Modern Research in Catalysis
Vol.2 No.3(2013), Article ID:33536,5 pages DOI:10.4236/mrc.2013.23010
Effect of Nb2O5·nH2O Termal Treatment on the Esterification of a Fatty Acid
Chemistry Department, Espírito Santo Federal University, Vitória, Brazil
Email: vljuniorqui@gmail.com
Copyright © 2013 Deborah A. dos Santos et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received February 7, 2013; revised March 20, 2013; accepted April 23, 2013
Keywords: Nb2O5·nH2O; Calcination; Fatty Acid; PLS Calibration
ABSTRACT
In general, esterification reactions are favored by the increase in reaction temperature, excess of one of the reactants (usually alcohol), and additions of acid or basis catalysts. Esterification of oleic acid with methanol catalyzed by Nb2O5·nH2O calcined at different temperatures showed good conversion rates, especially at reaction temperature of 100˚C and higher catalyst proportions. PLS calibration showed good results for predicting the amounts of methyl oleate in reaction products.
1. Introduction
Esterification of fatty acids is an important process for biodiesel production from acids generated during hydrolysis of crude biomaterials. This reaction can be autocatalytic, but without an appropriate catalyst, reaction yields are low [1-3].
Currently, the major concern in biodiesel production is economic feasibility. Most industrial biodiesel plants use refined vegetable oils as raw material. Consequently, the cost of refined vegetable oils contributes to about 80% of the total production costs [2]. Another concerning point is food production. One alternative that has been employed to overcome this problem is the use of fatty acids generated as by-product of vegetable oil treatment for the food industry. Recovering fatty acid waste is difficult and economically unfeasible. In Brazil, the esterification technology for fatty acids generated from palm oil processing has been used since 2005 at a biodiesel plant of 12.000 ton/year in Belém, PA [3,4].
Studies on several solid acid catalysts for biodiesel preparation have been reported in the relevant literature [5-8]. The use of heterogeneous catalysis has advantages over homogeneous homologues, such as easy handling, recovery and reuse, as well as the chance of obtaining purer products. Furthermore, heterogeneous catalysts are not corrosive and reduce the need of washing stages in product purification, which generates less waste. Also, this type of catalyst can simultaneously promote transesterification of tryglicerides and esterification of free fatty acids, both processes with low molecular weight alcohols [6]. However, an obstacle to using solid catalyst is their high costs [7,8].
Among the heterogeneous catalysts described for esterification and transesterification reactions are inorganic oxides, impregnated oxides, zeolites, layered double hydroxides, etc. [5].
Nb2O5·nH2O has high acid strength (Ho = −5.6 - −8.2) and it is an effective catalyst for reactions in which water molecules participate are released. Usually, hydrated niobium oxide crystallizes at 853 K, and its acid strength property disappears when it is heated to temperatures over 800 K. In short, Nb2O5·nH2O surfaces have both Lewis acid sites (LAS)—which increase with pretreatment temperature higher than 773 K and decrease at higher temperatures)—and Brönsted acid sites (BAS)— which are more abundant at 373 K and decrease at higher temperatures [9-12].
Literatures [3,4,13-15] have reported the use of niobium acid as catalyst in biodiesel prepared from several raw materials through transesterification and esterification, as well as in bio-oil production through thermal cracking of vegetable oils. These studies assess parameters related to niobium acid such as particle dimension, treatment with inorganic acids (H3PO4, H2SO4 e HNO3), and calcination is also usually described at temperatures between 200˚C and 300˚C. However, the effect of different pretreatment temperatures is not broadly studied, even though it is a relatively simple and cheap industrial process.
The use of spectroscopic methods in quantitative analyses is often associated to the use of chemometric tools [16]. The Partial Least Squares (PLS) regression method has become widespread in the past years because of its several benefits. In PLS, spectral decomposition takes the data of decomposition calibration sample concentrations into account, which results in higher weights for spectra with higher analyte concentrations and two sets of vectors and scores—one for spectral data and one for the concentrations [17].
Spectroscopy IR with Fourier Transform (FTIR) associated to multivariate calibration models is described as a fast and accurate method for monitoring ethanolisis of vegetable oils, as well as parameters associated to biodiesel stability, water content, acidity rate, and biodiesel content in diesel [17-19].
In this study, we aimed at assessing the effect of thermal pretreatment of Nb2O5·nH2O as catalyst in esterification reactions of a fatty acid—oleic acid—by altering parameters such as reaction temperature and amount of catalyst. Furthermore, we sought to identify and quantify methyl esters through chemometric tools.
2. Experimental
2.1. Materials
Nb2O5·nH2O (HY-340), supplied by Companhia Brasileira de Metalurgia e Mineração (CBMM) underwent thermal pretreatment at temperatures 115˚C, 300˚C and 500˚C, all of them for three hours in drying stove or muffle furnace. After calcination, niobium acid was kept in a desiccator until room temperature was reached. After that, it was used in the reactions described below.
2.2. Methods
The esterification reactions were carried out in a roundbottom flask with oleic acid, methyl alcohol, at molar ratio acid:alcohol of 1:30, auxiliary solvent when needed, and newly calcinated Nb2O5·nH2O at mass ratios acid: oxide of 5:1 and 1:1, also without thermal pretreatment. The reactions took place for 48 hours at the following temperatures: atmospheric temperature, 65˚C, 100˚C and 170˚C, in reflux system (except for reactions at room temperature), under constant agitation. In same conditions described above, a reaction without catalyst was also performed. This was named “blank test”. At the end of all esterification reactions the products were isolated through multiple extractions with hexane.
2.3. Analysis
The reaction products and the calibration samples were analyzed through spectroscopy in the infrared area (IR), using a FTLA2000-102 device by BOMEM. The spectra were obtained with resolution set at 4 cm−1, 16 scans, using accessory MI Racle, Pike Technologies = ATR Single Reflection, 45˚, ZnSe, using atmosphere as white. The reading range of the accessory was 550 - 4000 cm−1.
Quantification of esterification products was carried out through a calibration curve built by using prediction method through Partial Least Squares using Minitab software program employing transmittance data of spectral band of 1650 cm−1 to 1800 cm−1 of 26 samples containing known amounts of methyl oleate and oleic acid, 9 of which were used in cross-validation.
3. Results and Discussion
Generally, esterification reactions are favored by the increase in reaction temperature and by adding the excess of esterifying agent (alcohol) or by removing the products during the reaction. This makes the reaction balance be displaced towards ester formation. Reverse reaction is called hydrolysis. Both reactions—esterification and hydrolysis—are catalyzed by acid or basic sites.
In order to work using reaction temperature over boiling point of methanol (65˚C), toluene and DMSO were used as auxiliary solvents. No interaction was verified between solvent and starting materials and, consequently, by-product formation.
Spectroscopy IR analyses of oleic acid and methyl oleate showed different absorption values for the C = O bond, around 1709 cm−1 and 1742 cm−1, respectively (Figure 1). Therefore, in a mixture with both substances, it is possible to see the two absorption bands of the respective carbonyls with intensity proportional to analyte concentrations. Based on these data, a multivariate calibration PLS curve was built with the carbonyl stretch data.
The calibration results obtained were satisfactory. The curve coefficient of determination (R2) was 0.998 (Figure 2); the root mean square error of the cross validation (RMSECV), which measures the model’s ability to predict new samples, was 1.03; and the root mean square error of prediction (RMSEP), which is calculated when the model is applied to new data [20], was 0.89.
Through calibration we could quantify the conversion rates of esterification reactions of oleic acid and methanol shown below (Table 1).
It was observed that niobium acid was active for oleic acid esterification, especially with the increased reaction temperature. The highest increase in conversion rates took place with reactions carried out at 100˚C, even
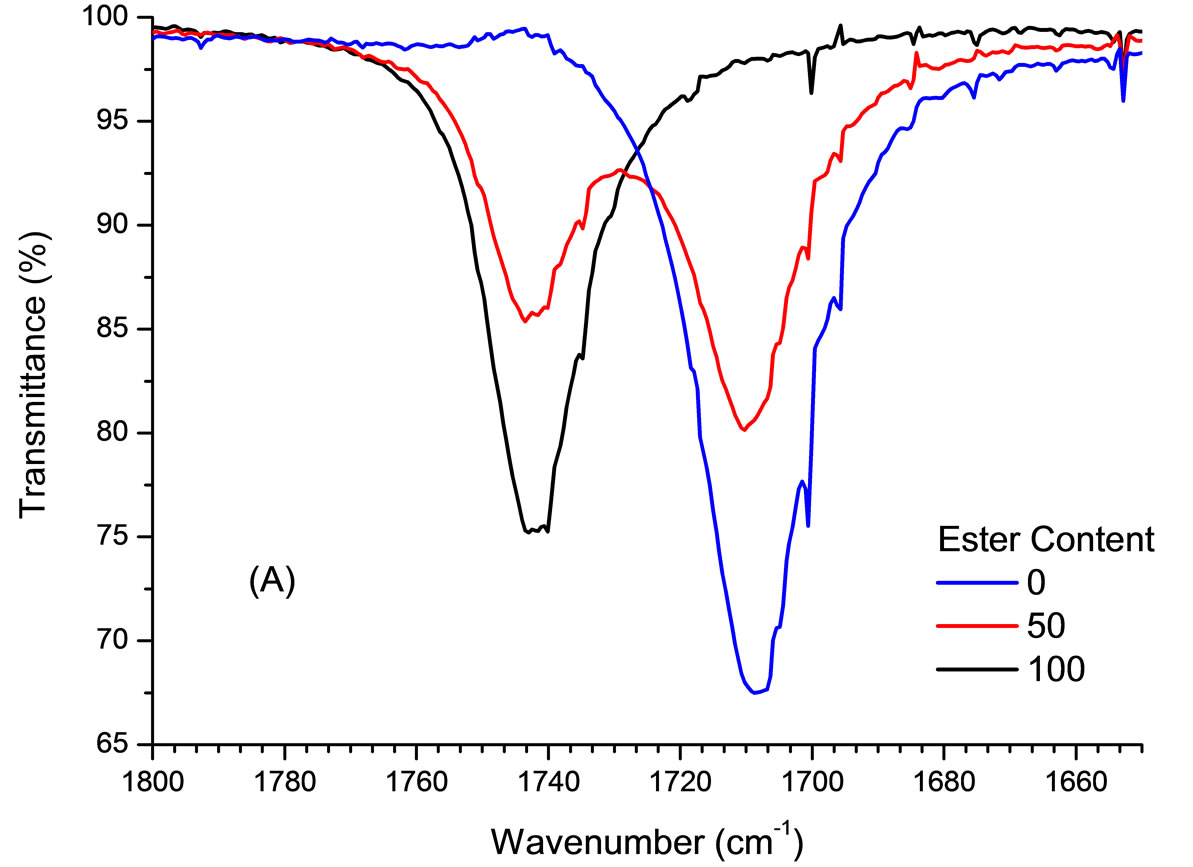
Figure 1. Part of the IR spectrum of a mixture of oleic acid and methyl oleate in different proportions.
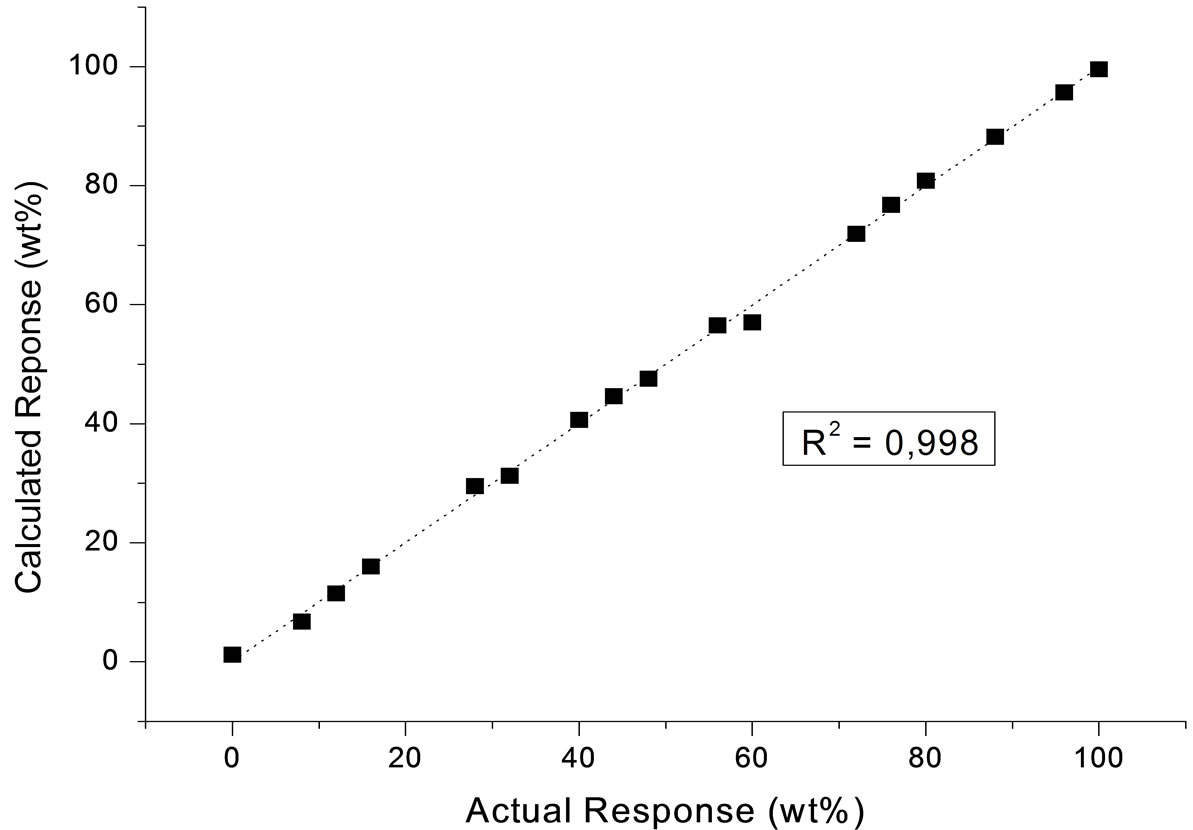
Figure 2. Result of PLS calibration for acid content.
though the highest conversion rates were expected at 170˚C. This decrease can be explained by the loss of the esterifying agent through evaporation, even when it is in excess, because the system was in reflux and the agent was not fully efficient.
We also verified that catalyst ratio influenced the conversion rates in a directly proportional relationship. Another important point concerns the calcination of niobium acid, all the reactions taking place with catalyst calcined at 100˚C and 300˚C showed higher conversion rates than those with niobium acid pretreated at 500˚C and with non-calcined catalyst. This suggests that the BAS (most abundant with calcination temperatures between 100˚C - 300˚C) are more active than the LAS (in higher concentration with calcination at 500˚C) as catalysts in oleic acid esterification. The “blank test” showed conversion rate of 22% for esterification of oleic acid under the temperature of 170˚C, an autocatalysis process could explain it.
4. Conclusion
The results show that Nb2O5·nH2O is efficient in esterification of oleic acid with methanol. Increasing reaction temperature up to 100˚C improves yields. However, at
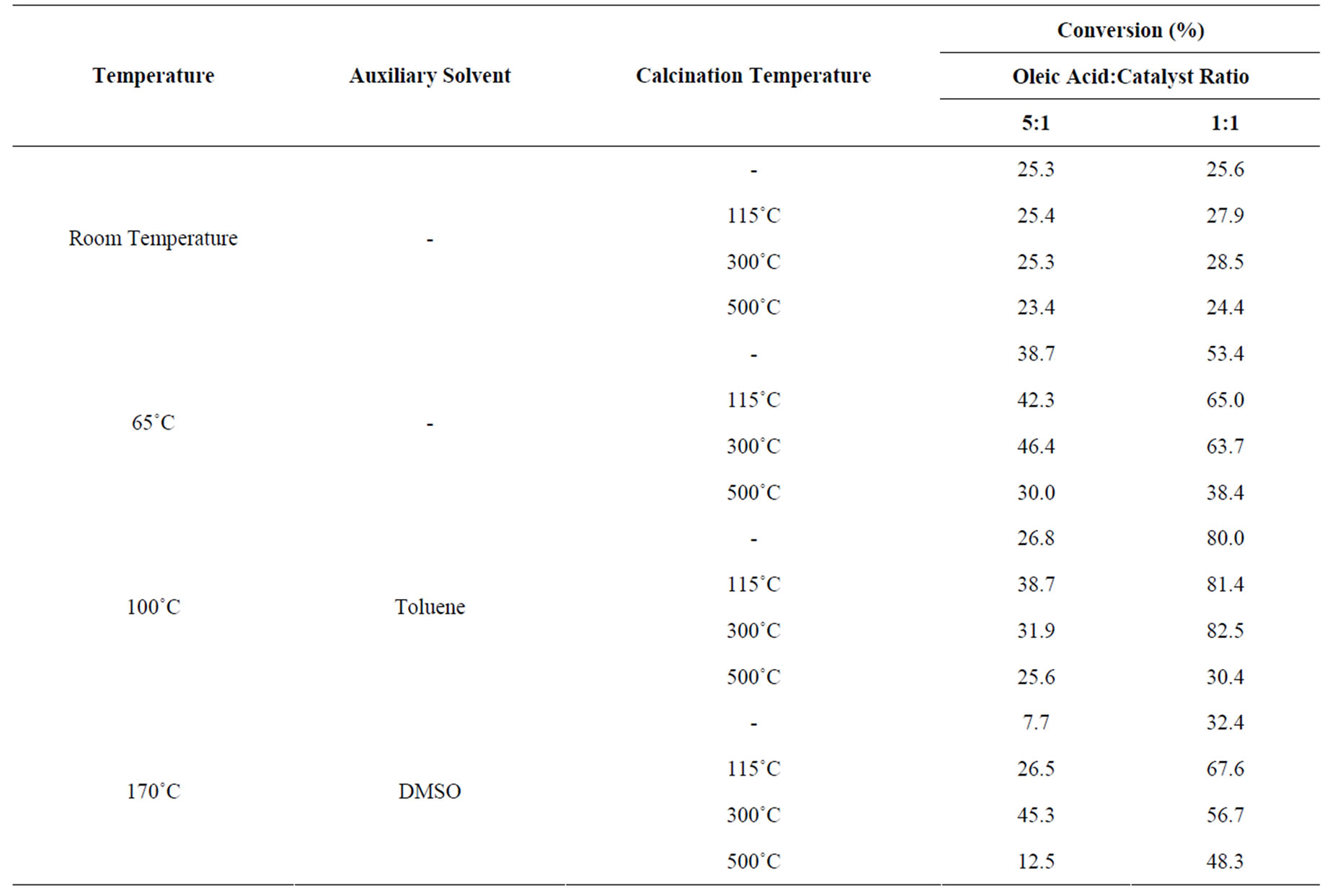
Table 1. Reaction conditions and conversion rates of oleic acid esterification reactions.
170˚C we assumed there were methanol losses due to evaporation and consequent decrease in conversion rates. Other important factors for the increase in reaction yields are thermal pretreatment of catalyst at 100˚C and 300˚C and increase in its ratio.
PLS calibration showed good results for predicting the amounts of methyl oleate in reaction products.
5. Acknowledgements
The authors thank CBMM for providing the niobic acid used in this work, the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), the Coordenadoria de Aperfeiçoamento de Pessoal do Nível Superior (CAPES), the Fundação de Amparo à Pesquisa do Espírito Santo (FAPES) and the Fundo de Apoio a Ciência e Tecnologia da Prefeitura de Vitória (FACITEC) for financial support.
REFERENCES
- J. Dupont, P. A. Z. Suarez, M. R. Meneghetti and S. M. P. Meneghetti, “Catalytic Production of Biodiesel and Diesel-Like Hydrocarbons from Triglycerides,” Energy & Environmental Science, Vol. 2, No. 12, 2009, pp. 1258- 1265. doi:10.1039/B910806H
- M. K. Lam, K. T. Lee and A. R. Mohamed, “Homogeneous, Heterogeneous and Enzymatic Catalysis for Transesterification of High Free Fatty Acid Oil (Waste Cooking Oil) to Biodiesel: A Review,” Biotechnology Advances, Vol. 28, No. 4, 2010, pp. 500-518. doi:10.1016/j.biotechadv.2010.03.002
- L. D. T. Câmara and D. A. G. Aranda, “Reaction Kinetic Study of Biodiesel Production from Fatty Acids Esterification with Ethanol,” Industrial & Engineering Chemistry Research, Vol. 50, No. 5, 2011, pp. 2544-2547. doi:10.1021/ie1005806
- D. A. G. Aranda, J. A. Gonçalves, J. S. Peres, A. L. D. Ramos, C. A. R. Melo Jr., O. A. C. Antunes, N. C. Furtado and C. A. Taft, “The Use of Acids, Niobium Oxide, and Zeolite Catalysts for Esterification Reactions,” Journal of Physical Organic Chemistry, Vol. 22, No. 7, 2009, pp. 709-716. doi:10.1002/poc.1520
- R. A. Soldi, A. R. S. Oliveira, L. P. Ramos and M. A. F. César-Oliveira, “Soybean Oil and Beef Tallow Alcoholysis by Acid Heterogeneous Catalysis,” Applied Catalysis A: General, Vol. 361, No. 1-2, 2009, pp. 42-48. doi:10.1016/j.apcata.2009.03.030
- X. Mo, E. Lotero, C. Lu, Y. Liu and J. G. Goodwin, “A Novel Sulfonated Carbon Composite Solid Acid Catalyst for Biodiesel Synthesis,” Catalysis Letters, Vol. 123, No. 1-2, 2008, pp. 1-6. doi:10.1007/s10562-008-9456-y
- J. Kansedo, K. T. Lee and B. Subhash, “Biodiesel Production from Palm Oil via Heterogeneous Transesterification,” Biomass and Bioenergy, Vol. 33, No, 2, 2009, 271-276. doi:10.1016/j.biombioe.2008.05.011
- K. G. Georgogianni, A. P. Katsoulidis, P. J. Pomonis and M. G. Kontominas, “Transesterification of Soybean Frying Oil to Biodiesel Using Heterogeneous Catalysis,” Fuel Processing Technology, Vol. 90, No. 5, 2009, pp. 671-676. doi:10.1016/j.fuproc.2008.12.004
- I. Nowak and M. Ziolek, “Niobium Compounds: Preparation, Characterization, and Application in Heterogeneous Catalysis,” Chemical Reviews, Vol. 99, No. 12, 1999, pp. 3603-3624. doi:10.1021/cr9800208
- T. Iizuka, K. Ogazawara and K. Tanabe, “Acidic and Catalytic Properties of Niobium Pentaoxide,” Bulletin of the Chemical Society of Japan, Vol. 56, No. 10, 1983, pp. 2927-2931. doi:10.1246/bcsj.56.2927
- K. Tanabe, “Catalytic Application of Niobium Compounds,” Catalysis Today, Vol. 78, No. 1-4, 2003, pp. 65-77. doi:10.1016/S0920-5861(02)00343-7
- C. Guo and Z. Quian, “Acidic and Catalytic Properties of Niobic Acid Crystallized at Low Temperature,” Catalysis Today, Vol. 16, No. 3-4, 1993, pp. 379-385. doi:10.1016/0920-5861(93)80077-E
- R. F. Brandão, R. L. Quirino, V. M. Mello, A. P. Tavares, A. C. Peres, F. Guinhos, J. C. Rubim and P. A. Z. Suarez, “Synthesis, Characterization and Use of Nb2O5 Based Catalysts in Producing Biofuels by Transesterification, Esterification and Pyrolysis,” Journal of the Brazilian Chemical Society, Vol. 20, No. 5, 2009, pp. 954-966. doi:10.1590/S0103-50532009000500022
- M. K. Pietri, L. C. P. Almeida, R. Landers, R. C. G. Vinhas and F. J. Luna, “H3PO4- and H2SO4-Treated Niobic Acid as Heterogeneous Catalyst for Methyl Ester Production,” Reaction Kinetics, Mechanisms and Catalysis, Vol. 99, No. 2, 2010, pp. 269-280. doi:10.1007/s11144-009-0143-9
- K. Srilatha, N. Lingaiah, P. S. S. Prasad, B. L. A. P. Devi, R. B. N. Prasad and S. Venkateswar, “Influence of Carbon Chain Length and Unsaturation on the Esterification Activity of Fatty Acids on Nb2O5 Catalyst,” Industrial & Engineering Chemistry Research, Vol. 48, No. 24, 2009, pp. 10816-10819. doi:10.1021/ie900864z
- M. M. C. Ferreira, A. M. Antunes and M. S. Melgo, “Quimiometria I: Calibração Multivariada, um Tutorial,” Química Nova, Vol. 22, No. 5, 1999, pp. 724-731. doi:10.1590/S0100-40421999000500016
- P. Geladi and B. R. Kowalski, “Partial Least-Squares Regression: A Tutorial,” Analytica Chimica Acta, Vol. 185, 1986, pp. 1-17. doi:10.1016/0003-2670(86)80028-9
- G. F. Zagonel, P. P. Zamora and L. P. Ramos, “Multivariate Monitoring of Soybean Oil Ethanolysis by FTIR,” Talanta, Vol. 63, No. 4, 2004, pp. 1021-1025. doi:10.1016/j.talanta.2004.01.008
- L. F. B. Lira, M. S. Albuquerque, J. G. A. Pacheco, T. M. Fonseca, E. M. S, Calvalcanti, L. Stragevitch and M. F. Pimentel, “Infrared Spectroscopy and Multivariate Calibration to Monitor Stability Quality Parameters of Biodiesel,” Microchemical Journal, Vol. 96, No. 1, 2010, pp. 126-131. doi:10.1016/j.microc.2010.02.014
- Ö. Yeniay and A. Göktas, “A Comparison of Partial Least Squares Regression with Other Prediction Methods,” Hacettepe Journal of Mathematics and Statistics, Vol. 31, 2002, pp. 99-111.

