Modern Research in Inflammation
Vol. 2 No. 2 (2013) , Article ID: 31122 , 8 pages DOI:10.4236/mri.2013.22004
Lead induced overactivation of phagocytes and variation in enzymatic and non-enzymatic antioxidant defenses in intestinal macrophages of Channa punctatus
![]()
Department of Biotechnology, Assam University, Assam, India; *Corresponding Author: senguptamahuya35@gmail.com
Copyright © 2013 Nilantika Paul, Mahuya Sengupta. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received 18 April 2013; revised 3 May 2013; accepted 18 May 2013
Keywords: Lead; Channa punctatus; antioxidant; oxidative Stress; lipid peroxidation; respiratory burst; glutathione; bioaccumulation
ABSTRACT
The aim of this study is to assess the adverse effects of lead, a well-documented non-essential element that occurs naturally in the environment, on Channa punctatus, in relation with ROS production and oxidative stress. Fishes were sampled, acclimatized and kept treated or untreated with lead (9.43 mg/L) under observation for 4 days. At day 4, respiratory burst activity, lipid peroxidation activity and superoxide dismutase level increased significantly in treated group as compared to the control. On the contrary, catalase, glutathione S-transferase, glutathione peroxidase, glutathione reductase and reduced glutathione activity decreased on treatment with lead. These results suggest that heavy metal like lead induces oxidative stress, influences the antioxidant defense system and may lead to physiological disorders rendering the health and survival of exposed fish to a compromised state.
1. INTRODUCTION
The pollution of the aquatic environment with heavy metals has become a worldwide problem during recent years, because they are indestructible and most of them have toxic effects on organisms [1]. Heavy metals occur as natural constituents of the earth crust, and are persistent environmental contaminants since they cannot be degraded or destroyed [2]. With rapid industrialization anthropogenic and geogenic activities, which are the major sources of heavy metal pollution, has increased exponentially. Lead is one of the first metals used by humans, is highly persistent, is not involved in normal metabolism and is very toxic [3,4]. Particularly in the 20th century, countless thousands of organic trace pollutants have been produced and in part released into the environment [5]. Lead intoxication is associated with several pathological conditions in children and adults and causes disturbances to the nervous and immune systems, anemia and reduced hemoglobin synthesis, cardiovascular diseases and bone metabolism, and also renal and reproductive dysfunction [6-15].
Fishes come into contact with multiple contaminants that are dissolved in the water or incorporated in the food chain, and so fishes are not only prone to endure negative toxicant-related health effects but also to bioaccumulate pollutants; fishes may therefore be used as bioindicators of environmental contamination [16-20]. Of interest to this study are reports that chemical exposure above a certain threshold may result in an integrated stress response, which can cause immunosuppression or immunoactivation [21].
Fish tissues are endowed with an antioxidant defense system to protect them from oxidative stress caused by metals [22-24]. Elevated levels of metals can induce oxidative stress by generating highly reactive oxygen species (ROS), such as hydrogen peroxide, superoxide radical and hydroxyl radical via Haber-Weiss and Fenton reactions that can oxidize proteins, lipids and nucleic acids, often leading to damage in cell stress or even cell death [25-28]. Organisms have developed several protective mechanisms to remove ROS before the detrimental effects occur in cell. Antioxidant enzymes, such as catalase (CAT), glutathione peroxidase (GPX), glutathione S transferase (GST), glutathione reductase (GR) and superoxide dismutase (SOD) are of great importance in antioxidative stress to cope with free radicals leading several disturbances [29,30].
In view of the above, and considering the lack of sufficient knowledge about the toxic potential effect of lead acetate to freshwater fishes, the objective of this work was to evaluate its effect on enzymatic and non-enzymatic antioxidant profiles of C. punctatus.
2. MATERIALS AND METHODOLOGY
2.1. Animals
Freshwater fish Channa punctatus, of length 12.5 - 15.5 cm and weight 20.0 - 30.0 g, were obtained from local fish market and housed in a 60-l glass aquarium. Fish of a single lot were used throughout the investigation. Prior to exposure, fish were held for 15 days for acclimatization and evaluation of overall fish health under laboratory conditions.
2.2. Exposure
After acclimatization, fish were divided into two groups (five fishes in each group, n = 5); one served as control and the other as treated group. Sub-lethal toxicity of lead acetate solution [9.43 mg/L, 1.02% of 96 h LD50 value (925 mg/L)] on the intestinal macrophages of Channa punctatus was analysed for 4 days [31].
2.3. Isolation of Intestinal Macrophages
The fish were dissected and the whole gut of the fish were isolated, immediately placed in Leibovitz medium (L-15) supplemented with heparin (10 IU/ml) and fetal bovine serum (2%), and then homogenised in ice cold condition. Macrophage was then isolated from the cell suspension by the method of Secombes [32].
2.4. Catalase (CAT) Activity
Catalase activity was measured by the method of Claiborne (1985) [33] with some modifications. One unit (U) of Catalase activity is defined as the amount of enzyme catalyzing 1 μmol of H2O2 per min at 25˚C.
2.5. Superoxide Dismutase (SOD) Activity
One unit of SOD activity was determined as the amount of enzyme that inhibited the auto-oxidation of pyrogallol by 50%. The activity was expressed as U/mg protein [34].
2.6. Estimation of Lipid Peroxidation
The LPO activity was determined by the procedure of Utley et al. (1967) [35], with some modifications. The rate of lipid peroxidation was expressed as nanomoles of thiobarbituric acid reactive substance (TBARS) formed per hour per milligram of protein using a molar extinction coefficient of 1.56 × 105 M−1·cm−1. Protein content of each sample was determined using method of Lowry et al. (1951).
2.7. Respiratory Burst Activity
Respiratory burst activity of intestinal phagocytes of control and treated fish was measured by the method of Fujiki and Yano (1997) [36], with some modifications. The respiratory burst activity was expressed as A630 nm per 106 cells.
2.8. Glutathione S-Transferase (GST) Activity
GST activity was measured by the method described by Mannervik and Guthenberg, 1981. Enzyme activity was calculated using a molar extinction coefficient of 9.6 × 103 M−1∙cm−1.
2.9. Glutathione Peroxidase (GPx) Activity
Total cellular GPx activity was determined by measuring the decrease in absorbance (340 nm) due to the decline in NADPH at 23˚C - 25˚C (Lorentzen et al., 1994). The activity of GPx was expressed as mU/mg protein and 1 mU was defined as 1 nmol of NADPH consumed/min/mL of sample.
2.10. Glutathione Reductase (GR) Activity
The principle of the method is the reduction of oxidized glutathione by glutathione reductase in the presence of NADPH (Carlberg and Mannervik 1975). One unit was defined as an amount of the enzyme which will reduce 1 μM of oxidized glutathione per minute at pH 7.6 at 25˚C, using a molar extinction coefficient of 6.22 × 103 for NADPH.
2.11. Reduced Glutathione (GSH) Assay
Non-enzymatic antioxidant, reduced glutathione, was assayed by the method previously described by Ellman (1959) [37]. The amount of glutathione was calculated using a GSH standard curve and expressed as micrograms of GSH formed/mg protein.
2.12. Analysis of Lead Bioaccumulation by Atomic Absorption Spectrophotometer
The intestine from treated and untreated group were allowed to dry at 120˚C until reaching a constant weight, concentrated nitric acid and hydrogen peroxide (1:1 v/v) (SD fine chemicals) was added. The digestion flasks were heated to 1300˚C until all the materials were dissolved and diluted with double distilled water appropriately. The element lead was assayed using Shimadzu AA 6200 Atomic Absorption Spectrophotometer at the Sophisticated Analytical Instrument Facility (SAIF), NEHU, Shillong, Meghalaya. The results were expressed as ppb/ g tissue.
2.13. Statistical Analysis
The data were expressed as mean ± standard deviation. Data were analyzed using Student’s t-test (two-sample assuming unequal variances) for determining the significant change over control values. The significance level was set at P < 0.05.
3. RESULTS
3.1. Effect of Lead on Catalase Released from Intestinal Macrophages
Catalase (CAT) release was found to decrease from 5.764 ± 0.127 U/mg protein in control to 4.496 ± 0.112 U/mg protein (P < 0.045) in lead treated group (Figure 1) which signifies that lead suppresses the activity of catalase significantly.
3.2. Effect of Lead on Superoxide Dismutase Released from Intestinal Macrophages
Superoxide dismutase (SOD) release was found to increase from 0.015 ± 0.01 U/mg protein in control group to 0.1835 ± 0.01 U/mg protein (P < 0.05) in lead treatedgroup (Figure 2). This shows that lead may probably overactivate SOD activity leading to formation of hy drogen peroxide in excessive amount rendering the host cell to damage.
3.3. Effect of Lead on Lipid Peroxidation in Intestinal Macrophages
There was a significant increase in lipid peroxidation activity as it is evident from the results that showed increase of TBARS from 0.385 ± 0.145 nmoles/hr in control group to 0.87 ± 0.112 nmoles/hr (P < 0.05) in lead treated group (Figure 3). Increased production of lipid peroxides signifies that lead can indeed disturb the integrity of plasma membrane, which is essential for cell viability, making cells prone to damage.
3.4. Effect of Lead on Respiratory Burst Activity in Intestinal Macrophages
There was a significant increase in respiratory burst activity from 0.521 ± 0.02 in control group to 1.148 ± 1.134 (P < 0.007) in lead treated group (Figure 4) which depicts the capability of lead in stimulating cells to pro-
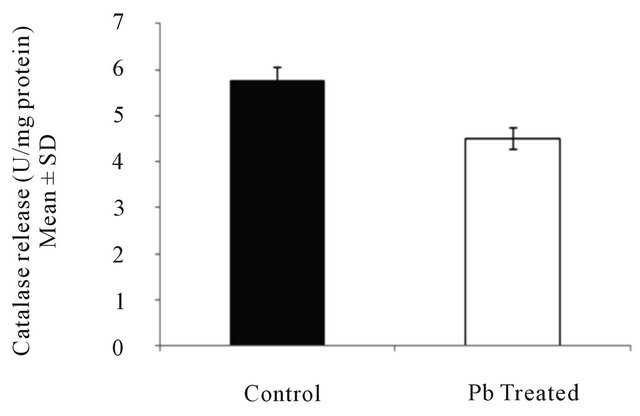
Figure 1. Catalase activity in intestinal macrophages of fish treated with lead. Values are expressed as mean ± S.D. Significant difference from control value is P < 0.045.

Figure 2. Superoxide dismutase activity in intestinal macrophages of fish treated with lead. Values are expressed as mean ± S.D. Significant difference from control value is P < 0.05.

Figure 3. Lipid peroxidation activity in intestinal macrophages of fish treated with lead. Values are expressed as mean ± S.D. Significant difference from control value is P < 0.05.
duce large amount of ROS leading to cell damage.
3.5. Effect of Lead on Glutathione S-Transferase Activity in Intestinal Macrophages
Glutathione S-transferase activity was found to decrease from 28.13 ± 0.15 in control group to 24.66 ± 0.01

Figure 4. Respiratory burst activity in intestinal macrophages of fish treated with lead. Values are expressed as mean ± S.D. Significant difference from control value is P < 0.007.
(P < 0.05) in lead treated group (Figure 5) which may indicates lead induced impaired detoxification mechanism in fish.
3.6. Effect of Lead on Glutathione Peroxidase Activity in Intestinal Macrophages
Glutathione peroxidase activity was found to decrease from 5.8 ± 0.15 in control group to 4.7 ± 0.01 (P < 0.001) in lead treated group (Figure 6) which indicates probable persistence of free radicals like hydrogen peroxide leading to severe cell damage.
3.7. Effect of Lead on Glutathione Reductase Activity in Intestinal Macrophages
Glutathione reductase activity was found to decrease from 3.96 ± 0.15 in control group to 3.6 ± 0.01 (P < 0.02) in lead treated group (Figure 7) indicating lead induced compromised antioxidant defence system in fish.
3.8. Effect of Lead on Reduced Glutathione Activity in Intestinal Macrophages
Reduced glutathione activity was found to decrease from 0.823 ± 0.15 in control group to 0.555 ± 0.01 (P < 0.005) in lead treated group (Figure 8) indicating that lead deactivates the formation of reduced glutathione which is an essential antioxidant molecule of fish defence system.
3.9. Concentration of Lead (ppb) in Intestinal Tissues of Treated and Untreated Group
Marked increase in lead accumulation was observed in intestine of treated group as compared to that of the control group (Table 1).
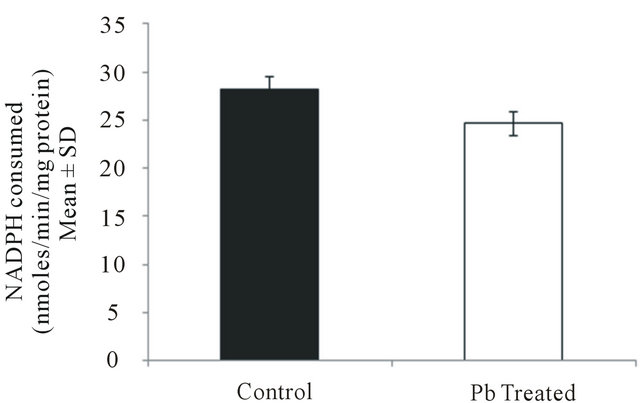
Figure 5. Glutathione S-transferase activity in intestinal macrophages of fish treated with lead. Values are expressed as mean ± S.D. Significant difference from control value is P < 0.05.
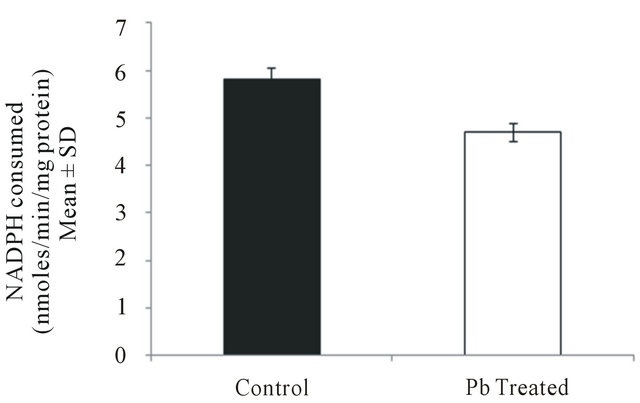
Figure 6. Glutathione peroxidase activity in intestinal macrophages of fish treated with lead. Values are expressed as mean ± S.D. Significant difference from control value is P < 0.001.

Figure 7. Glutathione reductase activity in intestinal macrophages of fish treated with lead. Values are expressed as mean ± S.D. Significant difference from control value is P < 0.02.
4. DISCUSSION
Fishes possess different defensive mechanisms to counteract the impact of toxicants. Heavy metals accumulated in fish tissues may trigger redox reactions that generate free radicals especially reactive oxygen species (ROS). These highly reactive compounds may damage lipids,
Figure 8. Reduced glutathione activity in intestinal macrophages of fish treated with lead. Values are expressed as mean ± S.D. Significant difference from control value is P < 0.005.

Table 1. Concentration of lead accumulated in intestinal tissues of treated and untreated group.
proteins, carbohydrates and nucleic acids and may induce morphological and physiological alterations in fish tissues [38,39]. Many aquatic organisms have been shown to possess defense pathway to protect them against damages induced by oxyradical production [40]. An earlier study report on enzymatic and non-enzymatic antioxidant processes contributes to reducing the impact of ROS in fishes [41]. Therefore, both the activity of antioxidant enzymes and the occurrence of oxidative damage have been proposed as indicators of pollutant-mediated oxidative stress [42].
Oxidative stress occurs when the critical balance between oxidants and antioxidants is disrupted due to the depletion of antioxidants or excessive accumulation of ROS, or both, leading to cellular damage [43]. The SODCAT system provides the first defense against oxygen toxicity and represents a cellular defense mechanism to counteract toxicity of ROS. SOD catalyzes the dismutation of the superoxide anion radical to water and hydrogen peroxide, which is detoxified by the CAT activity [44]. Palace et al. (1992) [45] have stated that SOD is the most responsive indicator of exposure to contaminants eliciting oxidative stress. Low levels of CAT in lead treated fishes could be attributed to high production of superoxide anion radical, which has been reported to inhibit CAT activity [46]. The reduction of superoxide radicals to H2O2 is catalyzed by SOD. The increase in SOD activity in lead treated fishes may be due to increased generation of reactive oxygen species.
Phagocytes, upon stimulation with various agents, produce ROS through activation of nicotinamide adenine dinucleotide phosphate reduced (NADPH) oxidase [47]. NADPH oxidase is a superoxide-producing enzyme consisting of the membrane (gp91PHOX and p22PHOX)
and the cytosolic (p47PHOX, p67PHOX, and p40PHOX) components [48,49]. In addition, small G-proteins such as rac1, as well as kinases including PKC, regulate its activity [50]. Our observations reveal that the respiratory burst activity of the treated group increased significantly as compared to that of the control group. Under normal circumstances, activation of cells of nonspecific immunity may be beneficial to the host, particularly the reactive intermediates released during phagocytic respiratory burst activity possess bactericidal activity [51,52]. Elevation in the respiratory burst activity on lead exposure may suggest over activation of the superoxide-producing enzyme NADPH oxidase and generation of large amount of ROS. Further, it could also be assumed that lead acetate in macrophage might have suppressed the activity of the regulatory proteins leading to uncontrolled enzyme activity which is destined to cell damage.
Heavy metal induced lipid peroxidation has already been described in various fish species [53-55]. LPO estimation in particular has also been found to have a high predictive importance as revealed from a credible number of research papers describing its suitability as a biomarker of effect [56-60].When the animal’s defenses are insufficient to neutralize ROS, oxidative damage may occur, and one of the most serious damages is membrane lipid peroxidation [44]. The integrity of plasma membrane is essential for cell viability, and as a consequence of over activation of phagocytes, its fluidity seems to be effected in fish [61]. Our results demonstrate that lead acetate exposure induces production of high levels of lipid peroxides thus causing deleterious membrane damaging effect. Thus it may be hypothesized that lead may stimulate the peroxidation of lipids by acting as catalysts in the formation of oxygen radicals.
Antioxidant enzymes that have often been studied as oxidative stress biomarkers link detoxification of ROS with the metabolism of reduced glutathione [62,63]. These include glutathione peroxidase (GPx), an enzyme removing hydrogen peroxide by the simultaneous oxidation of reduced GSH to its oxidized form glutathione disulfide (GSSG) and glutathione reductase (GR), an enzyme catalyzing the conversion of GSSG back to its reduced bioactive form maintaining thus GSH/GSSG equilibrium [64]. Glutathione S-transferase (GST), an enzyme involved in the detoxification process and in protecting against peroxidative damage, is ubiquitous in the cytosol and microsomes of eukaryotes [65]. Our results reveal significant depletion of GPx, GR and GST activity indicates an impaired detoxification mechanism of the fish upon low concentration of lead exposure.
One of the most remarkable effects of lead exposure on intestinal macrophage is a time-dependent decrease in GSH. GSH is the most well studied antioxidant molecule in fish. Heavy metal cations are characterized by an extremely high affinity to –SH residues [63] resulting in decrease of GSH level. Sandhir et al., [66] establish that glutathione reductase, the enzyme responsible for recycling of glutathione from the oxidized form (glutathione disulfide; GSSG) to the reduced form (reduced glutathione; GSH) is deactivated by lead, resulting in low levels of GSH. Our results clearly show depleted levels of GSH in lead treated group of fish as compared to control, and may contribute to the above facts.
The presence of metal pollutant in fresh water is known to disturb the delicate balance of the aquatic ecosystem. Among the various toxic pollutants, heavy metals are particularly severe in their action due to tendency of bio-magnification in the food chain. They readily tend to concentrate in different organs of fishes resulting in bioaccumulation and biomagnification of these metals to a toxic level even when the exposure is low [67,68]. Studies carried out on fish species have shown that heavy metal bioaccumulation may alter the physiological activities and biochemical parameters both in tissues and blood [22]. Our results show significant level of lead accumulation in intestinal tissues when compared with the untreated group and consequently correlates with the induced oxidative stress as well as altered enzyme activities in the intestinal macrophages.
In conclusion, the present study indicates that sub-lethal concentration of lead acetate has the capacity to bioaccumulate, thereby altering the normal functional activities of freshwater fish C. punctata. Further, the association of oxidative stress including variation in its antioxidant profile suggests that the defense system of C. punctata is significantly compromised upon metal exposure at low concentrations.
REFERENCES
- MacFarlane, G.B. and Burchettt, M.D. (2000) Cellular distribution of Cu, Pb, and Zn in the Grey Mangrove Avicemnia marina (Forsk.). Vierh Aquatic Botanica, 68, 45-59. doi:10.1016/S0304-3770(00)00105-4
- Duruibe, J.O., Ogwuegbu, M.O.C. and Egwurugwu, J.N. (2007) Heavy metal pollution and human biotoxic effects. International Journal of Physical Sciences, 2, 112-118.
- Guity, P., Mccabe, M.J., Pitts, D.K., Santini, R.P. and Pounds, J.G. (2002) Protein kinase C does not mediate the inhibitory action of lead on vitamin D3-dependent production of osteocalcin in osteoblastic bone cells. Toxicology and Applied Pharmacology, 178, 109-116. doi:10.1006/taap.1999.8819
- Saleh, A.M., Vijayasarathy, C., Masoud, L., Kumar, L., Shahin, A. and Kambal, A. (2003) Paraoxon induces apoptosis in EL4 cells via activation of mitochondrial pathways. Toxicology and Applied Pharmacology, 90, 47-57. doi:10.1016/S0041-008X(03)00126-1
- van der Oost, R., Beyer, J. and Vermeulen, N.P.E. (2003) Fish bioaccumulation and biomarkers in environmental risk assessment: A review. Environmental Toxicology and Pharmacology, 13, 57-149. doi:10.1016/S1382-6689(02)00126-6
- Nriagu, J.O. (1988) A silent epidemic of environmental metal poisoning. Environmental Pollution, 50, 139-161. doi:10.1016/0269-7491(88)90189-3
- Goyer, R.A. (1993) Lead toxicity: Current concerns. Environmental Health Perspectives, 100, 177-187. doi:10.1289/ehp.93100177
- Pirkle, J.L., Brody, D.J., Gunter, E.W., Kramer, R.A., Paschal, D.C. and Flegal, K.M. (1994) The decline in blood lead levels in the United States. Journal of American Medical Association, 272, 284-291. doi:10.1001/jama.1994.03520040046039
- Bernard, A.M., Vyskocil, A., Kriz, J., Kodl, M. and Lauwerys, R. (1995) Renal effects of children living in the vicinity of a lead smelter. Environmental Research, 68, 91-95. doi:10.1006/enrs.1995.1012
- USPHS (1997) Toxicological profile for lead [on CDROM]. Agency for Toxic Substances and Disease Registry, US Public Health Service.
- Krocova, Z., Macela, A., Kroca, M. and Hernychova, L. (2000) The immunomodulatory effect(s) of lead and cadmium on the cells immune system in vitro. Toxicology in Vitro, 14, 33-40. doi:10.1016/S0887-2333(99)00089-2
- Razmiafshari, M., Kao, J., d’Avignon, A. and Zawia, N.H. (2001) NMR identification of heavy metal-binding sites in a synthetic zinc finger peptide: Toxicological implications for the interactions of xenobiotic metals with zinc finger proteins. Toxicology and Applied Pharmacology, 172, 1-10. doi:10.1006/taap.2001.9132
- Collares-Buzato, C.B., Sueur, L.P.L. and Cruz-Höfling, M.A. (2002) Impairment of the cell-to-matrix adhesion and cytotoxicity induced by Bothtops moojeni snake venom in cultured renal tubular epithelia. Toxicology and Applied Pharmacology, 18, 124-132. doi:10.1006/taap.2002.9404
- Prozialeck, W.C., Grunwald, G.B., Dey, P.M., Reuhl, K.R. and Parrish, A.R. (2002) Cadherins and NCAM as potential targets in metal toxicity. Toxicology and Applied Pharmacology, 182, 255-265. doi:10.1006/taap.2002.9422
- Zheng, W., Aschmer, M. and Ghersi-Egea, J.M. (2003) Brain barrier systems: A new frontier in metal neurotoxincological research. Toxicology and Applied Pharmacology, 192, 1-11. doi:10.1016/S0041-008X(03)00251-5
- Hinton, D.E., Lantz, R.C., Hampton, J.A., McCuskey, P.R. and McCuskey, R.S. (1987) Normal versus abnormal structure: Considerations in morphologic responses of teleosts to pollutants. Environmental Health Perspectives, 71, 139- 146. doi:10.1289/ehp.8771139
- Hinton, D.E., Baumann, P.C., Gardner, G.R., Hawkins, W.E., Hendricks, J.D., Murchelano, R.A. and Okihiro, M.S. (1992) Histopathologic biomarkers. In: Huggett, R., Kimerle, R.A., Meherle, P.M. and Bergman, H.L. Eds., Biomarkers—Biochemical, Physiological and Histological Markers of Anthropogenic Stress. A Special Publication of SETAC Lewis Publishers Boca Raton, Ann Arbor, London and Tokyo, 155-212.
- Hinton, D.E. (1993) Toxicology-histopathology of fishes: A systematic approach and overview. In: Couch, J.A. and Fournie, J.W., Eds., Pathobiology of marine and estuarine organisms, CRC Press, Boca Raton, 177-215.
- Hinton, D.E. (1994) Cells, cellular responses, and their markers in chronic toxicity of fishes. In: Malins, D.C. and Ostrander, G.K., Eds., Aquatic Toxicology. Molecular, BioChemical and Cellular Perspectives, Lewis Publishers, Boca Raton, 207-239.
- Whitfield, A.K. and Elliott, M. (2002) Fishes as indicators of environmental and ecological changes within estuaries: A review of progress and some suggestions for the future. Journal of Fish Biology, 61, 220-250. doi:10.1111/j.1095-8649.2002.tb01773.x
- Wendelaar Bonga, S.E. (1997) The stress response of fish. Physiological Reviews, 77, 591-626.
- Basa, S.P. and Rani, U.A. (2003) Cadmium induced antioxidant defense mechanism in freshwater teleost Oreochromis mossambicus (Tilapia). Ecotoxicology and Environmental Safety, 56, 218-221. doi:10.1016/S0147-6513(03)00028-9
- Atli, G., Alptekin, Ö., Tükel, S. and Canli, M. (2006) Response of catalase activity to Ag+, Cd2+, Cr6+, Cu2+ and Zn2+ in five tissues of freshwater fish Oreochromis niloticus. Comparative Biochemistry and Physiology, C143, 218-224.
- Atli, G. and Canli, M. (2008) Responses of metallothionein and reduced glutathione in a freshwater fish Oreochromis niloticus following metal exposures. Environmental Toxicology and Pharmacology, 25, 33-38. doi:10.1016/j.etap.2007.08.007
- Nagalakshmi, N. (1998) Copper-induced oxidative stress in Scenedesmus bijugatus: Protective role of free radical scavengers. Bulletin of Environment Contamination and Toxicology, 61, 623-628. doi:10.1007/s001289900806
- Tripathi, B.N. and Gaur, J.P. (2004) Relationship between copperand zinc-induced oxidative stress and proline accumulation in Scenedesmus sp. Planta Medica, 219, 397- 404.
- Dewez, D., Geoffroy, L., Vernet, G. and Popovic, R. (2005) Determination of photosynthetic and enzymatic biomarkers sensitivity used to evaluate toxic effects of copper and fluid oxonilin alga Scenedesmus obliquus. Aquatic Toxicology, 74, 150-159. doi:10.1016/j.aquatox.2005.05.007
- Cao, L., Huang, W., Liu, J., Yin, X. and Dou, S. (2010) Accumulation and oxidative stress biomarkers in Japanese flounder larvae and juveniles under chronic cadmium exposure. Comparative Biochemistry and Physiology, C151, 386-392.
- Pinto, E., Sigaud-Kutner, T.C.S., Leitao, M.A.S., Okamoto, O.K., Morse, D. and Colepicolo, P. (2003) Heavy metal-induced oxidative stress in algae. Journal of Phycology, 39, 1008-1018. doi:10.1111/j.0022-3646.2003.02-193.x
- Tripathi, B.N., Mehta, S.K., Amar, A. and Gaur, J.P. (2006) Oxidative stress in Scenedesmus sp. during shortand longterm exposure to Cu2+ and Zn2. Chemosphere, 62, 538- 544. doi:10.1016/j.chemosphere.2005.06.031
- Devi, R. and Bannerjee, T.K. (2007) Toxicopathological impact of sub-lethal concentration of lead nitrate on the aerial respiratory organs of “murrel” Channa striata (Bloch, Pisces). Iranian Journal of Environmental Health Science and Engineering, 4, 249-256.
- Secombes, C.J. (1990) Isolation of salmoid macrophages and analysis of their killing activity. Techniques in Fish Immunology, 1, 137-155.
- Claiborne, A. (1985) Catalase activity. In: Greenwald, R.A., Ed., CRC Handbook of Methods for Oxygen Radical Research, CRC Press, Boca Raton, 283-284.
- Marklund, S. and Marklund, G. (1974) Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. European Journal of Biochemistry, 47, 469-474. doi:10.1111/j.1432-1033.1974.tb03714.x
- Utley, H.C., Bernheim, F. and Hachslein, P. (1967) Effect of sulfhydryl reagent on peroxidation in microsome. Archives of Biochemistry and Biophysics, 260, 521-531.
- Fujiki, K. and Yano, T. (1997) Effect of sodium alginate on the non-specific defense system of the common carp. Fish and Shellfish Immunology, 7, 417-427. doi:10.1006/fsim.1997.0095
- Lowry, O.H., Rosebrough, N.J., Farr, A.L. and Randall, R.J. (1951) Protein measurement with Folin phenol reagent. Journal of Biological Chemistry, 193, 265-275.
- Ellman, G.L. (1959) Tissue sulfhydryl groups. Archives of Biochemistry and Biophysics, 82, 70-77. doi:10.1016/0003-9861(59)90090-6
- Hermes, L.M. (2004) Oxygen in biology and biochemistry. In: Storey, K.B., Ed., Functional Metabolism: Regulation and adaptation, Wiley-Liss, Inc., New York, 319- 368.
- Varanka, Z., Rojik, I., Nemcsok, J. and Ábraham, M. (2001) Biochemical and morphological changes in carp liver following exposure to copper sulfate and tannic acid. Comparative Biochemistry and Physiology C, 128, 467- 478.
- Winston, G.W. and DiGiulio, R.T. (1991) Prooxidant and antioxidant mechanisms in aquatic organisms. Aquatic Toxicology, 19, 137-161. doi:10.1016/0166-445X(91)90033-6
- Lopez-Torrez, M., Perez-Campos, R., Cadenas, S., Rojas, C. and Barja, G. (1993) A comparative study of free radicals in vertebrates-II. Non-enzymatic antioxidants and oxidative stress. Comparative Biochemistry and Physiology, 105, 757-763.
- Ahmad, I., Hamid, T., Fatima, M., Chand, H.S., Jain, S.K., Athar, M. and Raisuddin, S. (2000) Induction of hepatic antioxidants in freshwater fish (Channa punctatus Bloch) is a biomarker of paper mill effluent exposure. Biochimica et Biophysica Acta, 1523, 37-48. doi:10.1016/S0304-4165(00)00098-2
- Scandalios, J.G. (2005) Oxidative stress: Molecular perception and transduction of signals triggering antioxidant gene defences. Brazilian Journal of Medical and Biological Research, 38, 995-1014. doi:10.1590/S0100-879X2005000700003
- Dimitrova, M.S.T., Tsinova, V. and Velcheva, V. (1994) Combined effect of zinc and lead on the hepatic superoxide dismutase-catalase system in carp (Cyprinus carpio). Comarative Biochemistry and Physiology Part C, 108, 43-46.
- Palace, V.P., Mjewski, H.S. and Klaverkamp, J.F. (1992) Interactions among antioxidant defenses in liver of rainbow trout (Oncorhyncus mykiss) exposed to cadmium. Canadian Journal of Fish and Aquatic Science, 50, 156-162. doi:10.1139/f93-018
- Kono, Y. and Fridovich, I. (1982) Superoxide radical inhibits catalase. Journal of Biological Chemistry, 257, 5751- 5754.
- Forman, H.J. and Torres, M. (2002) Reactive oxygen species and cell signaling. American Journal of Respiratory and Critical Care Medicine, 166, 24-28. doi:10.1164/rccm.2206007
- DeLeo, F.R. and Quinn, M.T. (1996) Assembly of the phagocyte NADPH oxidase: Molecular interaction of oxidase proteins. Journal of Leukocyte Biology, 60, 677- 691.
- Babior, B.M. (1999) NADPH oxidase: An update. Blood, 93, 1464-1476.
- El Benna, J., Dang, P.M., Gaudry, M., Fay, M., Morel, F., Hakim, J. and Gougerot-Pocidalo, M.A. (1997) Phosphorylation of the respiratory burst oxidase subunit p67phox during human neutrophil activation: Regulation by protein kinase C-dependent and independent pathways. Journal of Biological Chemistry, 272, 17204-17208. doi:10.1074/jbc.272.27.17204
- Baggiolini, M. (1984) Phagocytes use oxygen to kill bacteria. Experientia, 40, 906-909. doi:10.1007/BF01946438
- Secombes, C.J. and Fletcher, T.C. (1992) The role of phagocytes in the protective mechanisms of fish. Annual Review of Fish Diseases, 2, 51-71. doi:10.1016/0959-8030(92)90056-4
- Pandey, S., Parvez, S., Ansari, R.A., Ali, M., Kaur, M., Hayat, F., Ahmad, F. and Raisuddin, S. (2008) Effects of exposure to multiple trace metals on biochemical, histological and ultrastructural features of gills of a freshwater fish, Channa punctata Bloch. Chemico-Biological Interactions, 174, 183-192. doi:10.1016/j.cbi.2008.05.014
- Jastrzębska, E.B. (2010) The effect of aquatic cadmium and lead pollution on lipid peroxidation and superoxide dismutase activity in freshwater fish. Polish Journal of Environmental Studies, 19, 1139-1150.
- Rome´o, M., Bennani, N., Gnassia-Barelli, M., Lafaurie, M. and Girard, J.P. (2000) Cadmium and copper display different responses towards oxidative stress in the kidney of the sea bass Dicentrarchus labrax. Aquatic Toxicology, 48, 185-194. doi:10.1016/S0166-445X(99)00039-9
- Lackner, R. (1998) Oxidative stress in fish by environmental pollutants. In: Braunbeck, T., Hinton, D.E. and Streit, B., Eds., Fish Ecotoxicology, Birkhause Verlag, Basel, 203-224. doi:10.1007/978-3-0348-8853-0_6
- Ahmad, I., Pacheco, M. and Santos, M.A. (2004) Enzymatic and nonenzymatic antioxidants as an adaptation to phagocyte-induced damage in Anguilla anguilla L. following in situ harbor water exposure. Ecotoxicology and Environmental Safety, 57, 290-302. doi:10.1016/S0147-6513(03)00080-0
- Ahmad, I., Oliveira, M., Pacheco, M. and Santos, M.A. (2005) Anguilla anguilla L. oxidative stress biomarkers responses to copper exposure with or without β-naphthoflavone pre-exposure. Chemosphere, 61, 267-275. doi:10.1016/j.chemosphere.2005.01.069
- [61] Santos, M.A., Pacheco, M. and Ahmad, I. (2004) Anguilla anguilla L. antioxidants responses to in situ bleached kraft pulp mill effluent outlet exposure. Environment International, 30, 301-308. doi:10.1016/S0160-4120(03)00178-8
- [62] Elferink, J.G.R. (1987) Mode of activation of the metabolic burst in polymorphonuclear leukocytes by calcium oxalate crystals. Agents Actions, 22, 295-301. doi:10.1007/BF02009059
- [63] Stegeman, J.J., Brouwer, M., Digiulio, R.T., Forlin, L., Fowler, B.A., Sanders, B.M. and Vanveld, P.A. (1992) Molecular responses to environmental contamination— enzyme and protein systems as indicators of chemical-exposure and effect. In: Huggett, R.J., Kimerle, R.A., Mehrle, P.M. and Bergman, H.L., Eds., Biomarkers Biochemical, Physiological, and Histological Markers of Anthropogenic Stress, Lewis Publishers, Inc., Boca Raton, 235-335.
- [64] Viarengo,A. and Nott, J.A. (1993) Mechanisms of heavy metal cation homeostasis in marine invertebrates. Comparative Biochemistry and Physiology, 104C, 355-372.
- [65] Paskerová, H., Hilscherová, K. and Bláha, L. (2012) Oxidative stress and detoxification biomarker responses in aquatic freshwater vertebrates exposed to microcystins and cyanobacterial biomass. Environment and Science Pollution Research, 19, 2024-2037. doi:10.1007/s11356-012-0960-7
- [66] Sreejai, R. and Jaya, D.S. (2010) Studies on the changes in lipid peroxidation and antioxidants in fishes exposed to hydrogen sulfide. Toxicology International, 17, 71-77. doi:10.4103/0971-6580.72674
- [67] Sandhir, R., Julka, D. and Gill, K.D. (1994) Lipoperoxidative damage on lead exposure in rat brain and its implications on membrane bound enzymes. Pharmacology and Toxicology, 74, 66-71. doi:10.1111/j.1600-0773.1994.tb01077.x
- [68] Yousuf, M. H. A. and El-Shahawi, A. (1999) Trace metals in lethrinus lentjan fish from Arabian gulf: Metal accumulation in kidney and heart tissues. Bulletin of Environmental Contamination and Toxicology, 62, 293-300. doi:10.1007/s001289900873
- [69] Vinodhini, R. and Narayanan, M. (2008) Bioaccumulation of heavy metals in organs of fresh water fish Cyprinus carpio (Common carp). International Journal of Environmental Science and Technology, 5, 179-182.

