American Journal of Plant Sciences
Vol.3 No.6(2012), Article ID:20016,5 pages DOI:10.4236/ajps.2012.36085
In Vitro Organogenesis of Colocasia esculenta cv. antiquorum L.
![]()
Bangladesh Agricultural Research Institute, Gazipur, Bangladesh.
Email: dr.jahangir2011@yahoo.com
Received November 9th, 2011; revised December 27th, 2011; accepted January 10th, 2012
Keywords: Organogenesis; Auxin; Cytokinin; Meristem; Parenchymatous Storage Tissue; In Vitro; Colocasia esculenta cv. antiquorum L
ABSTRACT
In vitro organogenesis of an upland species of Colocasia esculenta cv. antiquorum L. was examined in relation to different explants like meristem and parenchymatous storage tissues with or without anthocyanin layer, four levels of each of Kn, 2,4-D, NAA and BAP and four incubation environments such as: 1) 16 h 3 Kl light intensity + 24˚C ± 2˚C; 2) 24 h dark + 24˚C ± 2˚C; 3) 24 h dark + 30˚C ± 3˚C and 4) 12 h diffuse light + 30˚C ± 3˚C. Only meristems showed proliferation with various degree of intensity both at 16 h 3 Kl light + 24˚C ± 2˚C and 24 h dark + 24˚C ± 2˚C conditions and poor response with different levels of Kn + NAA either in light or in the dark. Cultures with NAA + BAP were proliferated very quickly with very high degree of intensity. The cultures under dark did not proliferate for 20 days which upon transfer to light showed high degree of proliferation. Cultures with NAA + BAP formed calluses more pronouncedly at dark than that occurred in the light. Parenchymatous tissues with or without anthocyanin did not proliferate but the tissues with anthocyanin lost pigmentation after 25 - 30 days and turned to grey colour after 50 days while tissues without anthocyanin turned to green colour with shinny pimples indicating that protocorm may be developed. No culture under high temperature environment (30˚C ± 3˚C) neither survived nor proliferated. The meristems in culture were died within 15 - 20 days while others within 25 - 30 days. In conclusion, a combination of NAA (0.5 - 3.0 mg/l) and BAP (0.5 - 2.0 mg/l) and an incubation photoperiod of 16 h coupled with temperature of 24˚C ± 2˚C were found most suitable for in vitro culture of Colocasia esculenta cv. antiquorum L.
1. Introduction
The upland species of Colocasia esculenta cv. antiquorum L. is locally known as “mukhikachu” growing in about 30,000 ha of land in Bangladesh. Nutritionally, this crop is highly rich, particularly, the leaf blade and leaf stalk. The storage organ is known as corm or cormel. The seeds are called corm and the cormels are usually used as vegetables, which may give delicious dishes. This is mainly a carbohydrate rich food containing high proportion of amylopectin [1].
The cormels are the propagating materials and are maintained through vegetative propagation in the field. The cormels are not usually stored in coldstores and thus maintained at ambient temperature (av. 27˚C) during May to August. Due to high temperature and high humidity during this period the cormels released from dormancy very shortly and as a result, its quality is deteriorated quickly. Moreover, there is no recommended storage technology. Thus, the maintenance of this crop is very difficult. Moreover, conventional maintenance of germplasm through field multiplication makes this crop always under natural risk of losing valuable germplasm. Tissue culture method could make a successful aseptic preservation.
Most of the taro species are contaminated with endogenous pathogens and thus it is difficult to obtain disease free planting materials, although disease free planting materials may be developed through repeated meristem culture [2,3]. Several workers used different levels of sucrose in order to prolong the subculture interval [3-5]. While development of in vitro organogenesis of plant was partially examined [6].
The present research was aimed at: to develop a complete protocol for in vitro developement of Colocasia esculenta cv. antiquorum L. covering source of explants, culture media and culture environment.
2. Materials and Methods
Well sprouted cormels of a upland taro sp. Colocasia esculenta cv. antiquorum L. were used. Three different parts such as meristem and parenchymatous storage tissues with and without anthocyanin layer were used as explants. The size of the excised meristem varied from 0.5 to 0.8 mm long, while parenchymatous storage tissues were cut into cubes shape weighed to about 300 - 350 mg.
The 20 - 30 g weighted cormels were scrabbed with scalpel very carefully to remove the outer layer, keeping the anthocyanin layer almost intact. The explants were first washed with 70% ethanol for three times (10 minutes each time) followed by 10 minutes soaked in 1.5% chlorine water with few drops of tween 20. The explants were then washed with distilled and autoclaved water for five times under clean hood [7].
Organic salts of MS media were used. Four different plant growth regulators each at four different levels such as, auxins: 1) NAA: 0.5, 1.0, 2.0 and 3.0 mg/l; 2) 2,4-D: 0.1, 1.0, 1.5 and 2.0 mg/l and cytokinins; 3) Kn: 0.1, 0.5, 1.0 and 1.5 mg/l; 4) BAP: 0.5, 1.0, 1.5 and 2.0 mg/l were used. Three culture media were prepared combining Kn + NAA, Kn + 2,4-D and NAA + BAP in all possible ways. There were 17 treatments in each group including the control (without growth regulators).
The cultures were incubated in four different environments such as 1) 3.0 Kl light intensity for 16 h and 24˚C ± 2˚C; 2) 24 h dark and 24˚C ± 2˚C; 3) 24 h dark and 30˚C ± 3˚C; 4) 12 h diffuse light and 30˚C ± 3˚C. Five cultures were included in each replication. The data were analysed using a CRD and mean separation was done by LSD.
3. Results and Discussion
Three types of explants were cultured on MS supplemented with different levels of Kn + NAA, Kn + 2,4-D and NAA + BAP at four different environments. It was observed that only excised meristems showed differentiation in most of the media composition.
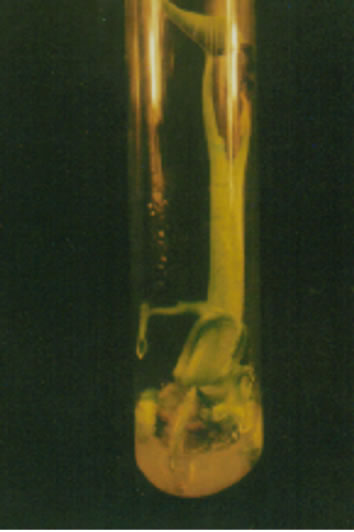
Parenchymatous tissues with anthocyanin layer after 25 - 30 days of culture lost pigmentation and after 50 days turned to grey colour while tissues without pigmentation turned to greenish with very shinny and watery pimples like structures, which is indicating that protocorms may be developed (Figure 1(a)).
The cultures which were incubated at either diffuse light or dark with 30˚C ± 3˚C did not survive or proliferate at all. The isolated meristems after 15 - 20 days became yellowish and ultimately died while the storage tissues on the other hand, changed colour from white to dull grey. Thus, it was assumed that this temperature range was not suitable at all for in vitro differentiation of Colocasia esculenta cv. antiquorum.
The excised meristems with MS+Kn+NAA both under light or dark and 24˚C ± 2˚C showed very poor response. Differentiation occurred randomly only in four treatments (Kn 1.0 + NAA 1.0, Kn 1.0 + NAA 2.0, Kn 1.5 + NAA 3.0 and the control) (Table 1) under dark condition and in six treatments (Kn 0.1 + NAA 1.0, Kn 0.5 + NAA 0.5, Kn 1.0 + NAA 0.5, Kn 1.5 + NAA 0.5, Kn 1.5 + NAA 3.0 and the control) (Table 2) under light condition. The reason for this random proliferation is quite obscure while it is clear from this result that proliferation of excised meristems of Colocasia esculenta cv. antiquorum may be occurred without PGR though control took much longer time for days to shooting and rooting (35 days). It was observed that plant shooting and rooting status, was much better in the control treatment, through their response delayed. None of the plantlet formed sucker (Figure 1(b)).
The excised meristems with Kn 1.0 + 2,4-D 1.0 onwards (Table 3) incubated under light and 24˚C ± 2˚C responded very well, though it took 28 - 40 days for differentiation. In the control it occurred within 26 days. No callus was formed though 2,4-D is a callus inducing hormone. Several workers obtained a very good callusing in Dioscorea alata L. using different levels of 2,4-D and BAP + NAA with MS and White’s media [8,9]. Similarly, new shoots first observed, except control (27 days), after 42 days at Kn 1.0 + 2,4-D 2.0. It was observed that
 (a)
(a) (b)
(b) (c)
(c)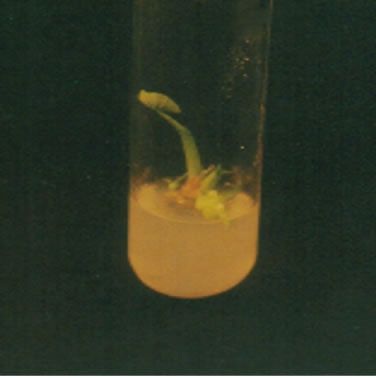 (d)
(d)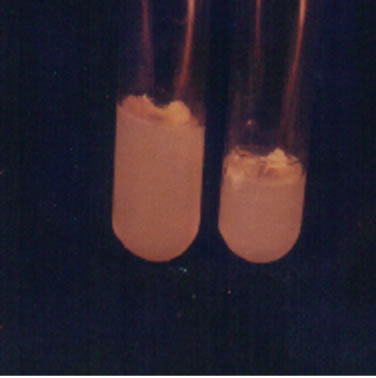 (e)
(e)
Figure 1. Different stages of in vitro development of Colocasia esculenta cv. antiquorum L., (a) Development of protocorm in callus; (b) None of the plantlets developed suckers; (c) Microplants showed robust with BAP plus NAA; (d) Microplants developed multiple shoots with BAP and NAA; and (e) Developed roots in microplants.
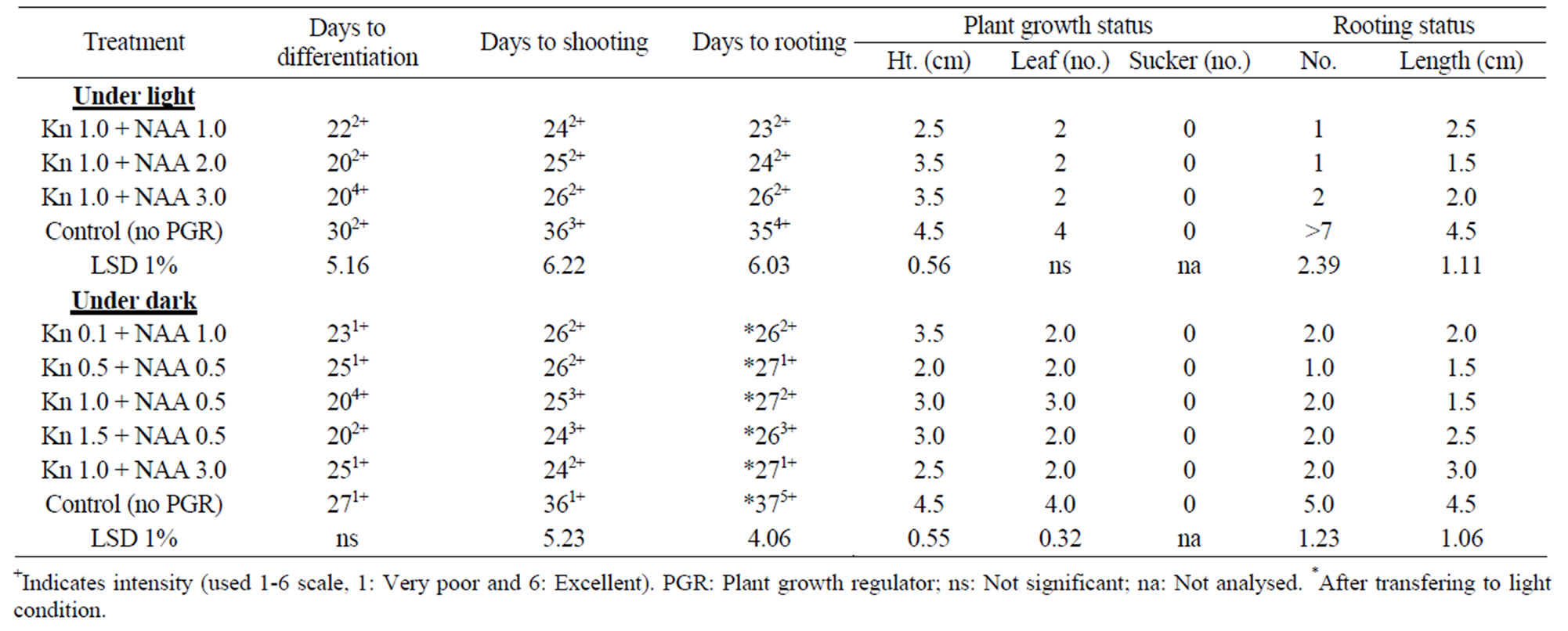
Table 1. In vitro morphogenesis of mukhikachu with meristem cultured on MS media with different combinations of Kn + NAA at 16 h 3 Kl light and at 24˚C ± 2˚C (only the effective treatment combinations of Kn + NAA are shown).
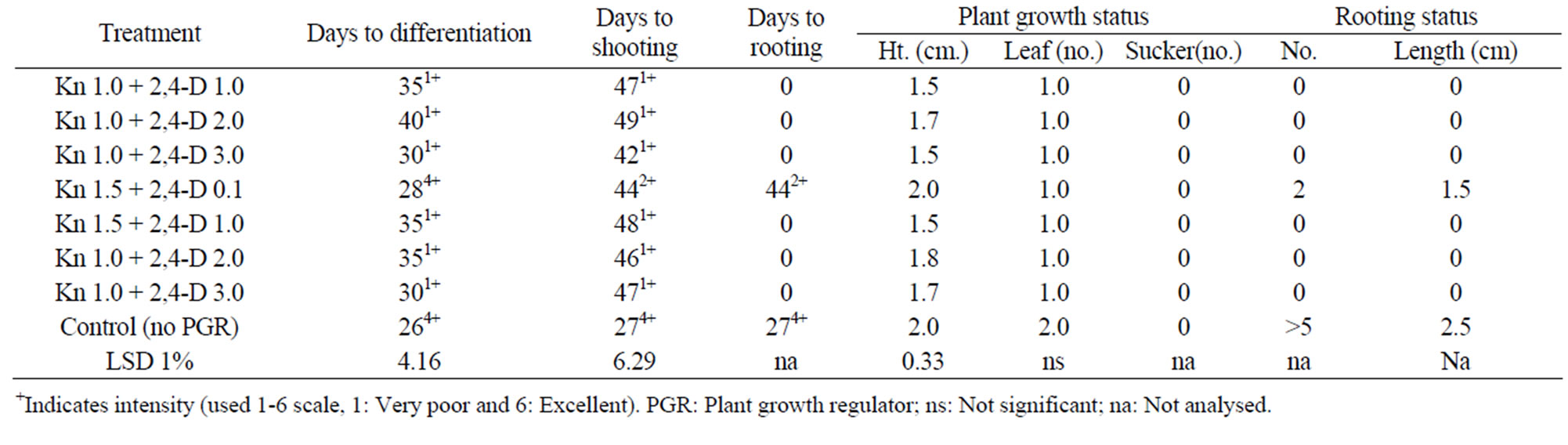
Table 2. In vitro morphogenesis of mukhikachu with meristem cultured on MS media with different combinations of Kn + 2,4-D at 16 h 3 Kl light and at 24˚C ± 2˚C (only the effective treatment combinations of kin and 2,4-D are shown).
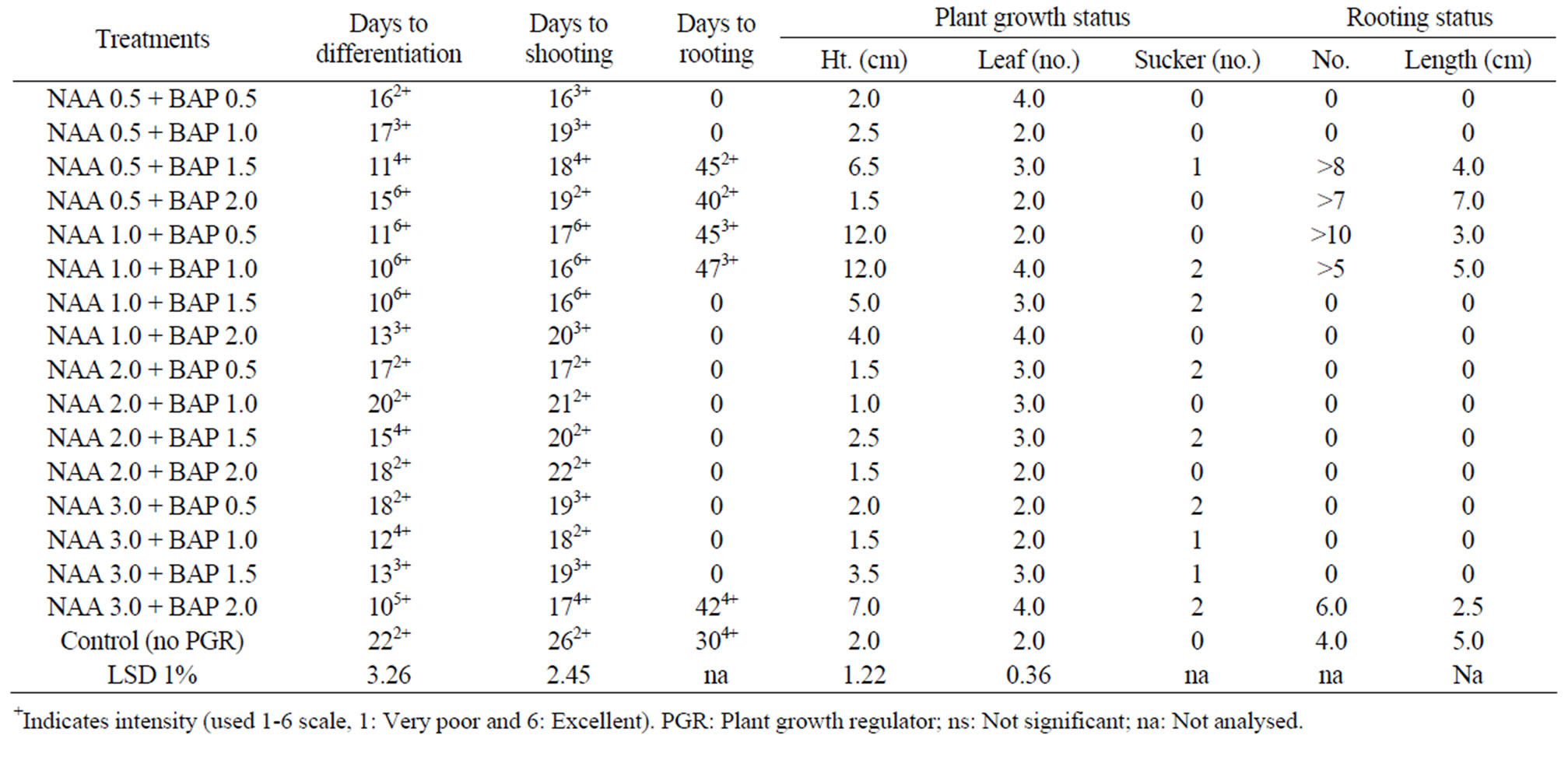
Table 3. In vitro morphogenesis of mukhi kachu with meristem cultured on MS media with different combinations of NAA + BAP at 16 h 3 Kl light and at 24˚C ± 2˚C.
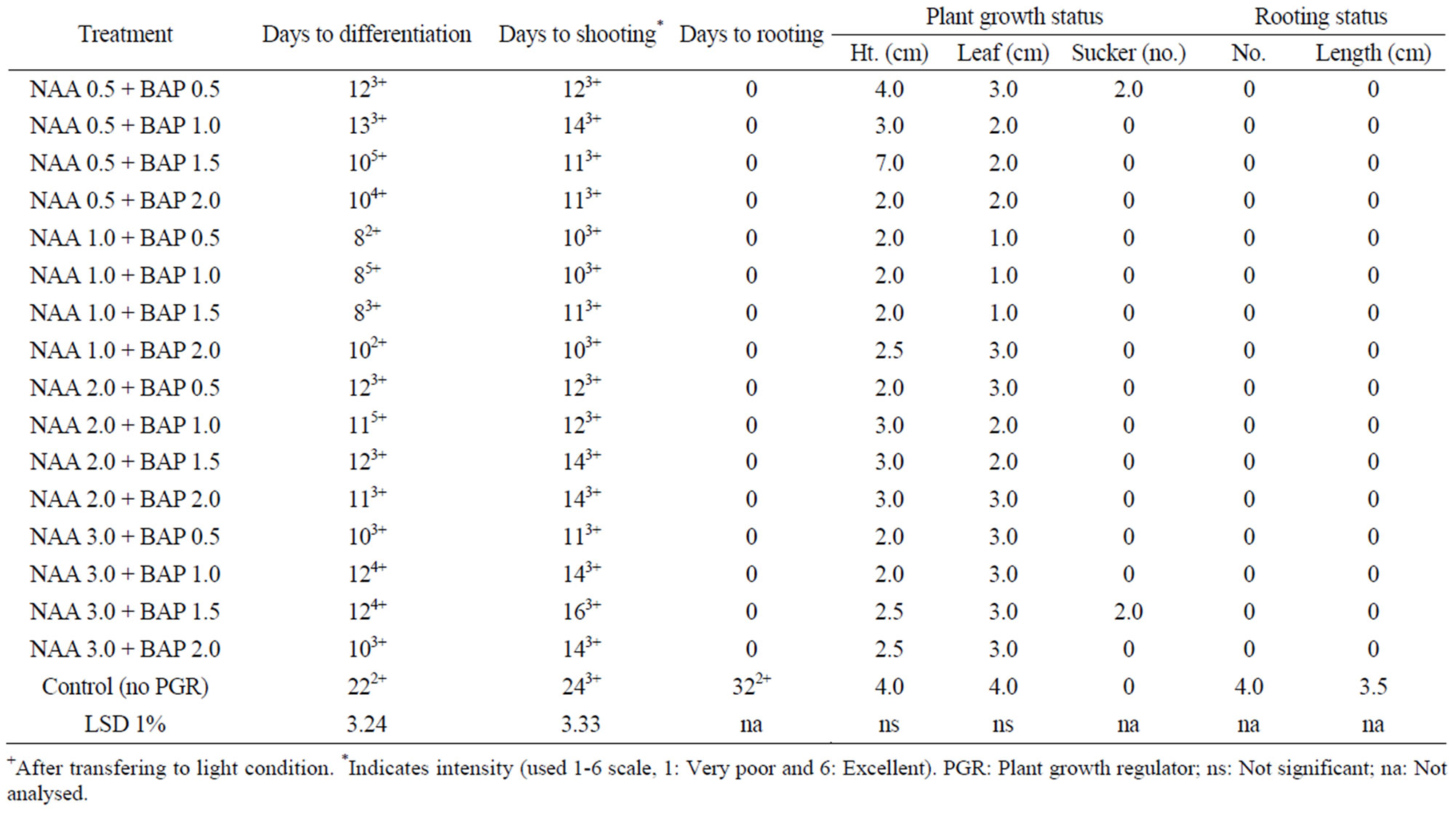
Table 4. In vitro morphogenesis of mukhi kachu with meristem cultured on MS media with different combinations of NAA + BAP at dark and at 24˚C ± 2˚C.
Kn + 2,4-D levels below 1.0 + 0.1 was ineffective. Moreover, Kin is a cytokinin group plant hormone enhanced plant cell elongation resulted in plant growth while 2,4-D is an auxin acts as mutagenic chemical, thus it seems Kn enhanced differentiation but due to presence of 2,4-D, it was delayed. Kn up to 0.5 mg/l was seemed to be nullified by 2,4-D and when Kn level increased to 1.0, it showed differentiation but delayed (35 days). Kn + 2,4-D combination had no effect on rooting, rather retarded because control treatment formed roots within 27 days. In a 50-day culture period, only one leaf was formed while in control it was 2, which seems to be related to days to differentiation. Profuse rooting was observed in the control treatment, having more than 5 roots per plant with an average length of 2.5 cm after 50 days of culture.
The excised meristem of Colocasia esculenta cv. antiquorum showed excellent response with the maximum intensity of growth under both light and dark condition and at 24˚C ± 2˚C (Figure 1(c)). In the light, differentiation started with the minimum of 10 days of incubation with NAA 1.0 + BAP 1.0 and NAA 1.0 + BAP 1.5 levels and in most cultures by 20 days while the control treatment took 26 days, having minimum intensity. The cultures with BAP + NAA combination developed multiple shoots (Figure 1(d)).
The growth of cultures was so vigorous with NAA + BAP combinations. Differentiation of shoot and root occurred almost simultaneously in most of the treatments except few, in which the variation was negligible. Rooting was negatively affected in twelve NAA + BAP combinations. Profuse rooting occurred in five treatments but much delayed (45 days after culture). The plants showed vigorous shoot growth with or without roots. The rooting status of NAA 0.5+BAP 1.5, NAA 0.5 + BAP 2.0, NAA 1.0 + BAP 0.5 and NAA 1.0 + BAP 1.0 was excellent (av. number was > 5 -> 10 and av. length was 3.0 - 7.0 cm) (Table 4) (Figure 1(e)). It was also good for the control treatment. Only a few treatments at random formed a good number of stolons, which showed a better growth rate than other parameters. The reasons for random differentiation is not clear.
The isolated meristems when incubated at dark differentiated very quickly with very good growth intensity for most of the treatments except a few including control. Differentiated meristems showed tendency of callusing and became depigmented due to absence of light which turned to normal upon transfer to 16 h light. The plant growth status was very good. The cultures in the dark also showed good response but after long duration of culture, stems and leaves became pale green as compared to deep green when cultured in the light. The plants became taller with small leaves as compared to the plants in the light. But none of the treatment formed root except control, which was indicative that NAA + BAP combination retarded root formation, which was pronounced under dark condition. Even stolon seldom formed.
REFERENCES
- M. M. Rashid, “Transferable Technologies in Tuber Crops,” Tuber Crops Research Centre, Bangladesh Agricultural Research Institute, Gazipur, 1991, pp. 1-14.
- R. D. Hartman, “Dasheen Mosaic Virus and Other Phytopathogens Eliminated from Caladium, Taro and Cocoyam by Cultures of Shoot Tips,” Phytopathology, Vol. 64, 1974, pp. 237-240. doi:10.1094/Phyto-64-237
- G. Staritsky, A. J. Dekkers, N. P. Louwaars and E. A. Zandvoort, “In Vitro Conservation of Aroid Germplasm at Reduced Temperatures and under Osmotic Stress,” In: L. A. Withers and P. G. Alderson, Eds., Plant Tissue Culture and Its Agricultural Applications, Butterworths, London, 1986, pp. 277-283.
- R. Pathirana, “Conservation of Plant Genetic Resources through in Vitro Methods,” In: A. H. Zakri, M. N. Normah, A. G. A. Karim and M. T. Senawi, Eds., Proceedings of the MNCPGR/CSC International Workshop on Tissue Culture for the Conservation of Biodiversity and Plant Genetic Resources, Lumpur, 28-31 May 1991, pp. 213-230.
- R. J. Westcott, G. G. Henshaw and W. M. Roca, “Tissue Culture of Potato Germplasm: Culture Iniatiation and Plant Regenaration,” Plant Science Letters, Vol. 9, No. 4, 1977, pp. 309-315. doi:10.1016/0304-4211(77)90101-8
- A. Y. Akhand, O. M. Islam and M. Ali, “In Vitro Conservation of Taro (Colocasia esculenta var. antiquorum L.) under Different Sucrose Levels,” Plant Tissue Culture, Vol. 7, 1997, pp. 81-88.
- M. J. Hossain, “In Vitro Node Culture of Potato (Solanum tuberosum L.),” Bangladesh Horticulture, Vol. 16, 1988, pp. 1-7.
- N. G. Nair and S. Chandrababu, “A Slow Growth Medium for in Vitro Conversation of Edible Yams,” Journal Root Crops, Vol. 20, 1994, pp. 68-69.
- V. Z. Acedo, A. Q. Villordon and E. S. Quevedo, “Callus Induction in Yam (Dioscorea alata L.),” Journal Root Crops, Vol. 20, 1994, pp. 64-67.

