American Journal of Plant Sciences
Vol.08 No.04(2017), Article ID:74547,15 pages
10.4236/ajps.2017.84045
Effect of Trichoderma harzianum in Combination with Fungicides in Controlling Gray Mould Disease (Botrytis cinerea) of Strawberry
Radwan M. Barakat*, Mohammad I. Al-Masri
Plant Protection Research Center, Faculty of Agriculture, Hebron University, Hebron, Palestine

Copyright © 2017 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/



Received: January 22, 2017; Accepted: February 28, 2017; Published: March 3, 2017
ABSTRACT
The effects of biofungicide formula containing the biocontrol agent Trichoderma harzianum (Jn14) as wettable powder in combination with the fungicides pyrimethanil and a mixture of cyprodinil and flydioxonil on Botrytis cinerea of strawberry in vitro, in vivo and in greenhouse were studied. The pathogen B. cinerea was more affected in vitro by low concentrations of the fungicides tested above 0.4 µg ml−1 than the bioagent T. harzianum (Jn14). The later was almost insensitive to pyrimethanil. In the same direction, gray mould disease severity on strawberry detached leaves and whole plants was reduced up to 89% by the tested fungicides, compared to the control, while the application of T. harzianum (Jn14) alone reduced disease severity up to 45% on strawberry detached leaves, compared to the control. In the integrated control approach, the combination of T. harzianum (Jn14) with higher concentrations of the tested fungicides (ED50) completely inhibited strawberry gray mould disease severity in pots and the greenhouse.
Keywords:
Fungicides, Trichoderma harzianum, Botrytis cinerea, Gray Mould, Strawberry

1. Introduction
Gray mould caused by Botrytis cinerea Pers. ex Fr. is one of the major fungal diseases of strawberry worldwide. The disease attacks flowers, setting fruits, mature fruits and leaves, and the main sources of inoculum for the disease in strawberry are dead leaves, mummified fruits, neighbouring crops and weeds [1] . The disease is responsible for serious product losses and reduces the product quality. Chemical control of the disease often depends on frequent fungicide applications, utilizing compounds for various chemical classes, and targeting specific cellular processes in fungi (e.g. respiration, sterol biosynthesis etc.). Unfortunately, the occurrence of resistance to different fungicides with specific mode of action, as well as residues of fungicides in food have been frequently reported [2] - [8] . Biological control using the well-known biocontrol agent Trichoderma harzianum against gray mould was intensively used as alternative [9] . Several investigators reported that Trichoderma isolates were effective in controlling anthracnose and grey mould in strawberry, under laboratory and greenhouse conditions [10] [11] [12] [13] . In addition, the bacterium Paenibacillus polymyxa (Isolate 18191), obtained from mature strawberry fruit, had shown antagonistic potential against B. cinerea, and reduced disease severity by reducing germination and germ tube growth [14] . The efficiency of Clonostachys rosea alone or integrated with fungicide sprays, reduced the incidence in both flowers and fruits [15] . The safety of T. harzianum as a biocontrol agent has been evaluated at different levels based on impact on non-target insects and mammals including humans, and no safety concerns were identified [16] . There is an increasing interest in the last two decades in the use of T. harzianum in biocontrol programs against several important plant diseases worldwide. Furthermore, several commercial formulations were developed in several countries lately such as Bio-fungus SupersivitÒ (BioPlant, Denmark), RootProÒ (Efal Agri, Israel), and RootShieldÒ (Bioworks Inc., USA).
In Palestine, during an intensive survey for many years, eighty five isolates of T. harzianum were recovered from soil samples collected from different agricultural areas in the West Bank. The efficiency of the Palestinian native isolates against several soilborne and foliar plant pathogens such as Sclerotium rolfsii, Fusarium oxysporum f. sp. lycopersici, Rhizoctonia solani and Botrytis cinerea was evaluated. A very virulent isolate (Jn14) was identified and was able over the years to induce high control rates of diseases, stimulated the plants growth and increased productivity through increasing the release of nutrients from available organic matter [17] . This isolate (Jn14) was formulated as a safe biofugicide and biofertilizer for vegetable nurseries and growers.
The objective of this study was to evaluate the effect of native Trichoderma harzianum formulated as wettable powder in combination with low dose of fungicides in controlling gray mould (Botrytis cinerea) of strawberry in vitro, in vivo and under greenhouse conditions (Appendix).
2. Materials and Methods
2.1. Botrytis cinerea Isolate
B. cinerea isolate used in the study was collected from a greenhouse cultivated with strawberry located in Hebron-West Bank of Palestine. Fruits, exhibiting symptoms of grey mould disease were detached from plants and placed in clean plastic bag. Infected fruits were placed in sterile Petri dishes, and small pieces of fruits were transferred and surface sterilized by using 1% sodium hypochlorite for 3 minutes, washed three time with sterilized distilled water and placed in the centers of Petri dishes containing potato dextrose agar (PDA) amended with 500 mg l−1 chloramphenicol (10 ml PDA medium per dish). Petri dishes were then incubated at 22˚C. After 4 days, mycelia growing at the edges of the colonies were subcultured on three Petri dishes containing PDA and incubated again at 22˚C with overhead fluorescent light. After 10 days, conidia were harvested from dishes by pipetting 5 ml sterilized distilled water and rubbing colonies gently with sterile glass rods. Conidial suspension was then filtered through double layers of sterilized muslin membrane to remove all traces of mycelium. Conidial suspension (200 µl) were then seeded on 90-mm PDA Petri dishes and incubated at 22˚C. After 2 days, single germinated conidia were removed using a fine sterile needle and transferred to new PDA Petri dishes, identified as (Bc1) and incubated for future experiments.
2.2. Trichoderma harzianum Formula
Trichoderma harzianum isolate (Jn14) was identified based on RAPD analysis and the sequence of (ITS) [18] , and formulated as wettable powder (WP) on Talc. The isolate was grown on 90 mm PDA medium plates for 10 days. Conidia were harvested from plates by pipetting 5 ml sterilized distilled water in plates and rubbing colonies gently with sterile glass rods. Conidial suspension was then filtered through a double layer of sterilized muslin membrane to remove all traces of mycelium. The filtrate suspension containing spores was centrifuged at 2308 ×g (RCF) for 15 minutes and the supernatant was discarded. The spore pellets were washed thoroughly by adding sterile distilled water, vortexed to ensure homogeneous suspension and centrifuged as discussed earlier (2 times). Pellets were then recovered in sterile water, and spores mixed well with talc powder (1:2, v:v) (Sigma, T-2015). The mixture was placed on aluminum foil in a sterile tray (in the form of small heaps) in a vacuum dryer oven at 35˚C for 48 - 72 hours. Once dried, the heaps which looked like “cakes” were crushed and sieved (200 μm) under sterile conditions to avoid contamination. The talc-formula was stored dried in a tightly closed flask either at 4˚C. The concentration of spores in the formula was evaluated by counting the number of CFU/g of talc by using dilute plates techniques.
2.3. Fungicides
Fungicides used in the experiments were pyrimethanil (MythosÒ SC 300 g∙l−1, Bayer Crop Science) and a mixture of cyprodinil and flydioxonil (SwitchÒ, WG 62.5 g∙kg−1 for both a.i, Syngenta, UK).
Effect of Fungicides on T. harzianum and B. cinerea
The effect of fungicides on T. harzianum and B. cinerea isolates was evaluated in vitro on mycelial growth rate and conidial germination, and in vivo on gray mould lesions on detached strawberry leaves and disease severity on whole plants.
2.4. In Vitro Assays
2.4.1. Mycelial Growth Rate
The effect of fungicides on T. harzianum and B. cinerea isolates was carried out in vitro on defined minimal medium (modified Difco Czapek-Dox recipe) to avoid possible interaction of fungicides with complex components of an undefined medium, such as PDA (Yourman and Jeffers, 1999). The medium (1 liter) contained 12 g of agar, 10 g of glucose, 3 g of NaNO3, 1 g of KH2PO4, 1 g of K2HPO4, 0.5 g of MgSO4∙7H2O, 0.5 g of KCl, 0.01 g of FeSO4∙7H2O and 0.5 g of chloramphenicol; the final pH was 6.6. Erlenmeyer flasks, each containing a standard volume of medium were heated on magnetic stirrer hot plate to dissolve the components. Flasks were then autoclaved, and allowed to cool to 55˚C - 60˚C. Appropriate volumes of both fungicides’ stock solutions (1000 µg∙ml−1, a.i of each) were added to the media to give final concentrations of 0.4, 0.8, 1.2, 1.6, 2, 2.4, 2.8, 3.2 and 3.6 µg∙ml−1. Growth media without fungicides were used as control. Growth media were dispensed into standard disposable Petri dishes (90 mm diameter) with 14 ml of medium in each dish. Petri plates were then inoculated in the center with 5 mm mycelium disks of 5 days old culture of T. harzianum and B. cinerea and incubated at 25˚C. Fungal colonies diameters were measured after 24 and 72 h and the mycelium growth rate (MGR, cm2 day−1) was calculated by using the following equation: R = {(D/2)2*л − (d/2)2*л}/T, where R―mycelium growth rate, D―average diameter of colony (cm) after 72 h, d―average diameter of colony (cm) after 24 h, л―3.14, and T―time of incubation (day) (Barakat and Al-Masri, 2006).
The experimental design was completely randomized design (CRD) with five replicates. The experiment was repeated twice and the means of MGR of T. harzianum and B. cinerea of both experiments were correlated with fungicides’ concentrations to calculate linear regression and identify the fungicides effective dosage that reduced fungal growth by 50% (ED50).
2.4.2. Conidia Germination
The germination of conidia was evaluated as conidial germination percentage in 24 wells microtiter sterilized plates (Greiner bio-one, Germany). The conidia of both T. harzianum and B. cinerea were harvested from 7 days old cultures growing on PDA medium and incubated at 25˚C, by gently rubbing the fungal colonies with a glass rod in 10 ml sterile DW. Conidial suspension was then filtered through a double layer of sterilized muslin membrane to remove all traces of mycelium. The filtrate suspension containing spores was centrifuged at 2308 ×g (RCF) for 3 minutes and the supernatant was discarded. The spore pellets were washed thoroughly by adding sterile distilled water, vortexed to ensure homogeneous suspension and centrifuged as discussed earlier (2 times). Conidial concentration was then adjusted using a haemocytometer to 2 × 104 conidia ml−1. The germination experiment design was completely randomized design that included 11 concentrations of the two fungicides. Fungicide concentrations (0, 1, 2, 3, 4, 5, 6, 8, 10, 12 and 15) µg ml−1 (a.i) amended with 1 mM glucose were replicated 4 times using 4 wells of a 24 well microtiter sterilized plates. Each fungicide concentration was tested against T. harzianum and B. cinerea. In each well, 480 μl of each fungicide concentration amended with 1mM glucose were added in addition to a 20 μl conidial suspension of either T. harzianum or B. cinerea. The plates were then incubated in at 25˚C. Germination percentages were assessed after 48 hours of incubation in five fields at 20× of an inverted microscope (Olympus CKX41, Japan). Germination (%) was calculated from 20 reading (4 wells × 5 fields). The experiment was repeated twice and the means of both experiments data were correlated with fungicide’s concentrations (linear regression) to identify the ED50 of fungicides.
2.5. In Vivo Assays
2.5.1. Gray Mould Lesion on Strawberry Detached Leaves
Healthy young leaves were collected and placed in the bottom of plastic boxes (40 × 25 × 15 cm), on double layer of a plastic mesh platform. The plastic mesh was placed on a sterilized wet paper towel to maintain high humidity in the box. Each box contained six leaves. The leaves were sprayed with fungicides or with the formula of T. harzianum using a micro sprayer. The concentrations used were 0, 100, 200, 300, 400, 500, 600, 700, 800, and 900 μg pyrimethanil and a mixture of cyprodinil and flydioxonil ml−1 water (a.i). The concentrations used from the T. harzianum formula were 0, 105, 106, 107, 108, and 109 spore ml−1. After the sprayed solutions were absorbed by leaves, the detached leaves were inoculated by placing a 5-mm diameter agar block taken from 3-day-old PDA cultures of B. cinerea. The boxes were moistened, covered by transparent plastic film and incubated at 22˚C with a 12 h photoperiod. Evaluation of disease development on detached leaves was carried out by measuring the rotting leaf area around the inoculum disk (lesion diameter in mm) after 7 days of incubation. The experimental design was a completely randomized design, where six leaves of strawberry plants considered as replicates for each concentration. The experiment was repeated and the effective dosage concentration (ED50) for both fungicides and the T. harzianum formula was calculated from linear regression.
2.5.2. Disease Severity on Strawberry Whole Plants
Ten strawberry plants (4 months old), were sprayed until runoff with the concentrations: 0, 200, 400, 600, 800, and 1000 μg ml−1 of pyrimethanil and a mixture of cyprodinil and flydioxonil ml−1 water (a.i) or with the T. harzianum formula at the concentrations (0, 105, 106, 107, 108, and 109) spore ml−1. After the sprayed solutions absorbed, the plants were inoculated with 30 ml of conidial suspension (2 × 105 CFU) in deionized sterile water containing 2 g∙l−1 glucose and 1 g∙l−1 potassium dihydrogen phosphate (KH2PO4) taken from 10-day-old PDA culture of B. cinerea (Bc1). The plants were covered with transparent plastic bags and incubated under greenhouse conditions at 22˚C - 25˚C. Gray mold severity was evaluated by estimation of the percentage of leaf mold coverage after 21 days of incubation. The experimental design used was completely randomized design, where the ten strawberry plants considered as replicates for each concentration. The experiment was repeated and the effective dosage concentration (ED50) for both fungicides and the T. harzianum formula was calculated from linear regression.
2.6. Gray Mould Integrated Management
2.6.1. Pot Experiment
In this experiment, fungicides were integrated with T. harzianum (Jn14) to protect strawberry plants from gray mould. The experimental treatments involved were a low dose (ED50) of the fungicides, pyrimethanil (943 μg∙ml−1) and a mixture of cyprodinil and flydioxonil (750 μg∙ml−1) (a.i) alone, the T. harzianum (Jn14) ml−1 formula alone at the concentrations of 108, and 109 spore, the T. harzianum (Jn14) ml−1 formula integrated with ED50 of fungicides, and water was used as control. Ten strawberry plants (4 months old), were sprayed until runoff with the previously mentioned treatments. After the sprayed solutions absorbed, the plants were inoculated with 30 ml of conidial suspension (2 × 105 CFU) in deionized sterile water containing 2 g∙l−1 glucose and 1 g∙l−1 potassium dihydrogen phosphate (KH2PO4) taken from 10-day-old PDA culture of B. cinerea (Bc1). The plants were covered with transparent plastic bags and incubated under greenhouse conditions at 22˚C - 25˚C. Gray mold severity was evaluated by estimation of the percentage of leaf mold coverage after 21 days of incubation. The experimental design used was completely randomized design, where the ten strawberry plants considered as replicates for each concentration. The experiment was repeated and the effective dosage concentration (ED50) for both fungicides and the T. harzianum formula was calculated from linear regression.
2.6.2. Greenhouse Experiment
In this experiment, fungicides were integrated with T. harzianum (Jn 14) to protect strawberry plants from gray mould under greenhouse conditions. Two years old strawberry plants were grown in greenhouse in double five rows at a distance of 40 cm between plants and 150 cm between rows. Each double row contained 50 plants. The experiment design was complete randomized with nine treatments each replicated 3 times. Each replicate contained 18 plants. The experimental treatments involved were a low dose (ED50) of the fungicides, pyrimethanil (943 μg∙ml−1) and a mixture of cyprodinil and flydioxonil (750 μg ml−1) (a.i) alone, the T. harzianum (Jn 14) ml−1 formula alone at the concentrations of 108, and 109 spore, the T. harzianum (Jn 14) ml−1 formula integrated with ED50 of fungicides, and water was used as control. Strawberry plants were sprayed (100 ml/plant) until runoff as mentioned above. After the sprayed solutions absorbed, the plants were inoculated with 30 ml of conidial suspension (2 × 105 CFU) in deionized sterile water containing 2 g∙l−1 glucose and 1 g∙l−1 potassium dihydrogen phosphate (KH2PO4) taken from 10-day-old PDA culture of B. cinerea (Bc1). Plants were repeatedly sprayed for 3 times within 10 days interval. The severity of gray mould was evaluated by estimation of the percentage of leaf mold coverage and fruit rot incidence (%) after 10, 20, and 30 days from inoculation.
2.7. Statistical Analysis
The data of mycelial growth rate, conidial germination, lesion diameter, and gray mould disease severity and fruit incidence were statistically analyzed by using one way ANOVA and Fisher LSD (statistical software Sigma StatÒ 2.0 program, SPSS Inc., USA). In addition, The ED50 values against mycelial growth rate, conidial germination, lesion growth, and disease severity were estimated statistically for both fungicides using linear regression equations.
3. Results
3.1. In Vitro
Both fungicides (Pyrimethanil and the mixture of Cyprodinil & Flydioxonil) at the concentrations 0.4 - 3.6 µg∙ml−1 reduced mycelium growth (MGR) of both fungi but in different proportions compared to the control (Table 1). T. harzianum MGR was less affected, however, by both fungicides than B. cinerea MGR. The fungicide Pyrimethanil was able to reduce MGRs by 24% - 29% and 55% - 73% for T. harzianum and B. cinerea, respectively. However, the fungicide mixture of Cyprodinil & Flydioxonil was able to reduce MGRs by 40% - 75% and 94% - 100% for T. harzianum and B. cinerea, respectively. Mycelial growth of B. cinerea was completely inhibited by the fungicide mixture at the concentration ≥ 2 µg∙ml−1.
Concerning conidial germination, both fungicides reduced germination percentages of both fungi compared to the control but in lower proportions. Similarly, T. harzianum conidial germination was less affected, by both fungicides than B. cinerea. The fungicide Pyrimethanil (0.4 - 3.6 µg∙ml−1) was able to reduce percentages of conidial germination by 1% - 13% and 17% - 48% for T. harzianum and B. cinerea, respectively. However, the fungicide mixture of Cyprodinil & Flydioxonil was able to reduce percentages of conidial germination by 25% - 47% and 68% - 88% for T. harzianum and B. cinerea, respectively.
3.2. In Vivo
The fungicide Pyrimethanil (100 - 900 µg ml−1) significantly reduced grey mould lesions development on strawberry detached leaves by 20% - 89% compared to the control (Table 2). The reduction in lesion development was positively correlated with increasing fungicide concentrations (r2 = 0.91, P ≤ 0.05). The effective dose of Pyrimethanil to induce 50% reduction in lesion development (ED50) on strawberry detached leaves was 577 µg∙ml−1. Furthermore, Pyrimethanil significantly reduced gray mould disease severity on whole plants by 18% - 65% at the concentrations (600 - 1000 µg∙ml−1), compared to the control. The reduction in disease severity was positively correlated with increasing fungicide concentrations (r2 = 0.85, P ≤ 0.05). The effective dose of Pyrimethanil to induce 50% reduction in disease severity (ED50) on strawberry whole plants was 943 µg∙ml−1.
The fungicide mixture of Cyprodinil & Flydioxonil (50 - 450 µg∙ml−1) significantly reduced grey mould lesions development on strawberry detached leaves by 16% - 75% compared to the control (Table 2). The reduction in lesion
Table 1. Effect of fungicides pyrimethanil and a mixture of cyprodinil and flydioxonil on mycelium growth rate (0 - 3.6 µg∙ml−1) and on conidia germination (0 - 15 µg∙ml−1) of the pathogen Botrytis cinerea and the biocontrol agent T. harzianum (Jn14) incubated at 25˚C.
*Means followed by the same letter in a column or row for each experiment are not significantly different according to Fisher LSD test (P ≤ 0.05).
Table 2. Effect of fungicides pyrimethaiil, a mixture of cyprodinil and flydioxonil and the biocontrol agent T. harzianum (Jn14) on gray mould lesions on detached leaves and on gray mould disease severity (%) on strawberries whole plants.
*Means followed by the same letter in a column or row for each experiment are not significantly different according to Fisher LSD test (P ≤ 0.05).
development was positively correlated with increasing fungicide concentrations (r2 = 0.95, P ≤ 0.05). The effective dose of fungicide mixture of Cyprodinil & Flydioxonil to induce 50% reduction in lesion development (ED50) on strawberry detached leaves was 267 µg∙ml−1. Furthermore, the fungicide mixture of Cyprodinil & Flydioxonil significantly reduced gray mould disease severity on whole plants by 6% - 76% at the concentrations (200 - 1000 µg∙ml−1), compared to the control. The reduction in disease severity was positively correlated with increasing fungicide concentrations (r2 = 0.98, P ≤ 0.05). The effective dose of fungicide mixture of Cyprodinil & Flydioxonil to induce 50% reduction in disease severity (ED50) on strawberry whole plants was 750 µg ml−1.
The T. harzianum biofungicide formula significantly reduced grey mould lesions development on strawberry detached leaves by 19% - 45% compared to the control at the concentrations 107 - 109 spores ml−1 (Table 2). The reduction in lesion development was positively correlated with increasing spore concentrations (r2 = 0.67, P ≤ 0.05). Furthermore, the T. harzianum biofungicide formula significantly reduced gray mould disease severity on whole plants by 13% - 15% at the concentrations (107 - 109 spores ml−1), compared to the control. The reduction in disease severity was positively correlated with increasing spore concentrations (r2 = 0.63, P ≤ 0.05).
3.3. Gray Mould Integrated Control
All treatments including the use of fungicides, T. harzianum or both significantly reduced gray mould disease severity in strawberries grown in pots or in greenhouse soil compared to the control (Table 3 and Figure 1). In the pot experiment (Table 3), the fungicides, Pyrimethanil and the fungicide mixture of Cyprodinil & Flydioxonil combined with the higher spores’ concentration (109 spores ml−1) of the T. hatzianum formula completely inhibited gray mould on strawberries compared to the control. However, both fungicides alone or in combination with the lower spores’ concentration of T. harzianum formula (108 spores ml−1) were able to reduce disease severity by 54% - 65%, compared with the control. T. harzianum alone reduced disease severity by 48% at the concentration of 109 spores ml−1, but declined to 29% when the lower spores concentration was used (108 spores ml−1).
In the greenhouse experiment (Figure 1), both fungicides in combination with T. harzianum reduced disease severity by 90% - 92% and disease incidence by 88%, compared to the control. The fungicides treatments alone reduced disease severity by 75% - 77%, and disease incidence by 76% - 80%, while the T. harzianum treatment alone reduced disease severity by 50%, and disease incidence by 54%, compared to the control.
4. Discussion
Biological control of strawberry gray mould disease using a bio-formula containing the biocontrol agent Trichoderma harizanum in combination with low
Table 3. Effect of integrated control using a low dose (ED50) of the fungicides pyrimethaiil, a mixture of cyprodinil and flydioxonil and the biocontrol agent T. harzianum (Jn14) on gray mould disease severity (%) on strawberries whole plants.
*Means followed by the same letter in a column are not significantly different according to Fisher LSD test (P ≤ 0.05).


Figure 1.Effect of integrated control using low dose (ED50) of the fungicides pyrimethaiil, a mixture of cyprodinil and flydioxonil and the biocontrol agent T. harzianum (Jn14) on gray mould disease severity (%) on strawberry whole plants (a) and on disease incidence on fruits (b) under greenhouse conditions.
dose of fungicides can provide a useful and environmentally safe tool. In addition, this reduces the possibility of resistance development to fungicides. The bio-formula containing T. harzianum (Jn14) reduced gray mould lesions development on strawberry detached leaves in addition to disease severity on plants grown in pots and under greenhouse conditions. From the in vitro study results, it was obvious that the pathogen B. cinerea was more affected by low concentrations of the fungicides tested (> 0.4 µg∙ml−1) than the bioagent T. harzianum (Jn14); this was true for the mycelium growth rate and the conidia germination. In this direction, Khirallah et al. [19] showed that sensitivity of T. harzianum to various fungicides was variable depending on the concentration and the active ingredients of tested fungicides. Furthermore, it was noted that moderate sensitivity of T. harzianum to cyprodinil and fludioxonil is due to active ingredients of two different families with two different modes of actions [20] . In addition, T. harzianum (Jn14) was almost insensitive to Pyrimethanil. Similar results were found by Yuan et al. [21] , who showed that Trichoderma (T-21) was resistant to Pyrimethanil.
As for the disease severity on strawberry detached leaves and whole plants, gray mould was reduced up to 89% by the tested fungicides compared to the control confirming the in vitro experiment’s results. Similar results on the effectiveness of Pyrimethanil and the fungicide mixture of Cyprodinil & Flydioxonil against gray mould disease severity was documented by Menzel et al. [22] , and Rosslenbroich and Stuebler [23] . However, in this study the application of T. harzianum (Jn14) alone reduced disease severity on strawberry detached leaves up to 45% compared to the control. This partial reduction in gray mould disease severity was thoroughly discussed by several researchers [22] [24] [25] .
In the integrated control experiments, the combination of the higher concentrations of tested fungicides and T. harzianum (Jn14) completely inhibited strawberry gray mould disease severity in pots and the greenhouse. Robinson-Boyer et al. [11] reported that the combinations of commercially available biological control agents (BCAs) can provide effective control to B. cinerea on detached strawberry leaves. They further elaborated that using Trichoderma atroviride (LC52) and Trichoderma harzianum (T22) were very effective either alone or in sequential combination with other BCAs. Furthermore, Xiangming et al. [13] reported that the combinations of commercially available biological control agents containing T. atroviride (LC52) and T. harzianum (T22), might control B. cinerea more effectively than individual BCAs on strawberry leaves and the efficacy affected by application regimes and temperature.
5. Conclusion
In conclusion, this study demonstrated the compatibility of T. harzianum (Jn14) with the tested fungicides (Pyrimethanil and a mixture of Cyprodinil & Flydioxonil) in the control of B. cinerea on strawberry. T. harzianum (Jn14) tolerated both fungicides at the in vitro, in vivo, and greenhouse levels, while B. cinerea was sensitive to these compounds at these levels.
Cite this paper
Barakat, R.M. and Al-Masri, M.I. (2017) Effect of Trichoderma harzianum in Combination with Fungicides in Controlling Gray Mould Disease (Botrytis cinerea) of Strawberry. American Journal of Plant Sciences, 8, 651-665. https://doi.org/10.4236/ajps.2017.84045
References
- 1. Sutton, J.C. (1995) Evaluation of Micro-Organisms for Biocontrol: Botrytis cinerea and Strawberry, a Case Study. In: Andrews, J.H. and Tommerup, I.C., Eds., Advances in Plant Pathology, Academic Press, San Diego, 173-190.
https://doi.org/10.1016/s0736-4539(06)80011-5 - 2. Dianez, F., Santos, M. and Blanco, R. (2002) Fungicide Resistance in Botrytis cinerea Isolates from Strawberry Crops in Huelva (Southwestern Spain). Phytoparasitica, 30, 529-534.
https://doi.org/10.1007/BF02979759 - 3. Vallejo, I., Munoz, F., Carbu, M., Rebordinos, L., Fernandez-Acero, F. and Cantoral, J. (2003) Study on Fungicide Resistance of Botrytis cinerea Isolates from Diseased Strawberry Plants. Archives of Phytopathology and Plant Protection, 36, 1-7.
https://doi.org/10.1080/0323540031000080155 - 4. Petsikos-Panayotarou, N., Markellou, E., Kalamarakis, A.E., Kyriakopoulou, D. and Malathrakis, N.E. (2003) In Vitro and in Vivo Activity of Cyprodinil and Pyrimethanil on Botrytis cinerea Isolates Resistant to Other Botryticides and Selection for Resistance to Pyrimethanil in a Greenhouse Population in Greece. European Journal of Plant Pathology, 109, 173-182.
https://doi.org/10.1023/A:1022522420919 - 5. Moyano, C., Gomez, V. and Melgarejo, P. (2004) Resistance to Pyrimethanil and Other Fungicides in Botrytis cinerea Population Collected on Vegetable in Spain. Journal of Phytopathology, 152, 484-490.
https://doi.org/10.1111/j.1439-0434.2004.00880.x - 6. Lerocha, M., Pleskena, C., Weberb, R.W.S., Kauffa, F., Scallietc, G. and Hahn, M. (2013) Gray Mold Populations in German Strawberry Fields Are Resistant to Multiple Fungicides and Dominated by a Novel Clade Closely Related to Botrytis cinerea. Applied and Environmental Microbiology, 79, 159-167.
https://doi.org/10.1128/AEM.02655-12 - 7. Barakat, R.M. and Al-Masri, M.I. (2014) Iprodione and a Mixture of Carbendazim and Diethofencarb Fungicides Resistance in Botrytis cinerea Population Collected from Palestinian Vegetable Greenhouses. IUG Journal of Natural and Engineering Studies, 22, 1-24.
- 8. Konstantinou, S., Veloukas, T., Leroch, M., Menexes, G., Hahn, M. and Karaoglanidis, G. (2015) Population Structure, Fungicide Resistance Profile, and sdhB Mutation Frequency of Botrytis cinerea from Strawberry and Greenhouse-Grown Tomato in Greece. Plant Disease, 99, 240-248.
https://doi.org/10.1094/PDIS-04-14-0373-RE - 9. Harman, G.E. (2006) Overview of Mechanisms and Uses of Trichoderma spp. Phytopathology, 96,190-194.
https://doi.org/10.1094/PHYTO-96-0190 - 10. Elad, Y. (2000) Trichoderma Harzianum T39 Preparation for Biocontrol of Plant Diseases-Control of Botrytis cinerea, Sclerotinia sclerotiorum and Cladosporium fulvum. Biocontrol Science Technology, 10, 499-507.
https://doi.org/10.1080/09583150050115089 - 11. Robinson-Boyer, L., Jeger, M.J., Xiang-Ming, X. and Jeffries, P. (2009) Management of Strawberry Grey Mould Using Mixtures of Biocontrol Agents with Different Mechanisms of Action. Biocontrol Science and Technology, 19, 1051-1065.
https://doi.org/10.1080/09583150903289105 - 12. Card, S.D., Walter, M., Jaspers, M.V., Sztejnberg, A. and Stewart, A. (2009) Targeted Selection of Antagonistic Microorganisms for Control of Botrytis cinerea of Strawberry in New Zealand. Australasian Plant Pathology, 38, 183-192.
https://doi.org/10.1071/AP08097 - 13. Xiangming, X., Robinson, L., Jeger, M. and Jeffries, P. (2010) Using Combinations of Biocontrol Agents to Control Botrytis cinerea on Strawberry Leaves under Fluctuating Temperatures. Biocontrol Science and Technology, 20, 359-373.
https://doi.org/10.1080/09583150903528114 - 14. Helbig (2001) Biological Control of Botrytis cinerea Pers. ex Fr. in Strawberry by Paenibacillus polymyxa (Isolate 18191). Journal of Phytopathology, 149, 265-273.
- 15. Cota, L.V., Maffia, L.A., Mizubuti, E.S.G. and Macedo, P.E.F. (2009) Biological Control by Clonostachys rosea as a Key Component in the Integrated Management of Strawberry Gray Mold. Biological Control, 50, 222-230.
https://doi.org/10.1016/j.biocontrol.2009.04.017 - 16. Harman, G.E., Howell, C.R., Viterbo, A., Chet, I. and Lorito, M. (2004) Trichoderma Species-Opportunistic, Avirulent Plant Symbionts. Nature Review Microbiology, 2, 43-56.
https://doi.org/10.1038/nrmicro797 - 17. Barakat, R.M. and Al-Masri, M.I. (2009) Trichoderma harzianum in Combination with Sheep Manure Organic Amendment Enhances Soil Suppressiveness of Fusarium Wilt of Tomato. Phytopathologia Mediterranea, 48, 385-395.
- 18. Ospina-Giraldo, M.D., Royse, D.J., Chen, X. and Romaine, C.P. (1999) Molecular Phylogenetic Analyses of Biological Control Strains of Trichoderma harzianum and Other Biotypes of Trichoderma spp. Associated with Mushroom Green Mold. Phytopathology, 89, 308-313.
https://doi.org/10.1094/PHYTO.1999.89.4.308 - 19. Khirallah, W., Mouden, N., Selmaoui, K., Achbani, E., Benkirane, R., Touhami, A.O. and Douira, A. (2016) Compatibility of Trichoderma spp. with Some Fungicides under in Vitro Conditions. International Journal of Recent Scientific Research, 7, 9060-9067.
- 20. Hilber, W. and Hilber-Bodmer, M. (1998) Genetic Basis and Monitoring of Resistance of Botryotinia fuckeliana to Anilinopyrimidines. Plant Disease, 82, 495-500.
https://doi.org/10.1094/PDIS.1998.82.5.496 - 21. Yuan, S., Zuo, G. and Chen, H. (2007) Studies on the Induction of Biocontrol Drug-Resistance Factor from Trichoderma and Its Biochemical Characteristics. Journal of Anhui Agricultural Science, 24, 7523-7524.
- 22. Menzel, C.M., Gomez, A. and Smith, L.A. (2016) Control of Grey Mould and Stem-End Rot in Strawberry Plants Growing in a Subtropical Environment. Australian Plant Pathology, 45, 489-498.
https://doi.org/10.1007/s13313-016-0440-5 - 23. Rosslenbroich, H.J. and Stuebler, D. (2000) Botrytis cinerea—History of Chemical Control and Novel Fungicides for Its Management. Crop Protection, 19, 557-561.
https://doi.org/10.1016/S0261-2194(00)00072-7 - 24. Navaneetha, T., Prasad, R.D. and Venkateswar, R.L. (2015) Liquid Formulation of Trichoderma Species for Management og Grey Mould in Castor (Ricinus communis L.) and Alternariaster Leaf Blight in Sunflower (Helianthus annuus L.). Journal of Biofertilizers and Biopesticides, 6, 149.
- 25. Rajkovic, S., Markovic, M., Rajkovic, R. and Rakonjac, L. (2013) Biofungicide Trichodex WP. International Journal of Biological, Biomolecular, Agricultural, Food and Biotechnological Engineering, 7, 449-452.
Appendix
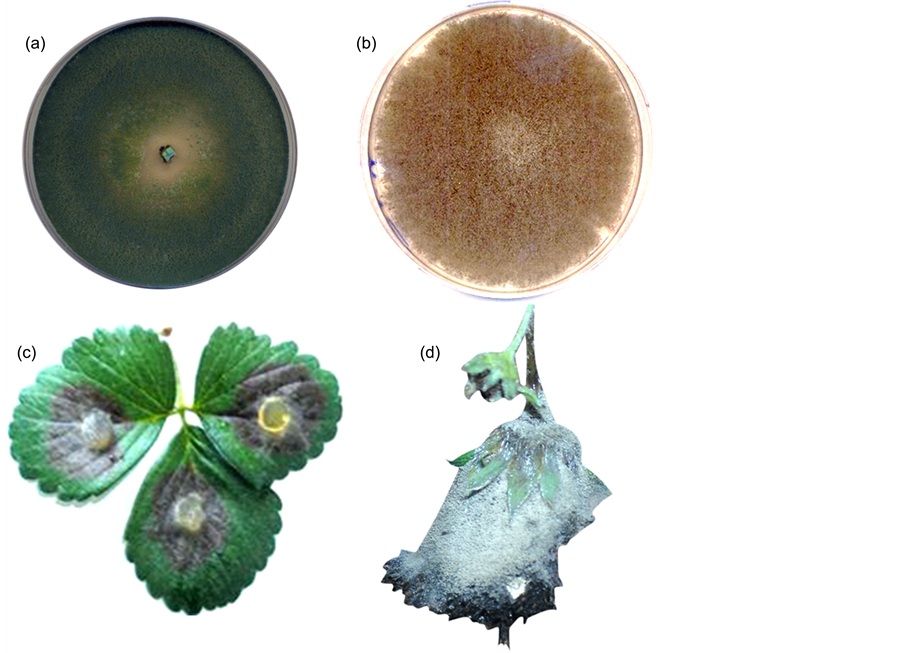
(a): Culture of Trichoderma harzianum (Jn14), and (b): culture of Botrytis cinerea (Bc1) grown on potato dextrose agar medium. (c) + (d): Symptoms of gray mould disease on strawberry detached leaf (c) and on fruit (d)

Submit or recommend next manuscript to SCIRP and we will provide best service for you:
Accepting pre-submission inquiries through Email, Facebook, LinkedIn, Twitter, etc.
A wide selection of journals (inclusive of 9 subjects, more than 200 journals)
Providing 24-hour high-quality service
User-friendly online submission system
Fair and swift peer-review system
Efficient typesetting and proofreading procedure
Display of the result of downloads and visits, as well as the number of cited articles
Maximum dissemination of your research work
Submit your manuscript at: http://papersubmission.scirp.org/
Or contact ajps@scirp.org


