity [13]. They argue the optimum selenium level is 100 ng·mL–1. The average Selenium content of donors from North/Northwest Rio de Janeiro was 70 ng·mL–1, which is below both references. In addition, only 26% donors presented Selenium content above 70 ng·mL–1 and only 10% presented average content above 100 ng·mL–1. Consequently, 74% of the population may have a failing antioxidant and vulnerable system to diseases development due to selenium sub-clinical deficiency, such as coronary diseases, Alzheimer and some tumor types. This is due to a lack of Selenium in diets, either by food ingestion with low Selenium content or by insufficient ingestion of food with high Selenium content.
On the other hand, in a research to detect Selenium in plasma and its influence in prostate cancer in Europe, average selenium content was 70 µg·mL–1 [14], in accordance to those found in donor’s blood in the Regional Blood Center in Campos dos Goytacazes, Brazil. A study for evaluating selenium in human serum in Switzerland, classified contents into three categories: low level, between 50 and 60 ng·mL–1; intermediary level, between 60 and 100 ng·mL–1; and high level, above 100 - 120 ng·mL–1 [15]. As a result, selenium status of Swiss was normal to high. Using the same classification, blood donors’ status from the Blood Center in Campos dos Goytacazes was intermediary and low.
Another study conducted in São Paulo, Brazil, with 46 individuals from 23 to 69 years old, detected Selenium average content of 73 ng·mL–1 [16], similar to the content found in this research, a fact justified by the similar diet habits. In addition, researchers evaluated selenium content in the blood of 236 residents of communities close to Tapajós, in the Amazon Region, Brazil, ranging from 142 to 2029 ng·mL–1, with an average of 284 ng·mL–1 [17]. This high concentration is due to specific diet habits from the population under study, based on high fish and Brazilian nuts ingestion, food containing high Selenium content.
The main dietary sources of selenium in Brazil are animal products, mainly fish. In vegetable origin foods, only those derived from wheat and those grown in some regions of the Amazon, such as Brazil nuts, have high levels of selenium. For example, the selenium content in fruits and vegetables is between 0 and 1 μg/100g. Thu [11], it is presumed that individuals who participated in this research consume small amounts of animal foods.
The difference of Selenium content among residents from different regions is due to Selenium content variation and distinct diet habits. Location, weather characteristics, such as annual rainfall, soil pH, type of cultivated plants, loss by food cooking are factors which lead to different selenium contents in food [18].
In a study conducted in China, the average Selenium content detected in plasma was 380% higher (98 ng·g–1 versus 20.4 ng·g–1) in women who lived in areas with soils with appropriate Selenium levels. The average Selenium content in breast milk from these women was 82% higher (14 ng·g–1 versus 7.7 ng·g–1 ) than breast milk from those who lived in areas with a lack of Selenium [19].
Considering that Selenium levels in blood is directly associated with diet, the results of this study indicate that donors are consuming fewer sources of selenium or insufficient amounts of food which contains it. As these donors are a sample of the population from the North and Northwest of Rio de Janeiro, it is possible to conclude that a high percentage of the population also has insufficient Selenium content in blood for the maximum selenoproteins performance, which makes them more vulnerable to sub-clinical deficiencies of this microelement, such as heart problems and problems originated from biomolecules oxidation by peroxides and other oxygen reactive substances.
Some strategies have been used to increase Selenium consumption by population from countries with a lack of Selenium in their soils, such as: individual supplementation, food addition, supplementation of cattle diet, fertilization and cultivation of plants capable of storing Selenium. Supplementation, using organic forms (selenium associated to yeast, selenoproteins and Selenium in food) has higher absorption by the organism once compared to inorganic forms (Selenite and Selenate), increasing selenium levels in blood [20].
Nutrition educational activities for guidance on the role, food sources and consumption levels of Selenium contribute for increasing its consumption. The work was continued with the identification of food sources of selenium in the Region and nutritional counseling for this population group.
Since blood donors are healthy subjects and, between them, 74% had blood levels of selenium below those recommended for the perfect functioning of the organism, can conclude that this impairment reaches a significant portion of population of this region, being a public health problem that must be solved.
5. Acknowledgements
The blood donor; the Regional Blood Center of Campos dos Goytacazes, Brazil; the Prof. Dr. José Carlos Gomes from Viçosa Federal University, Brazil; and Carlos Chagas Filho Support Research Foundation of the Rio de Janeiro State—FAPERJ, Brazil.
REFERENCES
- C. S. Broome, F. McArdle, F. Andrews, et al., “An Increase in Selenium Intake Improves Immune Function and poliovirus Handling in Adults with Marginal Selenium Status,” American Journal of Clinical Nutrition, Vol. 80, 2004, pp. 154-162.
- M. B. Mihailović, D. M. Avramović, I. B. Jovanović, et al., “Blood and Plasma Selenium Levels and GSH-Px Activities in Patients with Arterial Hypertension and Chronic Heart Disease,” Journal of Environmental Pathology, Toxicology and Oncology, Vol. 17, 1998, pp. 285-289.
- J. R. Arthur, G. Bermano, J. H. Mitchell, et al., “Regulation of Selenoprotein Gene Expression and Thyroid Hormone Metabolism,” Biochemical Society Transactions, Vol. 24, 1996, pp. 384388.
- O. Augusto, “Radicais Livres: Bons, Maus e Naturais,” Oficina do Texto, São Paulo, 2006.
- G. C. Ghisleni, “Alterações Comportamentais e Neuroquímicas Causadas Por Compostos Orgânicos de Selê- nio,” Ph.D. Dissertation, Santa Maria Biological Sciences UFSM, Santa Maria, 2007.
- R. V. Cauwenbergh, H. Robberechta, V. V. Vlaslaerb, et al., “Plasma Selenium Levels in Healthy Blood Bank Donors in the Central-Eastern Part of Belgium,” Journal of Trace Elements in Medicine and Biology, Vol. 21, 2007, pp. 225-233. doi:10.1016/j.jtemb.2007.06.003
- World Health Organization/Food and Agriculture Organization of the United Nations, “Human Vitamin and Mineral Requirements,” Food and Nutrition Division, FAO Rome, 2001.
- K. E. Hill, Y. Xia, B. Akesson, et al., “Selenoprotein P Concentration in Plasma is An Index of Selenium Status in Selenium-Deficient and Selenium-Supplemented Chinese Subjects,” Journal of Nutrition, Vol. 126, 1996, pp. 138-145.
- A. J. Duffield, C. D. Thomson, K. E. Hill, et al., “An Estimation of Selenium Requirements for New Zealanders,” American Journal of Clinical Nutrition, Vol. 70, 1999, pp. 896-903.
- A. L. Duffield-Lillico, M. E. Reid, B. W. Turnbull, et al., “Baseline Characteristics and the Effect of Selenium Supplementation on Cancer Incidence in a Randomized Clinical Trial: A Summary Report of the Nutritional Prevention of Cancer Trial,” Cancer Epidemiology Biomarkers & Prevention, Vol. 11, 2002, pp. 630-639.
- K. S. Ferreira, J. C. Gomes, C. R. Bellato, et al., “Concentrações de Selênio em Alimentos Consumidos no Brasil,” Pan American Journal of Public Health, Vol. 11, 2002, pp. 172-177.
- N. A. Clack, K. Teschke, K. Rideout, et al., “Trace Elements Levels in Adults from the West Coast of Canadá and Associations with Age, Gendre, Diet, Activities and Levels of Other Trace Elements,” Chemosphere, Vol. 70, No. 1, 2007, pp. 155-164. doi:10.1016/j.chemosphere.2007.06.038
- C. D. Thomson, M. F. Robinson, J. A. Butler, et al., “LongTerm SuppleMentation with Selenate and Selenomethionine: Selenium and GlutaThione Peroxidase,” (EC 1.11.1.9) in Blood Components of New Zealand Women,” British Journal of Nutrition, Vol. 69, No. 2, 1993, pp. 577588. doi:10.1079/BJN19930057
- E. N. Allen, P. N. Appleby, A. W. Roddam, et al., “Plasma Selenium Concentration and Prostate Cancer Risk: Results from the European Prospective Investigation into Cancer and Nutrition,” American Journal of Clinical Nutrition, Vol. 88, 2008, pp. 1567-1575. ”doi:10.3945/ajcn.2008.26205
- J. Burri, M. Haldimann and V. Dudler, “Selenium Status of the Swiss Population: Assessment and Change over a Decade,” Journal of Trace Elements in Medicine and Biology, Vol. 22, No. 2, 2008, pp. 112-119. doi:10.1016/j.jtemb.2007.11.002
- M. S. Saiki, M. Nairo, O. Jahul, et al., “Níveis de Selênio e Zinco Numa População de Idosos Saudáveis de um Hospital Universitário,” Jornal Brasileiro de Patologia e Medicina Laboratorial, Vol. 45, 2009, p. 118.
- M. Lemire, D. Mergler, M. Fillion, et al., “Elevated Blood Selenium Levels in the Brazilian Amazon,” Science of the Total Environment, Vol. 366, 2006, pp. 101- 111. doi:10.1016/j.scitotenv.2005.08.057
- M. Navarro-Alarcon, “M & C Cabrera-Vique Selenium in Food and the Human Body: A Review,” Science of the Total Environment, Vol. 400, No. 1-3, 2008, pp. 115-141. doi:10.1016/j.scitotenv.2008.06.024
- M. A. Moore, R. C. Wander, Y. M. Xia, et al., “Selenium Supplementation of Chinese Women with Habitually Low Selenium Intake Increases Plasma Selenium, Plasma Glutathione Peroxidase Activity and Milk Selenium, but not Milk Glutathione Peroxidase Activity,” Journal of Nutritional Biochemistry, Vol. 11, No. 6, 2000, pp. 341- 347. doi:10.1016/S0955-2863(00)00089-9
- J. Nève, “Human Selenium Supplementation as Assessed by Changes in Blood Selenium Concentration and Glutathione Peroxidase Activity,” Journal of Trace Elements in Medicine and Biology, Vol. 9, No. 2, 1995, pp. 65-73. doi:10.1016/S0946-672X(11)80013-1

