Advances in Parkinson's Disease
Vol. 2 No. 2 (2013) , Article ID: 31220 , 4 pages DOI:10.4236/apd.2013.22008
Comparison of continuous versus pulsatile dopaminergic therapy in the erderly with Parkinson’s Disease
![]()
1Geriatric Unit and Laboratory of Disability Prevention, Geriatric and Rehabilitation Department, University Hospital of Parma, Parma, Italy; *Corresponding Author: flauretani@ao.pr.it
2Department of Clinical and Experimental Medicine, Section of Geriatrics, University of Parma, Parma, Italy
3Geriatric Clinic Unit, Geriatric and Rehabilitation Department, University Hospital of Parma, Parma, Italy
4Neurology Unit, Ospedale di Fidenza—San Secondo, Azienda Unità Sanitaria Locale di Parma, Parma, Italy
Copyright © 2013 Fulvio Lauretani et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received 7 November 2012; revised 20 December 2012; accepted 1 January, 2013
Keywords: Parkinson’s disease; rotigotine; continuous dopaminergic therapy; elderly
ABSTRACT
Objective: Levodopa is the gold-standard of therapy in Parkinson’s Disease (PD), but it is associated with motor complications that affect 50% of patients after five years of treatment. Development of delirium and psychosis is the main limitation of dopaminergic treatment in older persons. These adverse effects may result from pulsatile stimulation of the dopamine receptors. Dopamine agonists with transdermal delivery that continuously stimulate the dopamine receptors may reduce these complications. The objective of this study was to evaluate the frequencies of acute delirium and psychosis in elderly patients treated with rotigotine vs. levodopa in a newly diagnosed drugnaïve Parkinson’s Disease (PD). Methods: Patients admitted to the Geriatric-Rehabilitation Department of the UniversityHospital of Parma were screened for the presence of Parkinsonism. All subjects admitted with diagnosis of PD according to the UK Brain Bank Criteria were randomly treated with Rotigotine or levodopa. All subjects were assessed by Movement Disorder Society (MDS)-Unified Parkinson's Disease Rating Scale (MDS-UPDRS) Part III. Acute delirium was asessed by CAM Diagnostic Algorithm during the first week after admission. After six months, diagnosis of psychosis was performed according to pro posed diagnostic criteria by NINDS and NIMH. Patients with cognitive impairment (MMSE < 21) and affected by any diseases potentially leading to psychosis, including Dementia with Lewy bodies (DLB), were excluded. Results: 60 consecutive newly diagnosed drug-naïve PD patients were evaluated. No statistical significant difference between the two groups were observed in term of age, gender, MMSE score, severity of disease expressed by H&Y staging. 30 patients were treated with rotigotine (6 mg/daily) and 30 patients were treated with L-Dopa (250 mg/daily). All participants completed the study. UPDRS Part III was statistical significant lower in both groups after treatment from 26.4 to 18.3 (rotigotine group) and from 26.3 to 17.3 (levodopa group), but comparable within groups (p = 0.83). After 6-month follow-up, acute delirium and/ or psychosis were observed in two cases (6.6%) of patients treated with rotigotine and in three cases (10%) of those treated with levodopa (p = 0.54). Conclusions: Transdermal rotigotine seems comparable to levodopa in regard to motor skill efficacy and neuropsychiatric safety, because provides a more continuous delivery of drug. Dopamine agonists may represent a valid therapeutic option in newly diagnosed older PD patients.
1. INTRODUCTION
Parkinson’s disease (PD) and parkinsonian signs that occur in old age are expected to increase dramatically in the next decades [1]. The prevalence of PD is low before 50 years of age and rises up to 4% in the highest age groups [2]. Parkinson’s disease is clinically diagnosed based on the presence of the classical motor features (i.e. bradykinesia, rigidity, rest tremor), without the presence of supportive features with the exception of the absence of exclusion criteria [3]. In most of the cases the diagnosis is straightforward, and no ancillary tests are required, while in older persons, the diagnosis is more complex given, for example, that complaints of aching stiffness with subtle changes in body posture and speed of movement are frequently and incorrectly dismissed as normal aging, leading to inappropriate referrals, for example, to rheumatologists [4]. In the latest decade, the approach to PD was dramatically changed [5]. In fact, although for many years PD has been considered only “a disease that affects walking”, with a key role of the neurotransmitter dopamine, recently Braak et al. [6] proposed neuro-anatomical stages of the disease able to explain motor and non-motor symptoms observed in this disease.
Levodopa is still considered the gold-standard therapy in PD, but it is often associated with motor complications that affect about 50% of PD patients after five years of treatment [7]. According to current guidelines, dopamine agonist therapy is not first line in 75+ patients because of the high risk of neuropsychiatric adverse effects [8]. Dopamine agonists are associated with a lower incidence of motor fluctuation in clinical trials irrespective of delivery mode. Recently, continuous dopaminergic stimulation has been proposed by Olanow et al. as being protective, mainly in regard to motor complications [9]. Moreover, prolonged release dopamine agonists, in particular with transdermal delivery, might have a better neuropsychiatric safety profile, which is the main concern for their use in older subjects. There is recent evidence that low doses of Rotigotine are well tolerated and may improve the quality of life of individuals with PD aged 75 and older [10], category of subjects usually excluded from clinical trials. We hypothesized that dopamine agonists with transdermal delivery, such as rotigotine, are associated with lower neuropsychiatric adverse effects than classical levodopa in older parkinsonian patients.
The objective of this pilot study was to evaluate the frequencies of acute delirium and psychosis in elderly patients treated with rotigotine vs. levodopa in a newly diagnosed drug-naïve Parkinson’s Disease (PD).
2. MATERIAL AND METHODS
Patients admitted to the Geriatric-Rehabilitation Department of the University Hospital of Parma were screened for the presence of Parkinsonism. All subjects with diagnosis of PD according to the UK Brain Bank Criteria [3] were randomly treated with Rotigotine or L-Dopa. All subjects were assessed by Movement Disorder Society (MDS)-Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) Part III [11]. The UPDRS is the most accepted scale to assess the severity of the disease. In this study, only the Section 3, which expressed motor skills of the patients, was used. The UPDRS Part III has been assessed by a trained physician and lower scores indicate higher levels of motor performances.
Acute delirium was assessed by CAM Diagnostic Algorithm [12] during the first week after admission. After six months, the diagnosis of psychosis was performed according to proposed diagnostic criteria by NINDS and NIMH [13]. Patients with severe and moderate cognitive impairment (MMSE < 21) and affected by any disease potentially responsible of psychosis, including dementia with Lewy bodies (DLB), were excluded [14]. The research complied with the ethical rules for human experimentation stated in the Declaration of Helsinki. All participants consented to participate in the study for scientific purposes. The study protocol was approved by the Local Ethical Committee of the Hospital.
All analyses were performed using the SAS, version 8.2, statistical software. Data are reported as mean ± standard deviation (SD). Baseline characteristics of the rotigotine and levodopa groups were compared by 1-way analysis of variance (ANOVA). The magnitude of change over time in UPDRS Part III and the development of psychosis in the rotigotine versus levodopa group was compared using a repeated-measures ANOVA and chisquare test.
3. RESULTS
General characteristics of subjects studied are reported in Table 1. 60 consecutive newly diagnosed drug-naïve PD patients were evaluated. No statistical significant difference between the two groups were observed in term of age, gender, MMSE score, severity of disease expressed by H&Y staging. 30 patients were treated with rotigotine (6 mg/daily) and 30 patients were treated with L-Dopa (250 mg/daily). All participants completed the study.
UPDRS Part III was statistically significant lower in both groups after treatment from 26.4 to 18.3 (rotigotine group) and from 26.3 to 17.3 (levodopa group), but comparable within groups.
Figure 1 showed the mean change from baseline to 6-month follow-up of UPDRS Part III scores in both groups. After 6-month follow-up, no statistical significant difference in term of motor skill were observed between the two groups (p = 0.83).
After 6-month follow-up, acute delirium and/or psychosis were observed in two cases (6.6%) of patients treated with rotigotine and in three cases (10%) of those
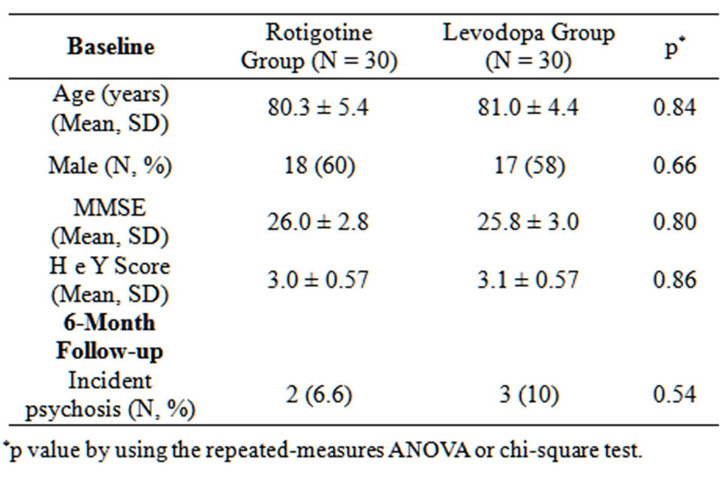
Table 1. Baseline and 6-mouth follow-up characteristics of the study population.
Figure 1. Mean change from baseline to 6-month follow-up of UPDRS Part III scores in both groups.
treated with levodopa (p = 0.54).
4. DISCUSSION
In older patients with PD, transdermal delivery of dopamine agonist have comparable improvement of motor skills and similar short-term risk of neuropsychiatric complications of levodopa. Continuous delivery of the drug, which is similarly achieved by other extended release formulations, is the probable cause of such tolerability of dopamine agonist. However, it should be point out that, in agreement with exclusion criteria, this may not apply to patients with moderate to severe cognitive impairment.
One of the underlying mechanisms, by which transdermal delivery of dopamine agonist could produce less neuropsychiatric adverse effects than levodopa, could be the reduction of the imbalance between dopamine and acetylcholine. The change of neutrasmettitorial profile has been proposed as contributing factor of delirium in older persons [15]. Moreover, the different dopamine receptors profiles of dopamine agonists could influence the psychiatric side effects. For example, rotigotine shows more affinity for D1 and D2 receptors, classically considered “motor” receptors, and minor affinity for D3 and D4 receptors, “behaviour” like receptors. This can explain why they could produce less psychiatric effects than pramipexole [9].
Transdermal rotigotine significantly improved “off” time in subjects with advanced Parkinson disease not optimally controlled with levodopa [16], and was also effective for the treatment of early-stage Parkinson disease [17].
From our point of view, considering the new multistages of the PD (6), the most important factor for planning a correct treatment of this disease in the elderly, that could be with multiple drug earlier than in young patients, is established the H&Y stage and assess both motor and cognitive performance of the patient. In fact, L-dopa or dopamine agonists may produce neuropsychiatric sideeffects not only in relation of the age of the patient but if the patient showed cognitive impairment or dementia associated with the motor deficit (5) at the moment of the first evaluation.
This concept should be implemented in the new guidelines for the treatment of the PD, because the Sydney multicenter study [18], for example, showed that even after few years the discovery of the clinical presentation of the disease, dementia or hallucinations could appear in the patient and this could be the most important factor that influence treatment decisions in older persons with Parkinson’s disease.
5. CONCLUSIONS
In this pilot study, transdermal rotigotine have comparable improvement of motor skills and similar risk of neuropsychiatric complications than levodopa in older parkinsonian patients.
Long-term clinical trials with adequate sample size are required to confirm present results, which suggest that dopamine agonists may represent a valid therapeutic option in newly diagnosed older PD patients.
6. CONFLICT OF INTERESTS AND ACKNOWLEDGEMENTS
None of the sponsoring institutions interfered with the collection, analysis, presentation, or interpretation of the data reported here. Founded by the Emilia-Romagna Region. These results are preliminarily presented at the XXX VIII LIMPE Congress-Venice, October 5-8, 2011.
REFERENCES
- Murray, A.M., Bennett, D.A., Mendes de Leon, C.F., Beckett, L.A. and Evans, D.A. (2004) A longitudinal study of Parkinsonism and disability in a community population of older people. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences, 59, 864-870. doi:10.1093/gerona/59.8.M864
- Lauretani, F., Maggio, M., Silvestrini, C., Nardelli, A., Saccavini, M. and Ceda, G.P. (2012) Parkinson’s disease (PD) in the elderly: an example of geriatric syndrome (GS)? Archives of Gerontology and Geriatrics, 54, 242-246. doi:10.1016/j.archger.2011.03.002
- Jankovic, J. (2008) Parkinson’s disease: Clinical features and diagnosis. Journal of Neurology, Neurosurgery & Psychiatry, 79, 368-376. doi:10.1136/jnnp.2007.131045
- Lees, A. (2010) The bare essentials: Parkinson’s disease. Practical Neurology, 10, 240-246. doi:10.1136/jnnp.2010.217836
- Lauretani, F., Maggio, M., Nardelli, A. and Ceda, G.P. (2012) Parkinson’s disease: Diagnosis, treatment and prognosis. In: Yoshida, C. and Ito, A., Eds., Treatment of Parkinson’s Disease and Parkinsonism in the Elderly, Nova Science Publishers, New York, pp. 165-178.
- Braak, H., Del Tredici, K., Rüb, U., de Vos, R.A., Jansen Steur, E.N. and Braak, E. (2003) Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiology of Aging, 24, 197-211. doi:10.1016/S0197-4580(02)00065-9
- Ahlskog, J.E. and Muenter, M.D. (2001) Frequency of levodopa related dyskinesias and motor fluctuations as estimated from the cumulative literature. Movement Disorders, 16, 448-458. doi:10.1002/mds.1090
- Horstink, M., Tolosa, E., Bonuccelli, U., Deuschl, G., Friedman, A., Kanovsky, P., et al. (2006) Review of the therapeutic management of Parkinson’s disease. Report of a joint task force of the European federation of neurological societies and the movement disorder society—European Section. Part I: Early (uncomplicated) Parkinson’s disease. European Journal of Neurology, 13, 1170-1185. doi:10.1111/j.1468-1331.2006.01547.x
- Olanow, C.W., Stern, M.B. and Sethi, K. (2009) The scientific and clinical basis for the treatment of Parkinson disease. Neurology, 72, S1-136. doi:10.1212/WNL.0b013e3181a1d44c
- Fasano, A., Guidubaldi, A., De Nigris, F. and Bentivoglio, A.R. (2011) Safety and efficacy of rotigotine in individuals with Parkinson’s disease aged 75 and older. Journal of the American Geriatrics Society, 59, 2386-2387. doi:10.1111/j.1532-5415.2011.03689.x
- Goetz, C.G., Tilley, B.C., Shaftman, S.R., Stebbins, G.T., Fahn, S., Martinez-Martin, P., et al. (2008) Movement disorder society-sponsored revision of the unified Parkinson’s disease rating scale (MDS-UPDRS): Scale presentation and clinimetric testing results. Movement Disorders, 23, 2129-2170. doi:10.1002/mds.22340
- Inouye, S.K., van Dyck, C.H., Alessi, C.A., Balkin, S., Siegal, A.P. and Horwitz, R.I. (1990) Clarifying confusion: The confusion assessment method. A new method for detection of delirium. Annals of Internal Medicine, 113, 941-948. doi:10.7326/0003-4819-113-12-941
- Ravina, B., Marder, K., Fernandez, H.H., Friedman, J.H., McDonald, W., Murphy, D., Aarsland, D., et al. (2007) Diagnostic criteria for psychosis in Parkinson’s disease: Report of an NINDS/NIMH work group. Movement Disorders, 22, 1061-1068. doi:10.1002/mds.21382
- Lauretani, F., Caffarra, P., Ruffini, P., Nardelli, A., Ceda, G.P., Maggio, M., et al. (2011) Brief practical clinical diagnostic criteria for the neurodegenerative diseases in the elderly. Drugs and Therapy Studies, 1, e6.
- Lauretani F., Ceda, G.P., Maggio, M., Nardelli, A., Saccavini, M. and Ferrucci, L. (2010) Capturing side-effect of medication to identify persons at risk of delirium. Aging Clinical and Experimental Research, 22, 456-458.
- LeWitt, P.A., Lyons, K.E. and Pahwa, R. (SP 650 Study Group) (2007) Advanced Parkinson disease treated with rotigotine transdermal system: PREFER study. Neurology, 68, 1262-1267. doi:10.1212/01.wnl.0000259516.61938.bb
- Watts, R.L., Jankovic, J., Waters, C., Rajput, A., Boroojerdi, B. and Rao, J. (2007) Randomized, blind, controlled trial of transdermal rotigotine in early Parkinson disease. Neurology, 68, 272-276. doi:10.1212/01.wnl.0000252355.79284.22
- Hely, M.A., Reid, W.G., Adena, M.A., Halliday, G.M. and Morris, J.G. (2008) The Sydney multicenter study of Parkinson’s disease: The inevitability of dementia at 20 years. Movement Disorders, 23, 837-844.
ABBREVIATIONS
PD: Parkinson’s Disease;
CAM: Confusion Assessment Method;
NINDS: National Institute of Neurological Disorders and Stroke;
NIMH: National Institute of Mental Health;
MMSE: Mini Mental State Examination;
H & Y stage: Hoehn and Yahr staging;
MDS: Movement Disorder Society;
MDS-UPDRS Part III: Movement Disorder Society-Unified Parkinson’s Disease Rating Scale Part III;
DLB: Dementia with Lewy Bodies.

