Modern Research in Catalysis
Vol.2 No.1(2013), Article ID:27286,9 pages DOI:10.4236/mrc.2013.21002
Efficient and Clean Catalytic Hydrogenolysis of Aromatic Ketones by Silica Supported Schiff Base Modify Chitosan-Palladium Catalyst
Shandong Provincial Key Laboratory of Chemical Energy-Storge and Novel Cell Technology, School of Chemistry and Chemical Engineering, Liaocheng University, Liaocheng, China
Email: *gongshw@lcu.edu.cn
Received November 14, 2012; revised December 19, 2012; accepted December 28, 2012
Keywords: CS-Schiff-Base; Pd Catalyst; Hydrogen; Aromatic Ketones
ABSTRACT
An silica supported chitosan-Schiff base Pd(II) catalyst was prepared in a simple way and characterized by XRD, FT-IR, SEM-EDS, XPS and TG, and the ability of this complex to catalyze hydrogenolysis of 1-tetralone into 1,2,3,4-tetrahydronaphthalene was also investigated in the presence of hydrogen. It has been revealed that the catalyst had high catalytic activity for hydrogenolysis of 1-tetralone at ambient temperature and normal pressure of hydrogen. Especially, the hydrogenolysis of 1-tetralone in ethanol solvent gave excellent results and the 100% conversion of 1-tetralone and the 100% selectivity for 1,2,3,4-tetrahydronaphthalene were obtained under optimized reaction conditions. The influences of reaction temperature, reaction time and solvent on the hydrogenolysis of 1-tetralone were also investigated. It has been also revealed that the catalyst was efficient and eco-friendly for the hydrogenolysis of carbonyl that connected with a benzene ring to give corresponding aromatic hydrocarbons.
1. Introduction
The reduction of aldehydes or ketones, especially selectively reduce C=O group to methylene (CH2) is an important organic reaction, which is used to the direct conversion of aromatic ketones to synthesize linear alkylbenzenes [1]. The linear alkylbenzenes are frequently used as intermediates in chemical industries. The classical reported procedures for the reduction of C=O group to CH2 are the Clemmensen and Wolff-Kishner reducetions [2,3]. However, not only Clemens reaction, but also Wolff-Kishner reaction is not environment-friendly, these reactions are either carried out in the presence of Zn-Hg/concentrated HCl or employed lot of hydrazine as reactant. Subsequently, the catalytic hydrogenation of C=O group to CH2 has been reported, Cu-Cr, Fe or Ni had proved less active for the conversion of C=O into CH2 at high temperatures (473 - 573 K) [4-6]. Avnir [7] once reported that the catalytic hydrogenolysis of aromatic ketones by a sol-gel entrapped combined Pd- [Rh(cod)Cl]2 catalyst, however, the selectivity of hydrogenation reaction towards the alkylbenzenes is low and the aromatic rings were fully hydrogenated. The result that reported by Prof. De Vos [8] suggests that the mixed choline-betainium ionic liquids has promotional effect on the hydrogenolysis of aromatic ketones catalytic by Pd catalyst, the conversion of aromatic ketones and selectivity to alkylbenzenes were all improved.
Chitosan (CS) is a natural biopolymer, which can be easily obtained from chitin that is widely dispersed in living organisms [9]. More recently, with the development of environmentally friendly industries, CS-supported metals catalysts have attracted a lot of attention because CS shows these advantages of nontoxicity and desirable physical and mechanical properties [10-13]. In previous papers [14-18], a silica-supported CS-palladium complex abbreviated as SiO-CS-Pd has been found to catalyze the hydrogenation of nitrotoluene, nitrobenzene, chlorophenol, 2-octene and 3-octene, et al. Some ketones can reduce to chiral alcohols catalyzed by silica supported CS-Pd complex via asymmetric hydrogenation [19,20]. However, the successful application of CS stabilized palladium catalysts in hydrogenolysis reactions of C=O to CH2 groups has not yet been reported.
In the presence of active amino group, CS also exhibits the possibility of chemical modifications, including the preparation of Schiff bases by reaction with aldehydes and ketones [21-23]. Recently, we has prepared a silica supported Schiff base modified CS-Pd catalyst and found that it exhibited good catalytic activity in the hydrogenaolysis of 1-tetralone. In this paper, we validated this finding and revealed the application scope of the catalyst. It can be found that this immobilized combined catalyst promotes the total hydrogenolysis of some aromatic ketones to give corresponding aromatic hydrocarbons under mild conditions.
2. Experimental
2.1. Materials
Chitosan (CS) finely purified to a de-acetyl degree of 90.0% was purchased from Sinopharm Chemical Reagent Co, Ltd. China. Salicylaldehyde and 1-tetralone was purchased from Aladdin Reagent Co, Ltd. China and salicylaldehyde was distilled before used. Other reagents were of analytical grade and were used as received.
2.2. Preparation of Catalyst
Silica supported chitosan (SiO2-CS) was first prepared, 4.0 g CS was added into 250 ml 1.5% CH3COOH and stirred until CS was all dissolved at room temperature, and then 8.0 g SiO2 was added. After continued stirred for 2 h, the PH of solution was modified by 1 mol/L NaOH to 13, the solid was separated by filtration, washed with water (until the PH = 8), ethanol and acetone, respectively, and then dried at 333 K under vacuum for 10 h to give white solid, the nitrogen content was determined to be 2.52 wt% by elemental analysis.
Then the silica supported chitosan Schiff base (SiO2-CS-Schiff base) was prepared refer to the procedure in the literatures 24 - 25. 10 g SiO2-CS and excess salicyal (15 ml) and acetic acid (12 ml) were added to methanol (120 ml), and then the mixture was refluxed for 10 h. After the resultant mixture was cooled, the solid was separated by filtration, washed with methanol and then dried at 333 K under vacuum for 12 h to give bright yellow solid.
Finally, the silica supported Schiff base modify chitosan-palladium (SiO2-CS-Schiff base-Pd) was prepared according to the method of reported in literatures [18,19, 23]. The SiO2-CS Schiff-base and PdCl2 were weighed in 10:1 mass ratio and were added in ethanol solvent. After the mixture was stirred at 303 K for 72 h, the solid product was filtered and washed with ethanol to the filtrate became transparent and colorless, and dried at 323 K under vacuum to obtained brown catalyst particles, which was then used to catalyze the hydrogenolysis of 1-tetralone with hydrogen. The metal contents of catalyst determined by ICP are 5.4%.
2.3. Characterization of the Catalyst
The X-ray diffraction analysis was carried out using a X-ray diffractometer (Beijing Purkinje General Instrument Co. Ltd) with Ni filtered Cu Kα radiation (λ = 1.542 Å ) and a scanning range 2θ of 5˚ - 80˚. The FT-IR spectra were measured on a FT6700 spectrophotometer in the range 400 - 4000 cm−1. X-Ray Photoelectron Spectroscopy (XPS) measurements were performed with a VG Scientific ESCALAB 250 instrument with Mg Kα radiation (1253.6 eV). The TG analyses were performed on a STA449 thermogravimetric analyzer (NETZSCH, Germany). The morphology and elemental composition of sample was analyzed by a JSM6380LV scanning electron microscopy equipped with energy dispersive X-ray (SEM-EDX) elemental analysis system. The content of Pd before and after reaction was identified by an OPTIMA2000DV Inductive Coupled Plasma Emission Spectrometer (ICP).
2.4. Hydrogenolysis of 1-Tetralone
The hydrogenolysis experiment was performed in a 50 mL tube with a side neck equipped with a magnetic stirrer and an automatic temperature controller. In a typical hydrogenolysis experiment, to a mixture of 1-tetralone substrates (0.5 ml) and catalyst (0.2 g) was added ethanol (15 ml). The reaction vessel was flushed (three times) with pure hydrogen and then retained the 40 ml/min current velocity of hydrogen. The reaction mixture was stirred magnetically at a rate of 150 rpm at 313 K for 5 h. After the reaction, the hydrogenolysis products were identified and quantified by 1H NMR (CDCl3, 400 MHz) and an HP 6890/5973 GC/MS instrument (FID, column: HP-5MS). The by-products of the reaction were 1-tetralin alcohol.
3. Results and discussion
3.1. Characterization Results
The XRD pattern of prepared catalyst is shown in Figure 1. From the pattern, it can be seen that SiO2-CS-Schiffbase-Pd only exhibits two broad peaks at about 20˚, which ascribes the amorphous silica and the crystalline macromolecule of CS, respectively. The diffraction peaks of palladium (0) (2θ = 38˚, 46˚, et al., JCPDS: 05-618) or other palladium-contained compounds are not observed.
The FT-IR spectrums of the prepared SiO2-CS-Schiffbase and SiO2-CS-Schiff-base-Pd catalyst had reported in previous works [25]. The results exhibited that a band due to the C=N stretching vibration appeared at 1630 cm−1, which indicated that the formation of Schiff base. And the characters bands of silica were also retained, which means that the structure of silica was no change in the preparation.
The TG data of the SiO2-CS-Schiff-base-Pd catalyst is depicted in Figure 2. From this result, the destruction
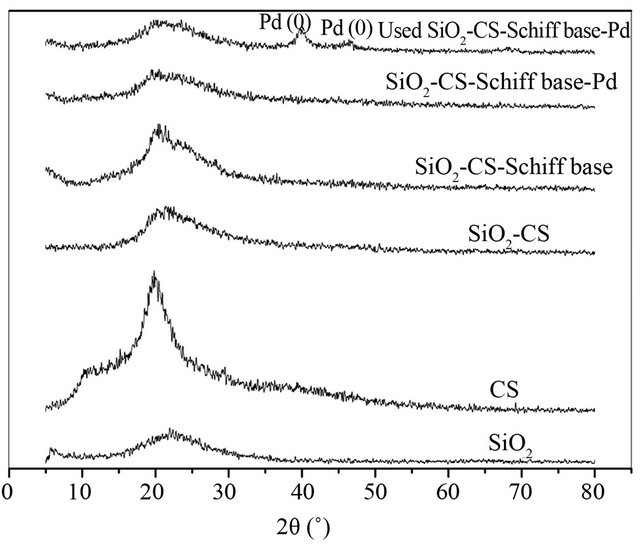
Figure 1. XRD pattern of silica supported CS-Schiff-basePd catalyst.
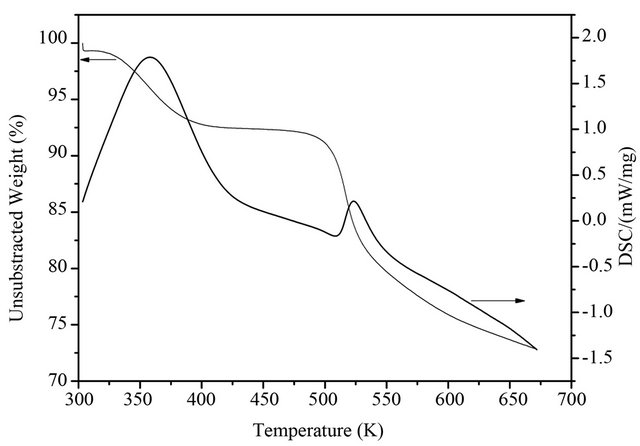
Figure 2. The TG data of silica supported CS-Schiff-basePd catalyst.
temperature of catalyst is 490 K, which suggests that the catalyst of SiO2-CS-Schiff-base-Pd have a good thermal stability between room temperature and 490 K. The weight loss of the catalysts before the destruction is attributed to desorption of water.
The surface morphology of the catalyst is shown in Figure 3. From the SEM image it is clear that the catalyst has irregular block structure. And the existence of palladium in the catalyst was confirmed by SEM-EDX analysis (Figure 3), chloride also was detected for SiO2- CS-Schiff-base-Pd catalyst. The elemental composition of catalyst analyzed by with SEM-EDX is listed in Table 1, and it can be seen that the molar ratio of chlorine: palladium is about 2:1. The appearance of the SiO2-CSSchiff-base-Pd catalyst and SiO2-CS-Schiff-base is different in color. The former was brown and the latter was bright yellow.
Figure 4 shows the XPS analysis of SiO2-CS-Schiffbase-Pd catalyst. The XPS analysis is a technique essentially limited to the very first external layers of the material (on a thickness limited to 2 - 3 times the wavelength
 (a)
(a)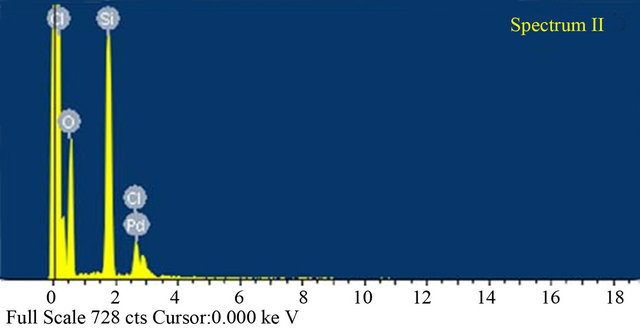 (b)
(b)
Figure 3. (a) SEM image; (b) SEM-EDS of silica supported CS-Schiff-base-Pd catalyst.
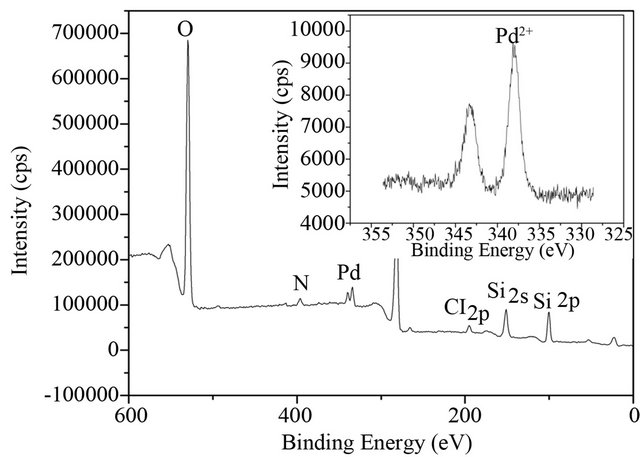
Figure 4. XPS analysis of SiO2-CS-Schiff-base-Pd catalyst.
Table 1. The element content and Surface area of SiO2-CSSchiff-base-Pd catalyst.
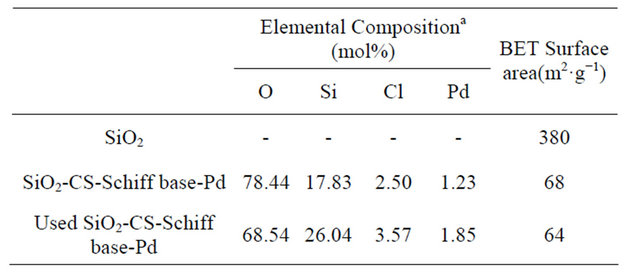
aAnalyzed by SEM-EDX.
of the analytical radiation) and the analysis result confirms the existence of O, N, Si, Cl and Pd. One peak was identified at 337.6 eV, corresponding to the Pd2+ forms, which means that the Pd2+ is the main existence of Pd in the prepared catalyst. The result is consistent with the analysis of SEM-EDX (Table 1) that the elemental composition of Cl is close to two times that of Pd.
3.2. Catalytic Activity
Catalytic activity of the SiO2-CS-Schiff-base-Pd catalyst was evaluated for the hydrogenolysis of 1-tetralone in the presence of H2 and ethanol. For comparison, CS-Pd, CS-Schiff base-Pd, ZSM and Al2O3 supported CS-Schiff base-Pd were prepared and also examined as hydrogenolysis catalyst with same conditions, and the results are showed in Table 2. As showed in Table 2, there is no reaction took place in the absence of catalyst or in the presence of CS-Pd. After 3 h hydrogenation, SiO2-CS-Pd exhibited activity of hydrogenation but only 1-tetrahydronaphthalenol was formed as the sole product, which was accordance with reported results that silica supported CS-Pd catalyst has catalytic activity for hydrogenation of ketones to form corresponding alcohols [19,20]. Yuan [19] once reported that the asymmetry transfer hydrogenation of acetophenone catalyzed by silica supported CS-Pd catalyst, and an optical yield of R-1-phenylethanol could reach 99% after 8 h reaction at 303 K under atmosphere hydrogen. In the presence of unsupported CS-Schiff base-Pd catalyst, 1-tetralone totally converted and the expected tetrahydronaphthalene in the yield of 37% and 1-tetrahydronaphthalenol in the yield of 63%. In this sense the modification of chitosan with salicylaldehyde to produce Schiff base can improve the hydrogenation selectivity of catalyst via the coordination between chitosan and palladium atom, in other words, the insertion of functional group of Schiff base in the chitosan matrix may improve its capacity of interaction with metallic ions by complexation [24]. For supported catalyst, the SiO2-CS-Schiff-base-Pd, gave 100% conversion of 1-tetralone and tetrahydronaphthalene formed by 72% yield, proved to be the most efficient for hydrogenolysis, which suggests that silica is an excellent support to optimize the catalytic activity and selectivity, which may be due to the high specific surface area of silica to enhance the catalytic chance of substrates and active sites of catalyst. Therefore, SiO2-CS-Schiff-base-Pd was chosen as catalyst for full reduction of C=O to CH2, and the effect of reaction parameters like temperature, the catalyst amount and the solvent on the conversion of 1-tetralone and selectivity to tetrahydronaphthalene has been investtigated in detail.
3.2.1. The effect of Reaction Temperature
As shown in Figure 5, the conversion increased to the maximum with the rise in the reaction temperature, and 69% of 1-tetralone conversed at room temperature, which suggests that the SiO2-CS-Schiff base-Pd catalyst exhibited excellent hydrogenation activity even at low temperature. 1-tetralone totally conversed when temperature was raised to 303 K. The selectivity for tetrahydronaphthalene and 1-tetrahydronaphthalenol as a function of the reaction temperature are also shown in Figure 6. It is clear that high temperature favors the formation of tetrahydronaphthalene and 1-tetralone all transformed into tetrahydronaphthalene at 313 K.
3.2.2. The Effect of Reaction Time
Figure 6 presents the effects of reaction time on 1-tetralone hydrogenolysis with hydrogen in ethanol. The reaction hardly occurred during the first 0.5 h, which might be the initiation period. After the initiation period, the 1-tetralone conversion increased as the reaction time was prolonged and the 100% conversion was obtained after 2 h reaction. In this period, the selectivity of tetrahydronaphthalene has slight decrease with the extension of time, and then increases with further prolonging the reaction time, 100% 1-tetralone was reduced to tetrahydronaphthalene after 4 h reaction. This result suggests that 1-tetralone may firstly converses to 1-tetrahydronaphthalenol by hydrogenation, and then tetrahydronaphthalene
Table 2. Hydrogenolysis of 1-tetralone catalyzed by different catalysts.
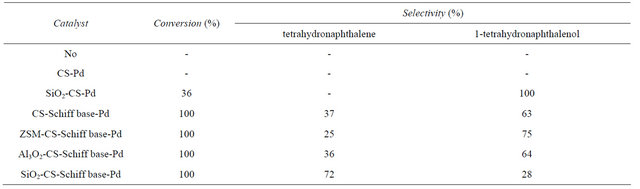
aReaction conditions: 0.5 mL substrate, 0.2 g catalyst, 15 mL ethanol, 40 mL/min H2, 313 K.
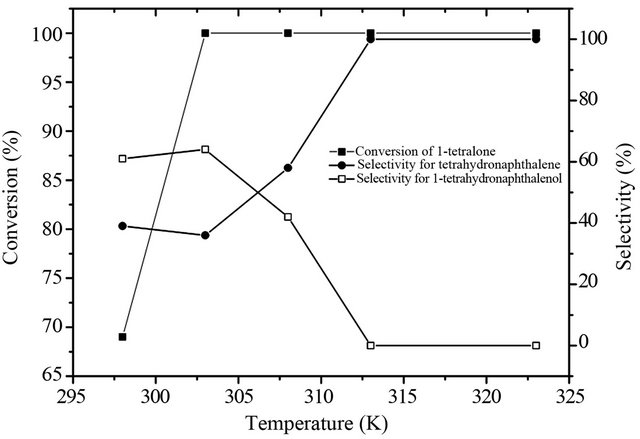
Figure 5. Effect of reaction temperature on deoxygenation of 1-tetralone.
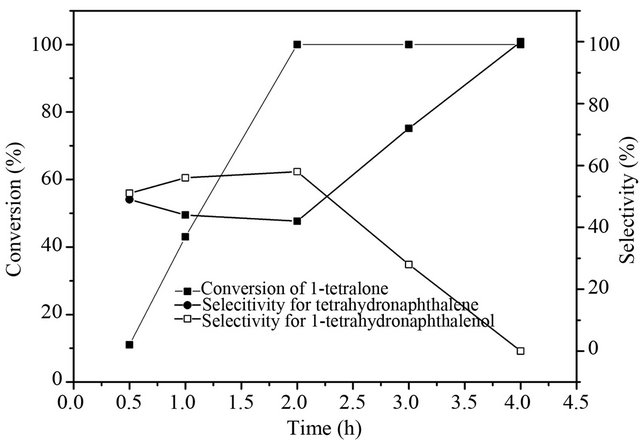
Figure 6. Effect of reaction time on deoxygenation of 1- tetralone.
is formed by hydrogenolysis after a prolonged reaction period.
3.2.3. Effect of Catalyst Amount
The effect of catalyst amount on conversion and selectivity was examined by varying the catalyst amount from 0.05 to 0.25 g (Figure 7). The conversion of 1-tetralone was observed to be only 18% with 27% selectivity to tetrahydronaphthalene for a 0.05 g amount of catalyst. The conversion of 1-tetralonn increased to 100% at a 0.15 g of the catalyst amount with 53% selectivity to tetrahydronaphthalene. The selectivity to tetrahydronaphthalene also increased to be 100% when 0.2 g catalyst was used, and no significant change in the conversion and selectivity was observed on a further increase in the catalyst amount. With increasing of catalyst amount, the conversion and selectivity to tetrahydronaphthalene all increased, which may be explained basis that the increase of adsorption sites with the increasing of catalyst amount. And it can be clear found that the totally conversion was first obtained and then the 100% selectivity to hydrogenolysis was obtained, which definitelysuggests that 1-tetrahydronaphthalenol shall further transform into tetrahydronaphthalene by hydrogenolysis.
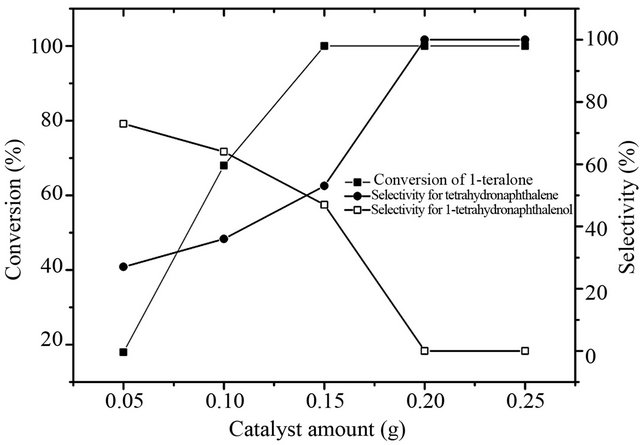
Figure 7. Effect of catalyst amount on deoxygenation of 1- tetralone.
3.2.4. Effect of Solvent
Different solvents were studied for SiO2-CS-Schiff basePd catalyzed hydrogenolysis of 1-tetralone. As showed in Table 3, proton solvents were essential to the reaction. In ethanol or methanol, the reaction gave 100% conversion but by-product 1-tetrahydronaphthalenol formed by 39% when the reaction was carried out in methanol. In acetonitrile, although the 100% conversion was obtained, the main reduced product was 1-tetrahydronaphthalenol and only 11% 1-tetralone was conversed into tetrahydronaphthalene. In cyclohexane, benzene, carbon tetrachloride or in the absence of solvent, the reaction hardly occurs or cannot take place.
3.3. Catalyst Recycling
In order to observe the recycle ability of the catalyst, the hydrogenolysis reaction was performed with 0.2 g of the catalyst at 313 K in ethanol and the results are given in Table 4. For the fresh catalyst the conversion and selectivity to tetrahydronaphthalene were all 100% after 4 h reaction. The filtered catalyst was only dried at 323 K under vacuum and reused under identical conditions with the fresh charge of other materials. There is a decrease in conversion and selectivity to tetrahydronaphthalene with the increase of reused times, after used three times, catalyst gave only 57% conversion and 37 % selectivity to tetrahydronaphthalene. However, the extension of reaction time was still favor the increase of conversion and selectivity to tetrahydronaphthalene, for second recycle catalyst, the total conversion and 81% selectivity to tetrahydronaphthalene was gave after 6 h reaction.
The third recycle catalyst was analyzed by ICP, the analysis result suggests the content of Pd increased to 6.6% from 5.4% of the fresh catalyst, and the similar result can be observed basis on the analysis result of SEM-EDX (Table 1), too. The increase of content of Pd may be due to the loss or the decomposition of CS and the functional group of Schiff base in the polarity reaction condition, and then results in the decrease of cata-
Table 3. Effect of solvent on hydrogenolysis of 1-tetralone.
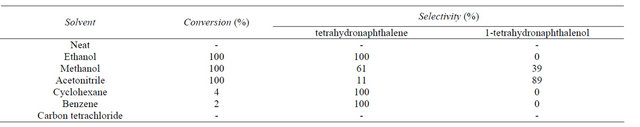
Reaction conditions: 0.5 mL substrate, 0.2 g catalyst, 15 mL solvent, 313 K, 40 mL/min H2, 4 h.
Table 4. Effect of recycle of the catalyst on hydrogenolysis of 1-tetralone.
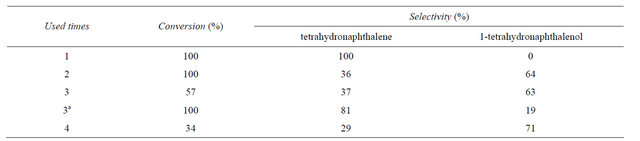
Reaction conditions: 0.5 mL substrate, 0.2 g catalyst, 15 mL ethanol, 313 K, 40 mL/min H2, 4 h aResult was obtained after 6 h reaction.
lytic activity in hydrogenolysis reaction. The XRD pattern of the third recycle catalyst clearly exhibits two diffraction peaks at 40.5˚ and 46.5˚, which indicates the presence of Pd(111) and Pd(110) phase which are attributed to Pd(0) (Figure 1), which indicates that partially Pd (II) was reduced to Pd(0).
3.4. Extension to Other Substrates
To deep reveal the catalytic hydrogenolysis activity of SiO2-CS-Schiff base-Pd catalyst, some hydrogenation experiments of representative carbonyl compounds were carried out and these results are summarized in Table 5. In the presence of SiO2-CS-Schiff base-Pd catalyst, the hydrogenation of examined aliphatic did not occur besides cyclohexanone (entry 1 - 4), and only 42% cyclohexanone was reduced to cyclohexanol, which suggests that the SiO2-CS-Schiff base-Pd catalyst is inactive for hydrogenation of aliphatic ketones. For examined aromatic carbonyl compounds, the dexoygenation of benzaldehyde (entry 5), acetophenone (entry 6) and propiophenone (entry 7) gave 100% conversion and 100% selectivity to corresponding aromatic hydrocarbon, which means that the C=O was totally transformed into CH2, in other words, SiO2-CS-Schiff base-Pd catalyst has excellent catalytic activity for hydrogenolysis of aromatic aldehyde or ketones. When the electron-donating group, such as OCH3 and (CH3)2N was introduced to benzene ring (entry 8 - 9), the C=O was also effectively reduced to CH2. When the electron-withdrawing group, such as Cl and NO2 attached with benzene ring, not only the C=O can be reduced to CH2, but also the Cl or NO2 can be reduced to H or NH2, respectively (entry 10 - 13). For hydrogenation of 2-hydroxy-1-naphthaldehyde (entry 14), the entirely conversion was found and the C=O was also transferred to CH2. The more interesting result is that 12 % of 1-methyl-5,6,7,8-tetrahydronaphthalen-2-ol was obtained besides the main product of 1-methylnaphthalen-2-ol, which indicates that the further hydrogenation was occurred for the condensed nucleus after the C=O was transferred to CH2 under this reaction condition. The similar result can be obtained in the hydrogenolysis of benzophenone (entry 15), benzophenone main formed diphenylmethane, and minority (cyclohexylmethyl) benzene was also observed.
The hydrogenolysis experiment of 2-hydroxy-1,2-diphenylethanone was also carried out and gave meaningful result (entry 17), the C=O and the OH were all transferred to CH2, which further confirms the conclusion that C=O of aromatic aldehydes or ketones was first reduced to CHOH, and then CHOH was conversed to CH2 by hydrogenolysis in this reaction system.
4. Conclusion
An effectively hydrogenolysis of aromatic aldehydes and ketones was developed. The silica supported Schiff base modified chitosan-palladium catalyst exhibited excellent catalytic ability of aromatic aldehydes or ketones are conversed into corresponding aromatic hydrocarbons in the presence of hydrogen with mild conditions. In this dexoygenation progress, C=O of aromatic aldehydes or ketones is firstly converses to CHOH, and then CHOH is further conversed to CH2. Compared with the comercial
Table 5. Hydrogenolysis of some different carbonyl compounds catalyzed by SiO2-CS-Schiff-base-Pd catalyst.
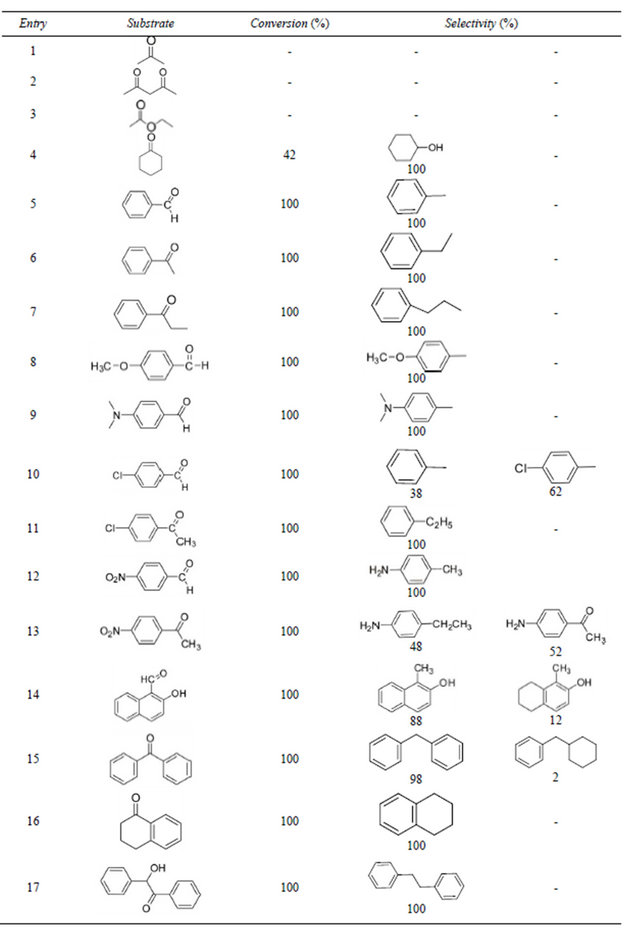
Reaction condition: 0.5 mL or 0.3 g substrate, 0.2 g catalyst, 15 mL ethanol, 313 K, 40 mL/min H2, 5 h.
Clemens or Wolff-Kishner reaction, the hydrogenolysis catalyzed by silica supported chitosan-Schiff base-palladium catalyst is more eco-friendly, there in no any toxic or corrosive reagents are used.
5. Acknowledgements
The authors are grateful for the support of the Doctor Foundation of Shandong Province (BS2010CL011) and the National Basic Research Program (No. 2010CB234601).
REFERENCES
- N. Desbois, A. Szollosi, A. Maisonial, V. Weber, E. Moreau, J. C. Teulade, O. Chavignon, Y. Blache and J. M. Chezal, “Simple and Convenient Conversion of Acridones into 9-Unsubstituted Acridines via Acridanes using Borane Tetrahydrofuran Complex,” Tetrahedron Letters, Vol. 50, No. 49, 2009, pp. 6894-6896. doi:10.1016/j.tetlet.2009.09.141
- W. P. Reeves, J. A. Murry, D. W. Willoughby and W. J. Friedrich, “Clemmensen Reductions Using Ultrasonic Irradiation,” Synthetic Communications, Vol. 18, No. 16- 17, 1988, pp. 1961-1966.
- C. A. Marques, M. Selva and P. J. Tundo, “Facile Hydrodehalogenation with H2 and Pd/C Catalyst under Multiphase Conditions. 3. Selective Removal of Halogen from Functionalized Aryl Ketones. 4. Aryl Halide-Promoted Reduction of Benzyl Alcohols to Alkanes,” The Journal of Organic Chemistry, Vol. 60, 1995, pp. 2430- 2435. doi:10.1021/jo00113a024
- L. S. Glebov, A. I. Mikaya, A. E. Yatsenko, V. G. Zaikin, G. A. Kliger and S. M. Loktev, “Effective Gas-phase Deoxygenation of Alcohols and Ketones on Iron Catalyst,” Tetrahedron Letters, Vol. 26, No. 28, 1985, pp. 3373- 3376. doi:10.1016/S0040-4039(00)98301-1
- R. Šuláková, R. Hrdina and G. M. B. Soares, “Oxidation of Azo Textile Soluble Dyes with Hydrogen Peroxide in the Presence of Cu(II)-chitosan Heterogeneous Catalysts,” Dyes and Pigments, Vol. 73, No. 1, 2007, pp. 19- 24. doi:10.1016/j.dyepig.2005.10.004
- A. Burkhardt, H. Görls and W. Plass, “Nickel(II) Complexes with Schiff-base Ligands Derived from Epimeric Pyranose Backbones as 2,3-Chelators: Modeling the Coordination Chemistry of Chitosan,” Carbohydrate Research, Vol. 343, No. 7, 2008, pp. 1266-1277. doi:10.1016/j.carres.2008.01.039
- R. Abu-Reziq, D. Avnir and J. Blum, “Catalytic Hydrogenolysis of Aromatic Ketones by a Sol-Gel Entrapped Combined Pd-[Rh(cod)Cl]2 Catalyst,” Journal of Molecular Catalysis A: Chemical, Vol. 187, No. 2, 2002, pp. 277-281. doi:10.1016/S1381-1169(02)00235-2
- C. Van Doorslaer, J. Wahlen, P. G. N. Mertens, B. Thijs, P. Nockemann, K. Binnemans and D. E. De Vos, “Catalytic Hydrogenolysis of Aromatic Ketones in Mixed Choline-Betainium Ionic Liquids,” ChemSusChem, Vol. 1, No. 12, 2008, pp. 997-1005. doi:10.1002/cssc.200800140
- S. E. S. Leonhardt, A. Stolle, B. Ondruschka, G. Cravotto, C. De Leo, K. D. Jandt and T. F. Keller, “Chitosan as a Support for Heterogeneous Pd Catalysts in Liquid Phase Catalysis,” Applied Catalysis A: General, Vol. 379, No. 1-2, 2010, pp. 30-37. doi:10.1016/j.apcata.2010.02.029
- D. Wei, Y. Ye, X. Jia, C. Yuan and W. Qian, “Chitosan as an Active Support for Assembly of Metal Nanoparticles and Application of the Resultant Bioconjugates in Catalysis,” Carbohydrate Research, Vol. 345, No. 1, 2010, pp. 74-81. doi:10.1016/j.carres.2009.10.008
- N. Sudheesh, S. K. Sharma1 and R. S. Shukla, “Chitosan as an Eco-friendly Solid Base Catalyst for the Solventfree Synthesis of Jasminaldehyde,” Journal of Molecular Catalysis A: Chemical, Vol. 321, No. 1-2, 2010, pp. 77- 82. doi:10.1016/j.molcata.2010.02.005
- A. B. Sorokin, F. Quignard, R. Valentin and S. Mangematin, “Chitosan Supported Phthalocyanine Complexes: Bifunctional Catalysts with Basic and Oxidation Active Sites,” Applied Catalysis A: General, Vol. 309, No. 2, 2006, pp. 162-168. doi:10.1016/j.apcata.2006.03.060
- J. W. Park, M. O. Park and K. K. Park, “Mechanism of Metal ion Binding to Chitosan in Solution. Cooperative Interand Intramolecular Chelations,” The Bulletin of the Korean Chemical Society, 1984, vol. 5, pp. 108-112.
- F. P. Blondet, T. Vincent and E. Guibal, “Hydrogenation of Nitrotoluene Using Palladium Supported on Chitosan Hollow Fiber: Catalyst Characterization and Influence of Operative Parameters Studied by Experimental Design Methodology,” International Journal of Biological Macromolecules, Vol. 43, No. 1, 2008, pp. 69-78. doi:10.1016/j.ijbiomac.2007.11.008
- F. Peirano, T. Vincent, F. Quignard, M. Robitzer and E. Guibal, “Palladium Supported on Chitosan Hollow Fiber for Nitrotoluene Hydrogenation,” Journal of Membrane Science, Vol. 329, No. 1-2, 2009, pp. 30-45. doi:10.1016/j.memsci.2008.12.022
- H. S. Han, S. N. Jiang, M. Y. Huang and Y. Y. Jiang, “Catalytic Hydrogenation of Aromatic Nitro Compounds by Non-Noble Metal Complexes of Chitosan,” Polymers for Advanced Technologies, Vol. 7, No. 8, 1996, pp. 704- 706. doi:10.1002/(SICI)1099-1581(199608)7:8<704::AID-PAT567>3.0.CO;2-3
- M. Adlim, M. A. Bakar, K. Y. Liew and J. Ismail, “Synthesis of Chitosan-Stabilized Platinum and Palladium Nanoparticles and their Hydrogenation Activity,” Journal of Molecular Catalysis A: Chemical, Vol. 212, No. 1-2, 2004, pp. 141-149. doi:10.1016/j.molcata.2003.08.012
- T. Vincent, S. Spinelli and E. Guibal, “Chitosan-Supported Palladium Catalyst. II. Chlorophenol Dehalogenation,” Industrial & Engineering Chemistry Research, Vol. 42, No. 24, 2003, pp. 5968-5976. doi:10.1021/ie0301482
- M. Y. Yin, G. L. Yuan, Y. Q. Wu, M. Y. Huang and Y. Y. Jiang, “Asymmetric Hydrogenation of Ketones Catalyzed by a Silica-Supported Chitosan-Palladium Complex,” Journal of Molecular Catalysis A: Chemical, Vol. 147, No. 1-2, 1999, pp. 93-98. doi:10.1016/S1381-1169(99)00133-8
- Y. X. Sun, Y. Guo, Q. Z. Lu, X. L. Meng, X. H. Wu, Y. L. Guo, Y. S. Wang, X. H. Liu and Z. G. Zhang, “Highly Selective Asymmetry Transfer Hydrogenation of Prochiral Acetophenone Catalyzed by Palladium-Chitosan on Silica,” Catalsis Letter, Vol. 100, No. 3-4, 2005, pp. 213- 217. doi:10.1007/s10562-004-3458-1
- J. E. dos Santos, E. R. Dockal and É. T. G. Cavalheiro, “Synthesis and Characterization of Schiff Bases from Chitosan and Salicylaldehyde Derivatives,” Carbohydrate Polymers, Vol. 60, No. 3, 2005, pp. 277-282. doi:10.1016/j.carbpol.2004.12.008
- G. K. Moore and G. A. F. Roberts, “A Compound Hydrodynamic Shape Function Derived from Viscosity and Molecular Covolume Measurements,” International Journal of Biological Macromolecules, Vol. 3, No. 5, 1981, pp. 337-341. doi:10.1016/0141-8130(81)90053-2
- S. W. Gong, H. F. He, C. Q. Zhao, L. J. Liu and Q. X. Cui, “Convenient Deoxygenation of Aromatic Ketones by Silica Supported Chitosan Schiff-Base Palladium Catalyst,” Synthetic Communications, Vol. 42, No. 4, 2012, pp. 574- 581. doi:10.1080/00397911.2010.527423
- J. Tong, Z. Li and C. Xia, “Highly Efficient Catalysts of Chitosan-Schiff Base Co(II) and Pd(II) Complexes for Aerobic Oxidation of Cyclohexane in the Absence of Reductants and Solvents,” Journal of Molecular Catalysis A: Chemical, Vol. 231, No. 1-2, 2005, pp. 197-203. doi:10.1016/j.molcata.2005.01.011
- H. F. He, S. W. Gong, L. J. Liu and Q. X.Cui and H.D. Yin, “Hydrogenation of Aromatic Ketones to Aromatic Hydrocarbons over SiO2-Supported Chitosan Schiff-Base Palladium Catalyst,” Chinese Journal of Catalysis, Vol. 31, No. 7, 2010, pp. 846-850.
NOTES
*Corresponding author.

