Modern Research in Catalysis
Vol.04 No.01(2015), Article ID:53039,6 pages
10.4236/mrc.2015.41002
Transition Metal Doped MnOx-CeO2 Catalysts by Ultrasonic Immersing for Selective Catalytic Reduction of NO with NH3 at Low Temperature
Jinshuo Qiao1, Ning Wang1, Cuiya Zhuang1, Kening Sun2*
1Beijing Key Laboratory for Chemical Power Source and Green Catalysis, School of Chemical Engineering and the Environment, Beijing Institute of Technology, Beijing, China
2Academy of Fundamental and Interdisciplinary Sciences, Harbin Institute of Technology, Heilongjiang, China
Email: *keningsunhit@126.com, qjinshuo@bit.edu.cn
Copyright © 2015 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 21 October 2014; revised 22 November 2014; accepted 21 December 2014
ABSTRACT
Transition metals doped Mn-based catalysts were prepared via ultrasonic immersing method for the selective catalytic reduction (SCR) of NOx from fuel gas. The Catalysts’ DeNOx efficiency and tolerance to sulfur were investigated in the paper. XRD results demonstrate high dispersion of Mn, Ce and M (Pr, Y, Zr, W) elements on TiO2 carrier, which is favor for reduction of active materials content. Mn-Ce-W catalyst presents uniform particle size about 500 nm to 800 nm from SEM pictures and shows the best NOx conversion of 93.2% at 200˚C and 98.4% at 250˚C, respectively. Sulfur tolerance analysis indicated that transition metals M can improve the catalysts’ performance when 0.01% SO2 exists in the fuel gas, because metal doping into the Mn-Ce catalyst can inhibit the sulfate deposition, especially metal sulfate, on the catalyst, which can be seen from the Fourier in- frared spectrum.
Keywords:
Mn-Based Catalysts, Ultrasonic Immersing Method, Selective Catalytic Reduction, Sulfur Tolerance

1. Introduction
Strict control of NOx emission is urgently needed due to the increasing effect on the environment from urban smog, ozone depletion and greenhouse. The selective catalytic reduction (SCR) technology with NH3 is considered as the most effective method to remove NOx in flues gases. The SCR catalysts have been developed in the past decades and Vanadium-based catalyst is the commercial one for application at temperature 300˚C - 400˚C due to its high DeNOx efficiency and good sulfur-resistance. However, these catalysts show the toxicity of raw materials and high cost, which limit their extensive applications. So developing catalysts working less than 300˚C is of interest due to their possible application in downstream after desulfurizer and electrostatic. Various catalysts have been investigated at low temperature such as Manganese oxides (MnOx) supported on Al2O3, TiO2 and SiO2. The results show that MnOx is a kind of promising catalyst applied at low temperature. At the same time, ceria is found to increase the oxygen storage capability of MnOx and enhance the migration rate of oxygen. In present, MnOx-CeO2 catalysts have been demonstrated possessing the high NOx removal efficiencies [1] - [4] . However, MnOx-CeO2 catalysts have no ideal sulfur-resistance with a long time, especially under the co-existence of H2O and SO2 [5] [6] .
In this work, transition metals will be doped into Mn-based catalysts to improve the catalyst’s tolerance to sulfur. Then ultrasonic immersing method was used to prepare catalysts, which apparently reduce the preparation period comparing to sol-gel method. The transition metals effect on DeNOx efficiency and sulfur tolerance of catalysts was discussed in details as following.
2. Experimental Section
2.1. Catalyst Synthesis
The TiO2 powder (Industrial grade, 96% purity, 15 - 25 nm, 93.9 m2・g−1) was dried at 105˚C for 1 h and milled for 150 mesh. Manganese nitrate, cerium nitrate and nitrate of transition metal (M) (Sinopharm Chemical Reagent Co.) at a certain ratio are solved into Deionized water (DW). Then, the obtained solution was placed into the ultrasonic reaction pool. The processed TiO2 powder was added into the solution when the metal nitrate dissolved fully under the stirring condition with 160 W ultrasonic at 50˚C for 24 h. Then the immersed TiO2 powder was dried at 105˚C for 24 h, calcined at 450˚C for 6 h and milled for 150 mesh to obtain catalysts powder. The obtained catalyst was expressed as Mn-Ce-M0.025, and M represent W, La, Pr and Zr. The mole ratio of Ti:Mn:Ce:M is 1:0.4:0.07:0.025.
2.2. Catalyst Characterization
The catalyst’s catalytic activity was measured in a quartz reactor (35 cm i.d.) using 6.75 g catalyst, see in Figure 1. The feed gas mixture in a N2 stream contained 1000 ppm NO, 1100 ppm NH3, and 5%O2. The total flow rate of the feed gas was 90 L/h (GHSV = 10,000 h−1). The reaction temperature was scanned from 100˚C to 400˚C.
Figure 1. Experimental setting and flow chart. 1―N2; 2―O2; 3―SO2; 4―NO; 5―NH3; 6―Silica gel drying apparatus; 7―CaCl2 dryer; 8―CaO dryer; 9―Stop valve; 10―pressure gage; 11―mass flow meter; 12―one-was valve; 13―mixer; 14―quartz tube; 15―silica wool; 16―catalysts; 17―temperature control for furnace; 18―thermocouple; 19―tube furnace; 20―NaOH washing bottle; 21―H3PO4 washing bottle; 22―flue gas analyzer.
The concentration of the NOx in the inlet and outlet of the reactor were measured on-line by a flue gas analyzer (MRU, Germany). H2-temperature-programmed reduction (H2-TPR, ChemBET Pulsar) experiments were carried out using every catalyst (40 mg) under a mixed gas flow of H2 and Ar.Morphologies of the samples were characterized by scanning electron microscopy (SEM, quanta FEG 250). Powder XRD was characterized with Cu-Ka radiation at the range of 10˚ - 90˚. Fourier infrared spectrum (FT-IR, Prestige-21) was used to analyze functional group change of catalyst after operating for a time.
3. Results and Discussion
3.1. Structure and Morphology Analysis
XRD patterns of the Mn-Ce-M catalysts are shown in Figure 2. Only TiO2 peaks in all catalysts suggests a low loading and a high dispersion of Mn,Te and M elements on the surface of TiO2. At the same time, compared with pure TiO2 (industrial grade), the TiO2 peaks become weak for all the catalyst, which further shows uniform active ingredients with small size for the catalyst can be obtained by impregnation technology. Figure 3 gives the SEM pictures of Mn-Ce-M catalyst. W-doped catalyst reveals uniform particle size from 500 nm to 800 nm as same as Mn-Ce catalyst. Adding Pr into Mn-Ce catalyst lead to slightly agglomeration of the catalyst particle, see the Figure 3(c). The prepared Mn-Ce-Zr and Mn-Ce-Y catalysts show obvious particle agglomeration, in the Figure 3(d) and Figure 3(e). At the same time, metal doping lead to bulk density increase or the porosity decrease of the catalyst. The catalyst morphology may possibly influence its performance, which need be investigated in the following.
3.2. Catalyst Performance
The catalytic performance of Mn-Ce-M0.025 catalysts, expressed as the percent conversion of NO as function of temperature, is shown in Figure 4. The Mn-Ce catalyst behaves above 80% NO conversion at 150˚C - 300˚C and achieved the maximum value with 91% conversion at 200˚C. Compared with Mn-Ce catalyst, Mn-Ce-M catalyst presents the lower NO conversion at 100˚C - 150˚C. Above 200˚C, the Mn-Ce-W catalyst shows the most excellent performance and obtains the 93.2% and 98.4% NO conversion at 200˚C and 250˚C respectively. Pr elements doped Mn-Ce catalysts decrease the catalytic performance relative to Mn-Ce catalyst at all test temperature. At the same time, Zr and Y elements addition into the Mn-Ce catalyst reduce the lower-tempera- ture (100˚C - 200˚C) performance and enhanced NO conversion at the high temperature (300˚C - 400˚C). At 250˚C, the Mn-Ce-Zr catalyst with 92.2% NO conversion is higher than that of Mn-Ce catalyst. The performance of Mn-Ce-Ycatalyst is near to that of Mn-Ce catalyst. These results demonstrate W element efficiently improves the catalyst’s NO conversion and Pr element presents poor action. Zr and Y elements promote Mn-Ce
Figure 2. XRD patterns of I-Mn-Ce-M. (a) CeO2 standard PD F#34- 0394; (b) TiO2 standard-PDF#21-1272; (c) Industrial-grade TiO2; (d) Mn-Ce; (e) Mn-Ce-W; (f) Mn-Ce-Pr; (g) Mn-Ce-Zr; (h) Mn-Ce-Y.
Figure 3. SEM micrographs of Mn-Ce-M. (a) Mn-Ce; (b) Mn-Ce-W; (c) Mn-Ce-Pr; (d) Mn-Ce-Zr; (e) Mn-Ce-Y.
Figure 4. NO conversion of Mn-Ce-M (reaction conditions: φ(NO) = 1000 ppm; φ(NH3) = 1200 ppm; φ(O2) = 5%; N2 as balance; GHSV = 10,000 h−1).
catalyst activity only at the high temperature. However, at high temperature range (above 250˚C), the NO conversions for Mn-Ce-Pr and Mn-Ce-Zr are near to that of Mn-Ce. According to SEM picture, it is possible that uniform particle size and good porosity tends to present good catalyst, especially at low temperature. The reason of the transition metal effect on catalyst performance will be further investigated in the following.
3.3. H2-TPR Analysis
The doped metal effect on the redox property of Mn-Ce-M0.025 catalysts was observed by H2-TPR, see in Figure 5. Use of Mn-Ce catalyst resulted in a broad reduction profile peak in the temperature range of 163˚C - 486˚C,
Figure 5. H2-TPR curves on Mn-Ce-M. (a) Mn-Ce; (b) Mn-Ce-W; (c) Mn- Ce-Pr; (d) Mn-Ce-Zr; (e) Mn-Ce-Y.
which was attributed to the continuous reduction steps of MnO2 → Mn2O3 → Mn3O4 → MnO [7] . The reduction peak at 553˚C was attributed to a reduction of Ce4+ to Ce3+ on the surface. Above 850˚C, the reduction profile peak was from the reduction of Ce4+ to Ce3+ in the bulk. When W was introduced to the Mn-Ce catalyst, all reduction peaks were connected together and not back to the baseline. Comparing to Mn-Ce catalyst, the initial reduction temperature of Mn-Ce-W catalyst increased, which may be the reason of its lower catalytic performance at low temperature. On the other hand, the reduction peak area of Mn-Ce-W catalyst at 163˚C - 486˚C is larger than that for Mn-Ce catalyst. The larger reduction peak area presents the higher oxygen capacity, so the catalytic performance of Mn-Ce-W catalyst begins to exceed that of Mn-Ce catalyst from 200˚C. At the same time, the new reduction peaks at 639˚C and 804˚C can be attributed to tungsten reduction, which result in a greatly increase of reactive oxygen species on the catalyst surface and an improvement of redox ability of the catalyst. Hence, comparing to the Mn-Ce catalyst, the Mn-Ce-W catalyst presents the more excellent catalytic performance. When Pr and Zr element were introduced to the Mn-Ce catalyst, the reduction peak of manganese oxide was divided to two peaks of MnO2 or Mn2O3 reduced to Mn3O4 below 398˚C and Mn3O4 to MnO above 398˚C. Two peaks area is lower than that of the Mn-Ce catalyst, which led to lower oxygen capacity. At the same time, the initial reduction temperature of Mn-Ce-Pr and Mn-Ce-Zr catalysts also increased, which indicate low reduction ability at low temperature. These may be two reasons of lower catalytic performance at lower temperature after doping Pr and Zr into the catalyst. At higher temperature, the H2-TPR profiles of Mn-Ce, Mn-Ce-Pr and Mn-Ce-Zr is similar, so the NO conversion of Mn-Ce-Pr is small lower to that of Mn-Ce and only a little promotion after doping Zr element to Mn-Ce catalyst. For the Mn-Ce-Y catalyst, two independent peaks present at 431˚C and 501˚C attributed to manganese oxide, which obviously shift to high temperature and the peaks area is reduced. The reduction peak area of Ce4+ to Ce3+ is also reduced that may be due to the action between Y and Ce element. Hence, in Mn-Ce-M catalysts, Mn-Ce-Y behaves the lowest NO conversion at the low temperature (below 250˚C). The reduction peak at 748˚C can be attributed to Y2O3, which promote catalytic performance of Mn-Ce-Y catalyst at high temperature. At 350˚C and 400˚C, the NO conversion of Mn-Ce-Y is second to that of Mn-Ce-W.
3.4. Sulfur Tolerance Analysis
The tolerance to the SO2 for these prepared SCR catalysts is further investigated. When SO2 concentration is 0.01%, activities of Mn-Ce-M were performed at 250˚C, as shown in Figure 6. For all the catalysts, after importing SO2 into the reactant gas, the NO conversion is kept or slightly increases within 50 min and then begins to decrease. The performance improvement for the time being can be attributed to the enhancement of NH3 adsorption on the acid site formed from the SO2 oxidation. After a period of time, a lot of sulfate were formed and adhered to the catalyst surface which leads to catalytic active decrease. Use of Mn-Ce-Pr and Mn-Ce-Y catalysts, the NO conversion are lower than that of Mn-Ce catalyst at initial stage, but decreased slowly and begin to ex-
Figure 6. SCR activities of Mn-Ce-M0.025 catalysts in the presence of SO2 (Reaction condi- tions: φ(NO) = 1000 ppm; φ(NH3) = 1200 ppm; φ(O2) = 5%; N2 as balance; GHSV = 10,000 h−1; SO2 = 0.01%; reaetion temperature = 250˚C).
ceed that of Mn-Ce catalyst after 150 min in SO2 condition. After 275 min in SO2 condition, the NOx conversion decrease from 88.8% to 73.5% over Mn-Ce catalyst, 87.1% to 77.1% over Mn-Ce-Pr catalyst and 88.7% to 75% over Mn-Ce-Y. The results demonstrated that Pr presents better tolerance to the SO2 than Y element. Doping W and Zr into the Mn-Ce catalyst not only improve the catalytic performance but also enhance the catalyst’s sulfur tolerance. In all catalysts, Mn-Ce-W catalyst presented the best performance with 88.1% NO conversion in SO2 condition after 275 min.
3.5. FT-IR Analysis
In order to study the catalyst sulfur tolerance, the fresh catalyst and deactivated Mn-Ce-M catalysts with SO2 was carried out by FT-IR spectroscopy to detect the adsorbed species on the Mn-Ce-M catalyst. The FTIR spectra for the fresh Mn-Ce catalyst, deactivated Mn-Ce and Mn-Ce-W catalysts with SO2 deactivated catalysts with SO2 are shown in Figure 7. The adsorption peak at 1634 cm−1 band was commonly considered as O-H stretching vibration peak [8] . Comparing to fresh Mn-Ce catalyst, four new bands appeared when the catalysts were exposed to flue gas with SO2. NH4+ was adsorbed on catalyst acid site at 1400 cm−1 and three bands between 1048 - 1129 cm−1 were identified as 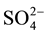 adsorption peaks [9] [10] . The results from the FTIR spectra indicated sulfate formation on the catalysts.
adsorption peaks [9] [10] . The results from the FTIR spectra indicated sulfate formation on the catalysts.
There into, the adsorption peak of NH4+ was weakest on Mn-Ce catalyst, but the strongest 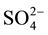 peaks were formed. That is to say, over Mn-Ce catalyst, a little ammonium sulfate or ammonium bisulfate was formed, but a lot of metal sulphates deposited. So, the maximum sulfate formation was observed on Mn-Ce catalyst, which may be the main reason of the catalyst inactivation. Metal doping into the Mn-Ce catalyst could inhibit the sulfate formation, especially the suppression to the deposition of metal sulfate, which could lead to the block of catalyst pores or channels. The weakest
peaks were formed. That is to say, over Mn-Ce catalyst, a little ammonium sulfate or ammonium bisulfate was formed, but a lot of metal sulphates deposited. So, the maximum sulfate formation was observed on Mn-Ce catalyst, which may be the main reason of the catalyst inactivation. Metal doping into the Mn-Ce catalyst could inhibit the sulfate formation, especially the suppression to the deposition of metal sulfate, which could lead to the block of catalyst pores or channels. The weakest 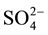 peaks presented over Mn-Ce-W catalyst, which proved that W addition could greatly inhibit the sulfate formation. Then Mn-Ce-W catalyst presented the optimal performance in the aspect of sulfur tolerance.
peaks presented over Mn-Ce-W catalyst, which proved that W addition could greatly inhibit the sulfate formation. Then Mn-Ce-W catalyst presented the optimal performance in the aspect of sulfur tolerance.
4. Conclusion
Mn-based catalysts were prepared via ultrasonic immersing method for the selective catalytic reduction (SCR) of NOx from fuel gas. Transition metals effect on the Catalysts’ DeNOx efficiency and tolerance to sulfur were investigated. SEM and XRD results demonstrate metal elements high dispersion on TiO2 and uniform particle size about 500 nm to 800 nm. Sulfur tolerance analysis indicated that transition metals M can improve the catalysts’ performance when SO2 exists in the fuel gas. Mn-Ce-W catalyst performs the best NOx conversion of 93.2% at 200˚C and 98.4% at 250˚C, respectively. From the FTIR results, the weakest 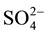 peak over the Mn-Ce-W catalyst which proved W element inhibit sulfate formation, especially metal sulfate, and this is the reason of good sulfur tolerance for the catalysts.
peak over the Mn-Ce-W catalyst which proved W element inhibit sulfate formation, especially metal sulfate, and this is the reason of good sulfur tolerance for the catalysts.
Figure 7. FT-IR spectra of fresh and deactivated catalysts with SO2. (a) Fresh Mn-Ce; (b) de- activated Mn-Ce catalysts; (c) deactivated Mn-Ce-W0.025 catalysts.
Acknowledgements
We acknowledge the support of the National Support Plan (2011BAA04B07) and Beijing Institute of Technology Basic Research Fund (20111042014).
References
- Qi, G., Yang, R.T. and Chang, R. (2004) MnOx-CeO2 Mixed Oxides Prepared by Co-Precipitation for Selective Catalytic Reduction of NO with NH3 at Low Temperatures. Applied Catalysis B, 51, 93-106. http://dx.doi.org/10.1016/j.apcatb.2004.01.023
- Carja, G., Kameshima, Y., Okada, K., et al. (2007) Mn-Ce/ZSM5 as a New Superior Catalyst for NO Reduction with NH3. Applied Catalysis B, 73, 60-64. http://dx.doi.org/10.1016/j.apcatb.2006.06.003
- Tang, X.L., Hao, J.M., Yi, H.H., et al. (2007) Low-Temperature SCR of NO with NH3 over AC/C Supported Manganese-Based Monolithic Catalysts. Catalysis Today, 126, 406-411. http://dx.doi.org/10.1016/j.cattod.2007.06.013
- Wu, Z.B., Jin, R.B., Liu, Y. and Wang, H.Q. (2008) Ceria Modified MnOx/TiO2 as a Superior Catalyst for NO Reduction with NH3 at Low-Temperture. Catalysis Communications, 9, 2217-2220.
- Uddin, A.M., Ishibe, K., et al. (2007) Effects of SO2 on NO Adsorption and NO2 Formation over TiO2 Low-Tempe- rature SCR Catalyst. Industrial & Engineering Chemistry Research, 46, 1672.
- Wu, D.W., Zhang, Q.L., Lin, T., et al. (2011) CexTi1-xO2 Supported Manganese-Based Catalsyt: Perparation and Catalytic Performance for Selective Reduction of NO with NH3 at Lower Temperature. Chinese Journal of Inorganic Chemistry, 27, 53.
- Maitarad, P., Zhang, D.S., Gao, R.H., et al. (2013) Combination of Experimental and Theoretical Investigations of MnOx/Ce0.9Zr0.1O2 Nanorods for Selective Catalytic Reduction of NO with Ammonia. Journal of Physical Chemistry C, 117, 9999-10006. http://dx.doi.org/10.1021/jp400504m
- Othman, I, Mohamed, R.M. and Ibrahem, F.M. (2007) Study of Photocatalytic Oxidation of Indigo Carmine Dye on Mn-Supported TiO2. Journal of Photochemistry and Photobiology A: Chemistry, 189, 82-83. http://dx.doi.org/10.1016/j.jphotochem.2007.01.010
- Topsoe, N.Y. (1994) Mechanism of the Selective Catalytic Reduction of Nitric Oxide by Ammonia Elucidated by in Situ On-Line Fourier Transform Infrared Spectroscopy. Science, 265, 1217-1219. http://dx.doi.org/10.1126/science.265.5176.1217
- Wu, Z., Jiang, B., Liu, Y., Wang, H. and Jin, R. (2007) DRIFT Study of Manganese/Titania-Based Catalysts for Low-Temperature Selective Catalytic Reduction of NO with NH3. Environmental Science and Technology Journal, 41, 5812-5817. http://dx.doi.org/10.1021/es0700350
NOTES

*Corresponding author.








