American Journal of Plant Sciences
Vol.3 No.5(2012), Article ID:19474,4 pages DOI:10.4236/ajps.2012.35068
Photoperiod Affects in Vitro Flowering in Wild Peanut Arachis paraguariensis
![]()
Department of Agronomy, University of Florida, Gainesville, USA.
Email: ainab@ufl.edu
Received March 5th, 2012; revised March 22nd, 2012; accepted April 12th, 2012
Keywords: Flower; Tissue Culture; Day-Length; Photoperiod; Peanut; Arachis; In Vitro
ABSTRACT
Arachis paraguariensis, a wild peanut species, is a potential experimental system for studying the molecular mechanisms of flowering in the genus Arachis. The present study was carried out to investigate the effect of photoperiod on in vitro reproductive behavior of five genotypes of A. paraguariensis. Day-lengths of 12, 16 and 24 h were tested to monitor in vitro flowering using growth chambers kept at 26˚C ± 1˚C and 60% ± 5% relative humidity under an illumination of 40 μmol·m–2·s–1. Flowering percentage of plantlets ranged from 35% to 93%, 20% to 75%, and 5% to 53% for 12, 16 and 24 h day-lengths, respectively. Genotype PI 262842 displayed the highest frequency of flowering under all the day-length treatments but in vitro flower bud initiation was delayed. The highest mean flowering percentage of 65% across all the genotypes for plantlets exposed to 12 h photoperiod is indicative that flowering induction actually occurred. The results presented in this paper provide evidence for photoperiodic flowering response as well as the occurrence of short day-length-enhanced flowering in A. paraguariensis.
1. Introduction
Several wild Arachis species constitute important sources of novel genes for improving cultivated peanut (Arachis hypogaea L.). Of significant importance is Arachis paraguariensis Chodat & Hassl., a perennial wild relative of the peanut having novel traits including disease [1,2], insect [3], and nematode [4] resistance. Low fruiting efficiency due to asynchronous flowering and pod formation is a persistent problem within the genus Arachis that often results in considerable seed-yield losses and low germination rates [5,6]. Although photoperiod sensitivity within the genus Arachis is widely reported [7,8], the specific role of environmental factors in the regulation of its reproductive performance is poorly understood. For example, results from field studies focusing on the effects of photoperiod on flowering in cultivated peanut were inconsistent due to differential responses among different cultivars [9,10].
Gaining a proper understanding of the molecular basis for flowering mechanisms should enable the plant breeder to efficiently manipulate reproduction processes for achieving significant impacts on yield and other important traits. In vitro flowering, a common phenomenon in several plant species offers an ideal experimental system for studying these mechanisms. Additionally, tissue culture techniques such as in vitro fertilization, peg culture and embryo rescue can be explored as a means of overcoming the long existing hybridization barriers between cultivated peanut and several of its wild relatives.
In vitro tissue explants for plant regeneration are grown on nutrient medium inside confined vessels; therefore, precise control of all environmental factors is expected. Nevertheless, seasonal effects of explant collection and culture initiation on plantlet regeneration have been reported for many species [11-15]. Furthermore, different cytokinins, sucrose concentrations, photoperiod, and subculture time have been used to promote in vitro flowering [4,11]. Flowering in vitro has been observed in a few Arachis spp., however, the photoperiodic flowering response in tissue culture is relatively rare and poorly understood. Still et al. [16] and Li et al. [17] reported the occurrence of flowering in tissue culture of A. paraguariensis, but the environmental and genetic factors involved were not investigated. In this study, a factorial experiment in a completely randomized design with sub-sampling was replicated two times to investigate the influence of photoperiod on in vitro flowering of five genotypes of A. paraguariensis.
2. Materials and Methods
2.1. Explant Source
Seeds of five genotypes of A. paraguariensis used for this study were obtained from the USDA Plant Genetic Resources Conservation Unit, Griffin Georgia, a division of the Germplasm Resources Information Network (GRIN) National Plant Germplasm System (NPGS). Table 1 presents the Plant Introduction (PI) numbers and origin of the genotypes. Seeds were shelled manually before storage at 5˚C.
2.2. Initiation of Tissue Cultures
Quartered-seed explants of A. paraguariensis were derived from whole seeds after surface-sterilization in 70% ethanol for 1 min followed by immersion in 0.1% mercuric chloride for 10 min. The sterilized explants were then rinsed six times in sterile distilled water before they were implanted on MS [18] medium (Sigma # M5524) supplemented with 3% (w/v) sucrose (Sigma # S5390), 0.8% (w/v) agar (Sigma # A7921), 2.2 mg·L–1 thidiazuron (TDZ), and 4.4 mg·L–1 6-benzylaminopurine (BAP). Vitamins were added according to Gamborg B5 medium [19]. As a routine, 1 mL–1 of Plant Preservative Mixture (PPMTM, Plant Cell Technology, Washington DC, USA) was added to the culture medium before the pH was adjusted to 5.8 using 0.1 N KOH. Medium was autoclaved at 121˚C with 1.06 kg·cm–2 pressure for 20 min.
Stock solutions of TDZ and BAP were sterilized through a double 0.2 µm filter and added to the autoclaved medium. Each 2.5 × 10 cm petri dish containing 4 explants on 25 ml semisolid medium were sealed with Parafilm® bands and maintained at 26˚C ± 1˚C and 60% ± 5% relative humidity under continuous lightning in a growth chamber with illumination of 40 µmol·m–2·s–1 provided by daylight-type florescent lamps. After 10 d, regenerated micro-shoots (1 - 2 cm height) were excised from explant-callus masses and sub-cultured into 11.4 cm × 86 cm × 102 cm PhytatrayTM culture vessels (Sigma # P5929) containing 50 ml of MS medium with 3% sucrose (w/v), 0.8% (w/v) agar and no growth regulators. The experiment was a 5 (genotypes) × 3 (photoperiod) factorial experiment in a completely randomized design with sub-sampling, and was replicated twice in time.
2.3. Day Length Treatments
In order to determine the influence of photoperiod on
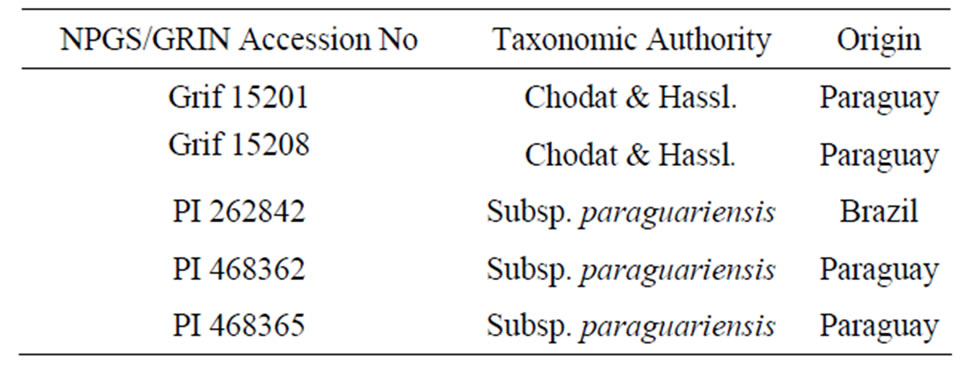
Table 1. The plant introduction (PI) numbers and origin of five selected genotypes of A. paraguariensis.
growth and flowering of A. paraguariensis plantlets, day-lengths of 16, 20 and 24 h were tested to monitor in vitro flowering in three growth chambers kept at 26˚C ± 1˚C and 60% ± 5% relative humidity under an illumination of 40 μmol·m–2·s–1 provided by cool-white fluorescent light.
For each day length treatment, culture initiation and flowering response were recorded for 10 vessels containing a sub-sample of 4 plantlets each per replicate. From the appearance of the first flower bud until when plantlets ceased to flower, cultures were evaluated in terms of percent flowering, weeks to flowering, and percent peg formation. The plantlets were later transplanted to jiffy pellets and acclimatized before being planted into pots filled with Metro-mix 300 and sand in a ratio of 1:1 (v/v) in the greenhouse.
2.4. Statistical Analysis
Analysis of data was performed according to a mixed effects model using SAS PROC MIXED [20]. Arcsine transformation was carried out on flowering frequency data before analysis while mean separation for days to flower bud initiation was performed using the Tukey’s Honestly Significant Difference Test (P = 0.05). Genotype and photoperiod were treated as fixed effects while replicate and the interactions were analyzed as random effects.
3. Results and Discussion
An in vitro flowering system is a convenient tool to study specific aspects of flowering, and floral organ development [21]. Certainly, the developmental decision to flower in plants is highly dependent on environmental and endogenous clues. Bünning [22] first proposed that the perception of day-length in plants was influenced by the interaction of light with a circadian rhythm, but innovative studies focusing on flowering in Arabidopsis have now provided genetic evidence for the role of the circadian clock in photoperiodic regulation of flowering. The FLOWERING LOCUS T (FT) gene, a major floral inducer in several species, interacts with diverse environmental signals to enable flowering and other developmental processes to be seasonally timed [23-25].
This study was carried out in the years 2009 and 2010 with the aim of investigating the influence of different day-length treatments on in vitro flower formation in five selected genotypes of A. paraguariensis. Observations from previous experiments [26] had proved that the five genotypes chosen for this study possessed a high frequency of regeneration, rooting and post acclimatization survival. Minimal callus formation was observed on explants after 24 h of culture initiation while shoot bud emergence began as early as 5 d afterwards. Most of the micro-shoots sub-cultured onto MS medium lacking growth regulators developed rapidly into plantlets within three wk. High frequency flowering was observed for all the genotypes that were studied (Figure 1(a)). The in vitro flowers appeared normal and were each borne on an erect pedicel as shown in Figure 1(b). Therefore, a pollen germination study was conducted to assess the fertility of in vitro derived plantlets and all the pollen samples tested germinated (data not shown). Additionally, further confirmation of a plantlet’s fertility was the occurrence of in vitro peg formation (Figure 1(c)) observed for genotype Grif 15201 while ex-vitro seed production (Figure 1(d)) was recorded for every plantlets generated across all the genotypes.
A remarkable observation during this study was that most of the plantlets failed to produce flowers during the last quarter of the year, but the regeneration frequency of explants was comparable from one season to another throughout the study. Hence, the scanty data obtained during those months were excluded from the results reported in this paper. It is important to note that the growth chambers used for the experiments were obtained from the same manufacturer and were equipped with digital sensors that offer stringent control over growth conditions for temperature, humidity, light intensity, and photoperiod. Several reports on seasonally dependent tissue culture responses have focused on the period of explant collection and culture initiation [27-29]. The storage duration for the seeds used for deriving the explants in this study varied, thus a possible explanation for the seasonal variation that was observed.
The flowering response of five A. paraguariensis genotypes as influenced by day-length treatment is presented in Table 2. The mean treatment effects across the genotypes are also displayed. In vitro flowering was recorded for all genotypes and every day length. The high-

Figure 1. (a) High frequency in vitro flowering observed in A. paraguariensis; (b) Normal flower borne on an erect pedicel with viable pollen; (c) In vitro peg formation observed for genotype Grif 15201; (d) Regenerated plantlets with pod formation after transplanting to pots in the greenhouse.
est flowering percentage of 65% recorded across all the genotypes for plantlets exposed to 12 h day-length is indicative that flowering induction actually occurred. Regression analysis revealed that reducing the day length treatment from 24 to 12 h resulted in a significant linear increase (P < 0.05, R2 = 0.92) in flowering frequency across all the genotypes.
Although flowering percentage differed among the five genotypes, all of them, except Grif 15208, showed increased reproductive response under reduced day length. The flowering percentage for 12 h, 16 h and 24 h day length treatment ranged from 35% to 93%, 20% to 75%, and from 5% to 53% respectively. The time taken for plantlets to initiate flower buds across all day lengths is shown in Figure 2. Flower bud initiation was relatively delayed in genotype PI 262842 even though this genotype gave the highest percentage of flowering across all the day lengths (Table 2). Interestingly, the time taken
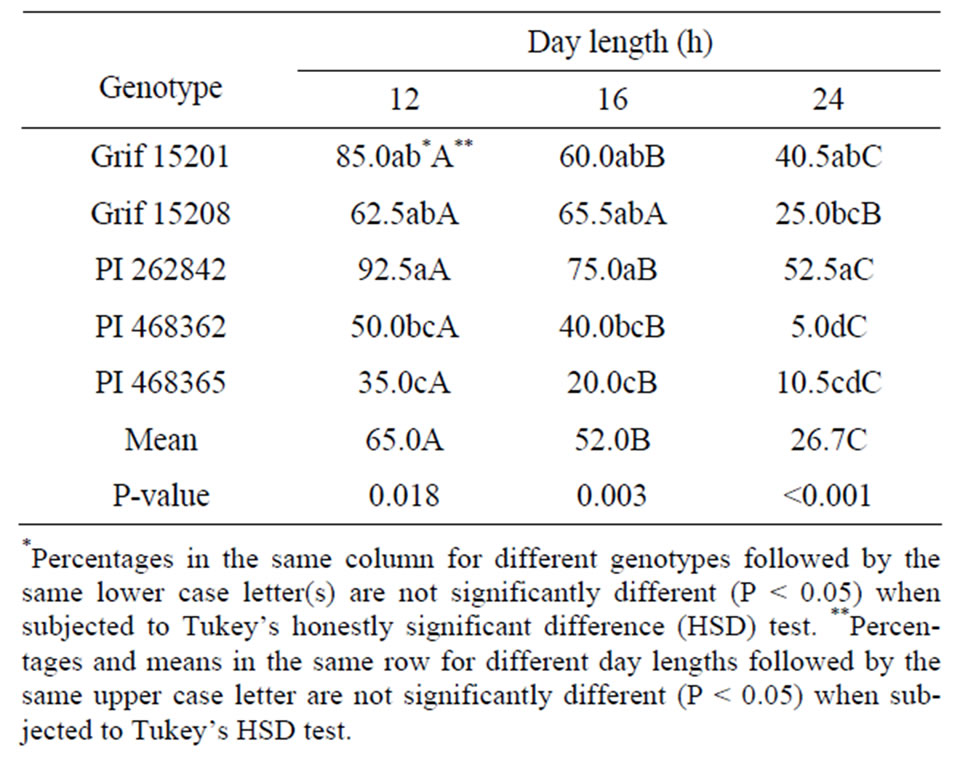
Table 2. The effects of photoperiod of in vitro flowering in five genotypes of A. paraguariensis.
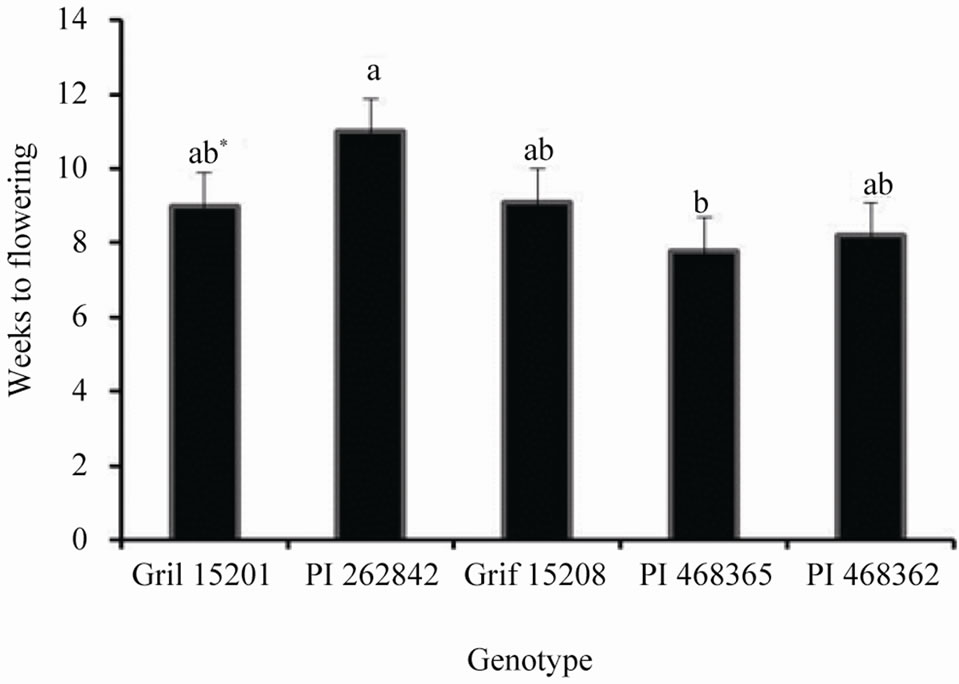
Figure 2. The time taken for in vitro plantlets to initiate flower buds across all day length treatments.
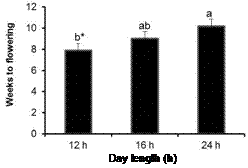
Figure 3. The time taken for in vitro plantlets to initiate flower buds across the five genotypes as affected by three day length treatments.
for in vitro plantlets to initiate flower buds across the five genotypes varied with the plantlets exposed to 12 h daylength flowering earlier than those incubated under the 24 h day-length (Figure 3).
Summerfield and Roberts [30] explained the difficulty involved in defining the exact day-length response of Arachis species since the interactions between environmental factors in many experiments were not clearly defined. Bagnall and King [31,32] later proposed that peanut should be classified as a short day plant because flowering was significantly enhanced during short-day photo periods. Thus far, many authors [33-35] continue to refer to species in the genus Arachis as day neutral plants with respect to their reproductive performance. The results presented in this paper agree with other field studies [31-33] indicating short day-length-induced flowering in Arachis.
4. Conclusion
The results from this study provide evidence of a photoperiodic reproductive response of tissue culture derived plantlets from five genotypes of A. paraguariensis. While in vitro flowering response differ among the genotypes, the highest flowering and peg formation across all the genotypes occurred under short day-length of 12 h. Hence, the observed short day-length-induced flowering should be considered while utilizing A. paraguariensis as a model plant for studying flowering mechanisms. Future work should focus on exposing plantlets to shorter daylengths as well as investigating the effect of light qualities on in vitro flowering in Arachis species.
5. Acknowledgements
This research was supported in part by a USDACSREES special grant for Tropical and Sub-Tropical Agricultural Research (TSTAR) # 2006 34135 17664 entitled Arachis and Desmodium for Forage and Conservation Use in the Sub-Tropics and Tropics. The authors express their appreciation to Dr. Roy Pittman, USDANPGS Plant Genetic Resource and Conservation Unit, Griffin, GA for providing seeds of A. paraguariensis.
REFERENCES
- P. Subrahmanyam, J. P. Moss, D. McDonald, P. V. Rao and V. R. Rao, “Resistance to Cercosporidium personatum Leafspot in Wild Arachis Species,” Plant Disease, Vol. 69, 1985, pp. 951-954. doi:10.1094/pd-69-948
- P. Subrahmanyam, R. A. Naidu, L. J. Reddy, P. L. Kumar and M. Ferguson, “Resistance to Groundnut Rosette Disease in Wild Arachis Species,” Annuals of Applied Biology, Vol. 139, No. 1, 2001, pp. 45-50. doi:10.1111/j.1744-7348.2001.tb00129.x
- P. C. Stevenson, J. C. Anderson, W. M. Blaney and M. S. J. Simmonds, “Developmental Inhibition of Spodoptera litura (Fab.) Larvae by a Novel Caffeoylquinic Acid from the Wild Groundnut, Arachis paraguariensis (Chodat & Hassl.,” Journal of Chemical Ecology, Vol. 19, No. 12, 1993, pp. 2917-2933. doi:10.1007/BF00980592
- S. B. Sharma, L. J. Reddy, P. J. Bramel and M. A. Ansari, “Resistance to Meloidogyne javanica race 3 in the Arachis Gene Pool,” Nematol Mediate, Vol. 30, 2002, pp. 221-225.
- S. B. Narasimhulu and G. M. Reddy, “In Vitro Flowering and Pod Formation from Cotyledons of Groundnut (Arachis hypogaea L.),” Theoretical and Applied Genetic, Vol. 69, No. 1, 1984, pp. 87-91. doi:10.1007/BF00262546
- P. C. Nautiyal, J. B. Misra and P. V. Zala, “Influence of Seed Maturity Stages on Germinability and Seedling Vigor in Groundnut,” Journal of SAT Agricultural Research, Vol. 8, 2010, pp. 1-10.
- P. A. Wigge, “FT, a Mobile Developmental Signal in Plants,” Current Biology, Vol. 21, 2011, pp. R374-R378. doi:10.1016/j.cub.2011.03.038
- M. J. Williams, T. R. Sinclair, P. Mislevy, K. H. Quesenberry and A. S. Blount, “Photoperiod Sensitivity of Rhizoma Peanut Germplasm,” Agronomy Journal, Vol. 100, No. 5, 2008, pp. 1366-1370. doi:10.2134/agronj2007.0113
- H. T. Stalker and J. C. Wynne, “Photoperiodic Response of Peanut Species,” Peanut Science, Vol. 10, No. 2, 1983, pp. 59-62. doi:10.3146/i0095-3679-10-2-4
- J. C. Wynne and D. A. Emery, “Response of Intersubspecific Peanut Hybrids to Photoperiod,” Crop Science, Vol. 14, No. 6, 1974, pp. 878-880. doi:10.2135/cropsci1974.0011183X001400060031x
- H. N. Vu, P. H. Anh and D. T. Nhut, “The Role of Sucrose and Different Cytokinins in the In Vitro Floral Morphogenesis of Rose (Hybrid Tea) cv. ‘First Prize’,” Plant Cell, Tissue and Organ Culture, Vol. 87 No. 3, 2006, pp. 315-320. doi:10.1007/s11240-006-9089-z
- V. K. Sharma, R. Hänsch, R. R. Mendel and J. Schulze, “Seasonal Effect on Tissue Culture Response and Plant Regeneration Frequency from Non-Bombarded and Bombarded Immature Scutella of Barley (Hordeum Vulgare L.) Harvested from Controlled Environment,” Plant Cell, Tissue and Organ Culture, Vol. 81, No. 1, 2005, pp. 19-26. doi:10.1007/s11240-004-2617-9
- A. Hohtola, “Seasonal Changes in Explant Viability and Contamination of Tissue Cultures from Mature Scots Pine,” Plant Cell, Tissue and Organ Culture, Vol. 15, No. 3, 1988, pp. 211-222. doi:10.1007/BF00033645
- R. Angrish and K. K. Nanda, “Seasonal Culture of Dormant Reproductive Buds of Salix Tetrasperma: Analysis of the Flowering Process,” Plant Cell, Tissue and Organ Culture, Vol. 1, No. 1, 1982, pp. 181-193. doi:10.1007/BF02318915:10.1007/s11240-004-2617-9
- P. K. Roy, A. N. K. Mamun and G. Ahmed, “In Vitro Plantlet Regeneration of Rose,” Plant Tissue Culture, Vol. 14, No. 2, 2004, pp. 149-154
- P. E. Still, M. I. Plata, R. J. Campbell, L. C. Bueno, E. A. Chichester and C. L. Niblett, “Regeneration of Fertile Arachis paraguariensis Plants From Callus and Suspension Cultures,” Plant Cell, Tissue and Organ Culture, Vol. 9, No. 1, 1987, pp. 37-43. doi:10.1007/BF00046077
- Z. Li, R. L. Jarret, R. N. Pittman, K. B. Dunbar and J. W. Demski, “Efficient Plant Regeneration from Protoplasts of Arachis paraguariensis Chodat and Hassl. Using a Nurse Culture Method,” Plant Cell, Tissue and Organ Culture, Vol. 34, No. 1993, pp. 83-90. doi:10.1007/BF00048467
- T. Murashige and F. Skoog, “A Revised Medium for Rapid Growth and Bioassays with Tobacco Tissue Cultures,” Plant Physiology, Vol. 15, No. 3, 1962, pp. 473- 497.
- O. Gamborg, R. Miller and K. Ojima, “Nutrient Requirement of Suspensions Cultures of Soybean Root Cells,” Experimental Cell Research, Vol. 50, No. 1, 1968, pp. 151-158. doi:10.1016/0014-4827(68)90403-5
- SAS Institute Inc, “Variance Estimation: SAS/STAT(R) 9.22 User’s Guide,” Cary, 2010.
- G. Y. Wang, M. F. Yuan and Y. Hong, “In Vitro Flower Induction in Roses,” In Vitro Cellular & Developmental Biology—Plant, Vol. 38, No. 5, 2002, pp. 513-518. doi:10.1079/IVP2002340
- E. Bünning, “Die Endogene Tagesrhythmik Als Grundlage Der Photoperiodischen Reaktion, Berichte der Deutschen Botanischen,” Gesellschaft, Vol. 54, 1936, pp. 590- 607.
- T. Imaizumi, “Arabidopsis Circadian Clock and Photoperiodism: Time to Think about Location,” Current Opinion in Plant Biology, Vol. 13, No. 1, 2010, pp. 83-89. 10.1016/j.pbi.2009.09.007
- R. Amasino, “Seasonal and Developmental Timing of Flowering,” The Plant Journal, Vol. 61, No. 6, 2010, pp. 1001-1013. doi:10.1111/j.1365-313X.2010.04148.x
- S. N. Nigam and R. Aruna, “Improving Breeding Efficiency for Early Maturity in Peanut,” In: J. Janick, Ed., Plant Breeding Reviews, Vol. 30, John Wiley and Sons, Inc., Hoboken, 2008, pp. 295-322. doi:10.1002/9780470380130.ch6
- O. O. Aina, K. H. Quesenberry and M. Gallo, “Thidiazuron Induced Tissue Culture Regeneration in Wild Peanut Arachis paraguariensis,” Crop Science, Vol. 52, No. 3, 2012, pp. 1076-1083. doi:10.2135/cropsci2011.07.0367
- R. Kumar, K. Sharma and V. AgrawalIn, “In Vitro Clonal Propagation of Holarrhena antidysenterica (L.) Wall. Through Nodal Explants from Mature Trees,” In Vitro Cellular & Developmental Biology—Plant, Vol. 41, No. 2, 2005, pp. 137-144. doi:10.1079/IVP2004624
- M. Doctrinal, R. S. Sangwan and B. S. Sangwan-Norreel, “In Vitro Gynogenesis in Beta Vulgaris L.: Effects of Plant Growth Regulators, Temperature, Genotypes and Season,” Plant Cell, Tissue and Organ Culture, Vol. 17, No. 1, 1989, pp. 1-12. doi: 10.1007/BF00042276
- A. Ritala, L. Mannonen and M. Oksman-Caldentey, “Factors Affecting the Regeneration Capacity of Isolated Barley Microspores (Hordeum vulgare L.),” Plant Cell Report, Vol. 20, No. 5, 2001, pp. 403-407. doi:10.1007/s002990100345
- R. J. Summerfield and E. H. Roberts, “Arachis hypogaea,” In: A. H. Halevy, Ed., CRC Handbook of Flowering, Vol. 1, CRC Press, Boca Raton, 1985, pp. 74-82.
- D. J. Bagnall and R. W. King, “Response of Peanut (Arachis hypogaea) to Temperature, Photoperiod and Irradiance 1. Effect on Flowering,” Field Crops Research, Vol. 26, No. 3-4, 1991, pp. 263-277. doi:10.1016/0378-4290(91)90004-F
- D. J. Bagnall and R. W. King, “Response of Peanut (Arachis Hypogea) to Temperature, Photoperiod and Irradiance 2. Effect on Peg and Pod Development,” Field Crops Research, Vol. 26, No. 3-4, 1991, pp. 279-293. doi:10.1016/0378-4290(91)90005-G
- M.-L. Flohr, J. H. Williams and F. Lenz, “The Effect of Photoperiod on the Reproductive Development of a Photoperiod Sensitive Groundnut (Arachis hypogaea L.) Cv. NC Ac 17090,” Experimental Agriculture, Vol. 26, No. 4, 1990, pp 397-406. doi:10.1017/S0014479700001320
- A. H. Bunting and J. Elston, “Ecophysiology of Growth and Adaption in the Groundnut: An Essay on Structure, Partition and Adaptation,” In: R. J. Summerfield and A. H. Bunting, Eds., Advances in Legume Science, HMSO, London, 1980, pp. 495-500.
- S. K. Leong and C. K. Ong, “The Influence of Temperature and Soil Water Deficit on the Development and Morphology of the Groundnut (Arachis hypogaea L.),” Journal of Experimental Botany, Vol. 34, No. 11, 1983, pp. 1551-1561.

