Modern Research in Catalysis
Vol.3 No.2(2014), Article ID:43876,9 pages DOI:10.4236/mrc.2014.32005
Triphase Catalysis Based on Gemini Surfactant-Clay Intercalates
Nahid Shabestary, Derek T. Rensing, Danielle N. Reed, Alex F. Austiff, Mark D. Cox
Department of Chemistry, Southern Illinois University Edwardsville, Edwardsville, USA
Email: nshabes@siue.edu
Copyright © 2014 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/


Received 6 January 2014; revised 6 February 2014; accepted 15 February 2014
ABSTRACT
A series of novel catalysts was developed using cationic Gemini surfactants intercalated in natural montmorillonite (MMT) clay. These Gemini surfactant-MMT intercalates were used to study the kinetics of a nucleophilic displacement reactions converting n-butyl bromide to n-butyl chloride in a triphase catalytic (TC) system. Most reaction rates compared favorably to those of biphase catalytic reactions where Gemini surfactants were used in the absence of MMT. Catalytic activity varied with Gemini surfactant structure, specifically with carbon spacer group and side chain length. In addition to the ease of catalyst separation that a triphase system affords, Gemini-MMT catalysts are stable and can be recycled and re-used several times.
Keywords:Triphase Catalysis; Gemini Surfactant; Intercalation Reaction; Nucleophilic Displacement; Montmorillonite; Phase Transfer Catalysis

1. Introduction
Triphase catalysis (TC) is a unique form of phase transfer catalysis (PTC) in which the catalyst and each of a pair of reactants are located in separate phases [1] -[4] . In this catalytic system, reactants are usually located in the aqueous and organic phases while the catalyst is supported by the solid phase. In the last few decades, comprehensive reviews both from chemistry and engineering viewpoints have been published on PTC [5] -[7] . While a large spectrum of reactions can be conducted using PTC, catalyst recovery from the liquid phase and recycling has been a problem limiting industrial application. TC on the other hand greatly facilitates catalyst recovery via simple filtration or centrifugation and thus provides considerable potential for commercial application. Low cost catalyst separation, mild reaction conditions, and catalyst recyclability in a triphase system also promote greener chemistry.
After the pioneering work of Regen [1] [2] on the application of polymer-supported catalysts in triphase catalysis, numerous quaternary onium salts immobilized on insoluble synthetic polymers as well as inorganic oxides have been widely explored [8] [9] . While TC drastically improves catalyst recovery, polymer supports and some inorganic oxides such as silica or alumina suffer from diffusion limitations and structural instability. Quaternary onium salts, such as conventional monomeric surfactants immobilized on natural clays via an intercalation reaction, have been extensively investigated as triphase catalysts [10] -[12] . These intercalated complexes are quite stable catalysts for various organic syntheses. They have also been shown to be efficient and recyclable [4] .
In the present research, we have developed a series of solid catalysts based on Gemini surfactant-Na+-Montmorillonite (Na+-MMT) clay intercalates to carry out simple nucleophilic substitution reactions in order to demonstrate the efficacy and stability of these materials. Gemini surfactants represent a new class of surfactants that are composed of two amphiphilic moieties connected by a spacer group [13] [14] . The spacer group can be hydrophilic or hydrophobic, rigid or flexible. Gemini surfactants have several technological advantages over conventional monomeric surfactants such as lower critical micelle concentration (CMC), greater surface tension, and in particular better wetting properties that can be useful in phase transfer catalysis to promote reactivity.
Montmorillonite (MMT) from the smectite clay family is a layered aluminosilicate in which isomorphous substitution in nature generates permanent negative charge on the clay layers. The negative charges are balanced by inorganic cations that are intercalated between the interlayer gallery surfaces. The inorganic cations can be replaced by larger cationic organic species with an associated increase in the interlayer spacing. MMT has a great affinity towards water as well as organic reagents. This unique characteristic that combines hydrophilicity with hydrophobicity makes it attractive for TC application.
2. Experimental
2.1. Chemicals and Instrument
All the chemical reagents and solvents were purchased from Aldrich Chemical Co. and used with no further purification. Sodium-rich Wyoming MMT clay (SWy-2) with a particle size of <2 µm was purchased in pre-centrifuged and spray-dried form from the Source Clay Repository, Purdue University. MMT clay has the chemical composition Na0.70[Mg0.70Al5.30(Si8.00)O20(OH)4 and a cation exchange capacity (CEC) of 76.4 meq/100g of airdried clay. The clay was purified by removing carbonates using pH5 acetate buffer solution and eliminating non-lattice iron oxides by employing sodium hydrosulfate based on literature procedure [15] .
A 300 MHz Varian Unity NMR was used to perform proton and carbon-13 NMR as well as correlation spectroscopy (COSY) to verify Gemini surfactant structures. A Rigaku Miniflex X-ray Diffractometer with a copper X-ray source (Kα = 1.5418Å) was used to determine basal spacing of Gemini-MMT intercalates. A Perkin Elmer Clarus 500 Gas Chromatograph (GC) equipped with a flame ionization detector and a 20 m× 0.18 mm Elite-5 capillary column were used for the kinetics analysis.
2.2. Gemini Synthesis
Gemini surfactants were synthesized and purified as described in the literature [16] . The surfactants were synthesized by reacting appropriate diamines and corresponding halides in dry ethanol solution (3 moles of alkyl bromide with 1 mole of alkyl diamine to minimize the potential of monosubstituted surfactant formation) at reflux temperature (~80˚C) for a duration of 48 to 200 hours depending on the structure. Once Gemini crystals formed, the solvent was removed from the reaction mixture under vacuum. The resulting solid was re-crystallized several times using an acetone-methanol mixture and washed with ether for further purification. The Gemini surfactants were characterized by NMR spectroscopy.
2.3. NMR Results
Due to structural similarity, only an exemplary of both hydrogen and carbon NMR results of Gemini C16-C4-C16 is given below:
1H NMR Spectrum of Gemini C16-C4-C16 (300 MHz, CDCl3): δ 3.29 (12H, s), 2.53 (4H, m), 2.14 (4H, m), 1.77 (4H, m), 1.33 (4H, m), 1.28 - 1.37 (52H, m), 0.86 - 0.90 (6H, q).
13C NMR Spectrum of Gemini C16-C4-C16 (300 MHz, CDCl3): δ 65.45 (N-CH2-side chain, C1), 63.69 (N-CH2-spacer group), 50.98 (N-CH3), 31.89 (CH2-side chain, C14), 29.21 - 29.68 (CH2 side chain, C4-C13), 26.33 (CH2-side chain, C3), 22.90 (CH2 side chain, C15), 22.65 (N-CH2-CH2 side chain, C2), 19.90 (N-CH2- CH2-spacer group), 14.09 (CH3 end of side chain, C16).
2.4. Intercalation Reaction
An aliquot of 1% aqueous suspension of Na-MMT clay was added to an aliquot of aqueous solution of the appropriate Gemini surfactant with a concentration twice that of the clay CEC to ensure that all the interlayer sodium cations were replaced by Gemini ions. The solution was stirred for 5 days at 50˚C until the exchange reaction was completed (verified by constancy of the X-ray diffraction pattern). Some of the less soluble Gemini surfactants were intercalated using a 50:50 ethanol/water solution instead of pure water. The organically-modified clay was centrifuged and washed with ethanol and water a few times to remove excess Gemini salt. The intercalates were then re-suspended in water and washed until free of halide ion as tested by AgNO3 solution. The products were then collected by centrifugation and freeze-dried before storage in a desiccator. X-ray powder diffraction (XRD) analysis was performed to determine the basal spacing of all the Gemini-MMT intercalates. The d spacing of the intercalated MMT was analyzed using Bragg’s equation (nλ = 2d sinθ).
2.5. Triphase Catalytic Reaction
The nucleophilic displacement reaction was conducted in 50-mL culture tube fitted with a Teflon-lined screw cap and magnetic stirrer using procedure detailed below. Rates of reaction were monitored by following the disappearance of the starting n-butyl bromide from the organic phase using gas liquid chromatography. To the culture tube was added 0.100 g Gemini-clay catalyst, an aliquot of 3 mL 3.3 M NaCl solution, and 2 mL toluene solution containing 1 mmol butyl bromide. The tube was sealed and stirred in an 80˚C ± 1 oil bath. Aliquots of 1 µL organic solution were withdrawn at 30 minute intervals for GC analysis to measure the extent of reaction. For sampling, the tube was removed from the oil bath, quickly cooled by ice water to a temperature below room temperature, opened, resealed and returned to the bath after sampling with a small syringe. The value of Kobs was calculated by plotting Ln{[n-butyl bromide]t/[n-butyl bromide]t=0} vs. the time of reaction. The rate law expression used to calculate the Kobs is given below:
CH3(CH2)3-Br + Cl−1àCH3(CH2)3-Cl + Br−1
Rate = −d[CH3(CH2)3-Br]/dt = K [Cl−1][ CH3(CH2)3-Br]
At high concentration of NaCl relative to CH3(CH2)3-Br the equation can be written as:
Rate = −d[CH3(CH2)3-Br]/dt = Kobs [CH3(CH2)3-Br] or Ln{[CH3(CH2)3-Br]t/[CH3(CH2)3-Br]t=0}= −Kobst Note: For all the corresponding bi-phase reactions, the procedure was similar to tri-phase reaction with the exception that 0.07 mmole of pure Gemini surfactants were used as catalyst instead of solid Gemini-clay catalyst.
For recyclability testing, the Gemini-clay intercalates were filtered and washed first with 10 mL ethanol and then with 10 mL deionized water prior to reuse.
3. Results and Discussion
3.1. Gemini Surfactant Synthesis and Characterization
Gemini surfactants represent a new class of surfactants. Menger and Littau [17] assigned the name Gemini to bis-surfactants. They are made of two amphiphilic moieties (side chains) connected at the level of the head groups or very close to the head groups by a spacer group, as schematically represented in Figure 1. Gemini surfactants are remarkably different from conventional surfactants or quaternary alkylammonium salts in surface activity and solution properties such as critical micelle concentration, surface tension and viscosity [1] [14] .
Figure 1. Schematic representation of Gemini (dimeric) surfactant.
We have synthesized several Gemini surfactants comprised of two N-alkyldimethylammonium bromide groups joined together by an alkyl spacer with the general formula Cn-Cx-Cn, where n is the number of carbons in the free N-alkyl side chain and x is the number of carbon atoms in the spacer group (see Table 1). An exemplary chemical structure of a Gemini surfactant is given below for C16-C4-C16.
The Gemini surfactants were characterized by NMR prior to the intercalation reaction. During the intercalation reaction (Figure 2), Gemini surfactant salts in excess of MMT clay CEC were added. Upon the exchange reaction, intercalates were washed a few times with water and ethanol to remove excess Gemini salts from the clay and air-dried prior to the catalytic reaction. The intercalation reaction for each Gemini surfactant was verified by XRD. An example of XRD pattern of C16-C4-C16 Gemini-MMT vs. pure MMT is shown in Figures 3 and 4 where a basal spacing of 34.2 Å was observed. Several Gemini surfactant-MMT intercalates were used as the solid phase in a triphase catalytic system as shown in Table 1. Not all possible combinations of side chains with spacer groups could be purified.
3.2. Triphase Catalytic Reaction and Kinetic Study
The chlorination of 1-bromobutane was selected as a suitable nucleophilic displacement reaction to demonstrate the effectiveness of Gemini-MMT clay intercalates as triphase catalysts.
C4H9-Br(org) + Cl−(aq) à Gemini-MMT à C4H9-Cl(org) + Br−(aq)
Pseudo-first order kinetics was observed for the chlorination reaction. This is similar to the behavior of conventional monomeric surfactant-clay intercalates that have been extensively studied by several authors [4] [12] [18] -[21] . Here the concentration of the nucleophile Cl− was kept in many fold equivalent excess over the organic substrate, C4H9Br. Thus the observed rate constant (kobs) could be derived and measured by the equation below:
−d[n-butyl bromide]/dt = kobs[n-butyl bromide]
In a control experiment where no catalyst was used, no catalytic activity was observed. We also studied the catalytic activity of Gemini surfactants in a biphase reaction where no solid MMT intercalated material was used. The catalytic activities of these biphase systems are also listed in Table 1. We found that the rate constant increases with both spacer group length and carbon side chain length in the biphase setting (Figure 5). Since the solubility of some of the long chain Gemini surfactants in water was low, no biphasic reaction could be carried out for those surfactants (i.e., 18 carbon side chain length).
Interestingly, we observed much higher catalytic activity for all MMT-supported Gemini surfactant catalysts used in our studies except for C8-C2-C8 compared to non-intercalated counterparts. An increase in catalytic activity in triphase systems has been reported for conventional monoionic surfactants when supported by smectite clay [22] . However, in most cases, a decrease in catalytic activity is seen in triphase systems perhaps due to onium ion immobilization and decreased accessibility of the catalytic site on clay interlayer surfaces.
We also observed no significant catalytic activity after removing Gemini-MMT solid from the reaction mix-
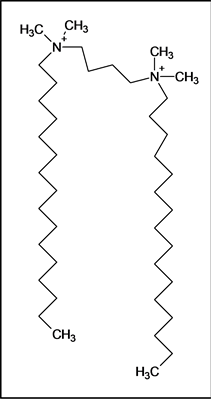

Table 1. Gemini surfactant-MMT as triphase catalysts for the chlorination of n-C4H9Br.
All reactions were carried out at 80˚C under the following stoichiometric conditions: 1.0 mmol of n-butyl bromide in 2.0 mL of toluene; 10 mmol of NaCl in 3.0 mL of water; 0.100 gram of Gemini surfactant-MMT. X-ray basal spacing of Gemini surfactant-MMT was measured at 25˚C under dry condition.

Figure 2. Schematic representation of Gemini surfactant intercalation.
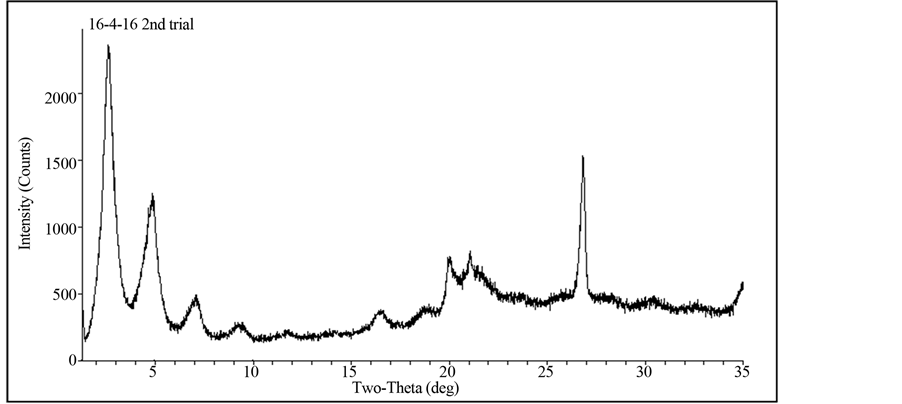
Figure 3. Representative X-ray diffraction pattern of Gemini (C16-C4-C16) intercalated MMT clay.
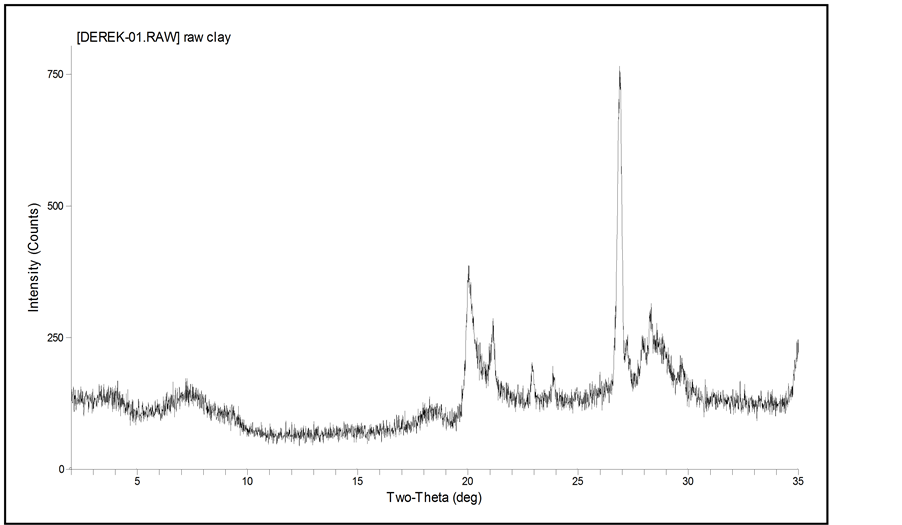
Figure 4. X-ray diffraction pattern of Na+-MMT clay.
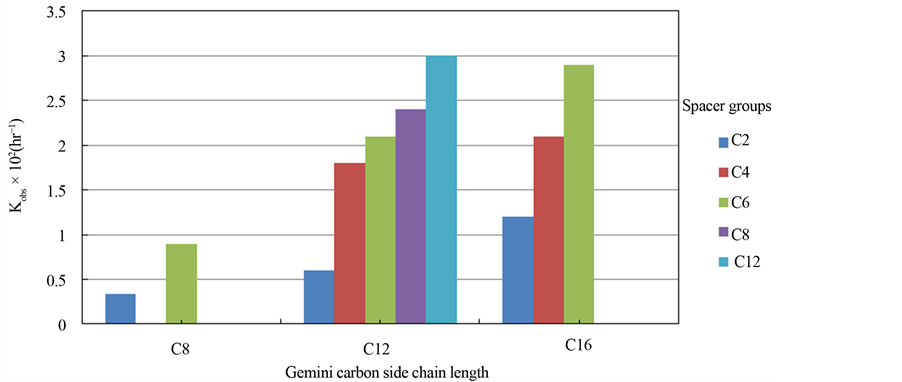
Figure 5. Biphase rate constant with varying Gemini surfactant structure.
ture by filtration. This suggests that Gemini surfactants are not desorbing from the MMT surface under triphase catalytic reaction conditions and that Gemini-MMT catalysts are quite stable and can be recycled. In fact, recycled supported catalysts retained better than 95% of original catalytic activity. This is also consistent with observations made in the literature regarding conventional monoionic surfactant-clay catalyst [11] [22] .
The reaction mixture (Gemini surfactant-MMT, organic and aqueous phase) forms a uniform emulsion that is easily broken by centrifugation. This type of emulsion formation has been reported previously for monomeric surfactant-clay catalysts [4] [18] [12] [23] . Pinnavaia et al. [4] have argued that organo clays can form thin, membrane-like assemblies of platelets at the liquid-liquid interface of an oil/water type of emulsion, and reagents in emulsified liquid phases are readily transferred to the interface of clay assemblies for facile reaction.
We observed that Gemini surfactant-MMT emulsions were broken with low-speed centrifugation (<2000 RPM) and sometimes with long term storage (about 2 weeks). This property allows efficient separation of catalyst from product. However, pure Na-MMT is unable to emulsify in the triphase system and as a result no catalytic activity was observed.
In general, organo-clay assemblies with higher basal spacing are expected to have higher catalytic activities; however, this is not always the case (Table 1). An order of magnitude difference in catalytic activity was observed between C18-C2-C18 and C16-C6-C16. While C18-C2-C18 has larger basal spacing (43.3 Å compared to 35.3), it shows much lower reactivity (Kobs × 102 h−1 = 1.85 and 15.4, respectively).
In Gemini surfactant-MMT systems, molecular structure plays a significant role in catalytic activity. We noticed marked variation in catalytic activity using Gemini surfactants with varying side chain lengths and spacer groups. For 4 and 6 carbon spacer groups, longer carbon side chain length generally resulted in higher catalytic activity (Figure 6). The length of the carbon side chain had little effect on activity when a 2 carbon spacer group was present. The highest catalytic activity was observed for Gemini surfactants with a 6 carbon spacer group regardless of carbon side chain length.
3.3. Mechanistic Approach
Lagaly [24] demonstrated that alkyl ammonium ions intercalated in smectite clay form ordered assemblies in which alkyl chains and onium head groups adopt specific orientations. The structure of these ordered assemblies depends in part on the length of the alkyl chains and the charge density of the clay layers. In the case of Gemini surfactants, surfactant orientation on the clay surface is more complex because of the presence of two cationic centers.
As described by Williams-Daryn and Thomas [25] , three possible conformations exist for an idealized Gemini vermiculite clay intercalate where the surfactant has some choice in how to interact within the clay interlayer surfaces. The Gemini surfactant can attach to either one or two negative clay surface charges along the same surface, it can act as a bridge between two surfaces, or only one of the two charges need be directly attached to the clay layer while the other exists as an ion pair. Due to the heterogeneous charge density distribution in natural MMT, a combination of the aforementioned conformations is likely (Figure 2).
These factors can not only affect basal spacing but may also change the hydrophilic/hydrophobic character of Gemini surfactant-MMT intercalates. The specific ordered assemblies may be related to the extent or conformation of the spacer group, distance between the two charged groups of the dimeric surfactant and the charged sites of the clay surface, or may be random. Regardless, the catalytic activity of Gemini surfactant-MMT intercalates varies with Gemini surfactant structure, which affects ordered catalyst assembly. The exact mechanism for the impact on catalytic activity is unclear at this time. Considering the near infinite possibilities that exist to generate Gemini surfactants using conventional amphiphilic moieties and various spacer groups to form any desired structure opens a vast opportunity for exploring efficient catalysts in a triphase catalytic system.
4. Conclusion
We have developed a series of novel catalysts to be used in a triphase system using Gemini surfactants interca-

Figure 6. Triphase rate constant with varying Gemini surfactant structure.
lated in MMT clay. Advantages of triphase catalysis include efficient and low cost catalyst recovery via filtration or centrifugation, catalyst recyclability and mild reaction conditions, which not only improve the potential for commercial application but also promote greener chemistry. We have characterized the kinetics of a nucleophilic displacement reaction using these catalysts and have demonstrated that catalytic activity varies with surfactant structure, notably with Gemini spacer group length. Interestingly, triphase catalytic activity is improved when compared to the biphase system for most Gemini surfactants studied. Gemini surfactant-MMT catalysts are also recyclable with no significant loss in reactivity. There remains much potential for exploring new efficient catalysts in a triphase system given the many possibilities of Gemini surfactant structure.
Acknowledgements
We would like to thank the donors of the American Chemical Society Petroleum Research Fund for support of this research (ACS PRF# 43890-B5) as well as the Division of Undergraduate Education, DUE-CCLI, at the National Science Foundation (DUE-0410642) for support of the X-ray powder diffraction instrument used in this research.
References
- Regen, S.L. and Besse, J.J. (1979) Liquid-Solid-Liquid Triphase Catalysis. Consideration of the Rate-Limiting Step, Role of Stirring, and Catalyst Efficiency for Simple Nucleophilic Displacement. Journal of the American Chemical Society, 101, 4059-4063.http://dx.doi.org/10.1021/ja00509a008
- Regen, S.L. (1979) Dreiphasen-Katalyse. Angewandte Chemie, 91, 464-472. http://dx.doi.org/10.1002/ange.19790910605
- Murugan, E. and Gopinath, P. (2009) Triphase Catalytic Activity of a New Insoluble Multi-Site Phase Transfer Catalyst inC-alkylation of Dihydrocarvone—A Kinetic Study. Journal of Molecular Catalysis A: Chemical, 309, 12-20. http://dx.doi.org/10.1016/j.molcata.2009.04.009
- Lin, C.L. and Pinnavaia, T.J. (1991) Organo Clay Assemblies for Triphase Catalysis. Journal of Materials Chemistry, 3, 213-215.http://dx.doi.org/10.1021/cm00014a003
- Starks, C.M., Liotta, C. and Halpern, M. (1994) Phase Transfer Catalysis, Fundamentals, Applications, and Industrial Perspectives. Chapman and Hall Publications, New York.
- Sasson, Y. and Neumann, R. (1997) Handbook of Phase Transfer Catalysis. Chapman and Hall Publications, New York.
- Dehmlow, E.V. and Dehmlow, S.S. (1993) Phase Transfer Catalysis. 3rd Edition, VCH, New York.
- Deschler, U., Kleinschmit, P. and Panste, P. (2003) 3-Chloropropyltrialkoxysilanes—Key Intermediates for the Commercial Production of Organofunctionalized Silanes and Polysiloxanes. Angewandte Chemie International Edition, 25, 236-252. http://dx.doi.org/10.1002/anie.198602361
- Ford, W.T. (1988) Immobilized Phase Transfer Catalysts. Chemtech, American Chemical Society, Washington DC, 436-439.
- Pinnavaia, T.J. and Lin, C.L. (1992) Organoclay Triphase Catalysts. US Patent No. 5,099,054. http://www.everypatent.com/comp/pat5099054.html http://www.google.es/patents/US5099054
- Yadav, G.D. and Naik, S.S. (2000) Clay-Supported Liquid-Liquid-Solid Phase Transfer Catalysis: Synthesis of Benzoic Anhydride. Organic Process Research and Development, 4, 141-146. http://dx.doi.org/10.1021/op990087c
- Varma, R.S., Pitchumani, K. and Naicker, K.P. (1999) Triphasic Catalyst Systems Based on Surfactant/Clay Composites-Facile Synthesis of Cyano, Thiocyano and Hydroxyl Compounds Using a Triphasic Catalyst. Green Chemistry, 1, 95-97. http://dx.doi.org/10.1039/a807092j
- Menger, F.M. and Littau, C.A. (1993) Gemini Surfactants: A New Class of Self-Assembling Molecules. Journal of the American Chemical Society, 115, 10083-10090. http://dx.doi.org/10.1021/ja00075a025
- Hait, S.K. and Moulik, S.P. (2002) Gemini Surfactants: A Distinct Class of Self-Assembling Molecules. Current Science, 82, 1101-1111. http://www.iisc.ernet.in/currsci/may102002/1101.pdf
- Mills, J.G. and Zwarich, M.A. (1972) Recognition of In-Terstratified Clays. Clays and Clay Minerals, 20, 169-174. http://dx.doi.org/10.1346/CCMN.1972.0200309
- Zana, R., Benrraou, M. and Rueff, R. (1991) Alkanediyl-.Alpha.,.Omega.-Bis(Dimethylalkylammonium Bromide) Surfactants. Effect of the Spacer Chain Length on the Critical Micelle Concentration and Micelle Ionization Degree. Langmuir, 7, 1072-1075. http://dx.doi.org/10.1021/la00054a008
- Menger, F.M. and Littau, C.A. (1991) Gemini-Surfactants: Synthesis and Properties. Journal of the American Chemical Society, 113, 1451-1452. http://dx.doi.org/10.1021/ja00004a077
- Cornelius, A. and Lazlo, P. (1982) Clay-Supported Reagents. II. Quaternary Ammonium Exchanged Montmorillonite as Catalysts in the Phase-Transfer Preparation of Symmetrical Formaldehyde Acetals. Synthesis, 1982, 162-166. http://dx.doi.org/10.1055/s-1982-29732
- Shabestary, N., Khazaeli, S. and Long, N. (2005) Triphase Catalytic Reactions Using Clay Intercalates. International Journal of Science and Technology (Scientia Iranica), 12, 290-294. http://www.siue.edu/artsandsciences/chemistry/faculty/shabestary/pdf/ShabestaryPaper1.pdf
- Shabestary, N., Khazaeli, S., Dutko, D. and Cutts, B.L. (2007) Clay-Supported Quaternary Ammonium and Phosphonium Cations in Triphase Catalysis and the Effect of Co-Solvent in Catalytic Activity. International Journal of Science and Technology (Scientia Iranica), 14, 297-302. http://www.siue.edu/artsandsciences/chemistry/faculty/shabestary/pdf/ShabestaryPaper2.pdf
- Ghiaci, M., Kalbasi, R.J. and Sedaghat, M.E. (2003) A Kinetic Study of 2-Ethyl-1-Hexanol Oxidation by Dichromate Using Clay-Supported 1-Butyl 4-Aza-1-Azonia Bicyclo[2.2.2]Octane Chloride as the Phase-Transfer Catalyst. Organic Process Research Development, 7, 936-938. http://dx.doi.org/10.1021/op034120d
- Lin, C., Lee, T. and Pinnavaia, T.J. (1992) Organoclay Assemblies and Their Properties as Triphase Catalysts. Superamolecular Architecture, ACS Symposium Series, American Chemical Society, Washington DC, 147-154.
- Kadkhodayan, A. and Pinnavaia, T.J. (1983) Clay Intersalation Compounds for Selective Triphase Catalysis: Reaction of Alkyl Bromides with NaCl. Journnal of Molecular Catalysis, 21, 109-117. http://dx.doi.org/10.1016/0304-5102(93)80114-A
- Lagaly, G. (1986) Interaction of Alkylamines with Different Type of Layered Compounds. Solid State Ionics, 22, 43-51. http://dx.doi.org/10.1016/0167-2738(86)90057-3
- Williams-Daryn, S. and Thomas, R.K. (2002) The Intercalation of a Vermiculite by Cationic Surfactants and Its Subsequent Swelling with Organic Solvents. Journal of Colloid and Interface Science, 255, 303-311. http://dx.doi.org/10.1006/jcis.2002.8673


