Modern Research in Catalysis
Vol.1 No.2(2012), Article ID:21294,8 pages DOI:10.4236/mrc.2012.12003
I2/Nanostructured Pyrophosphate: A Mild and Efficient Catalyst for the Selective Protection of Carbonyl Compounds
1Laboratoire de Catalyse, Chimiométrie et Environnement, URAC 24 (Unité de Recherche Associée au Centre National de Recherche Scientifique et Technique), Faculté des Sciences et Techniques, Université Hassan II, Mohammedia, Morocco
2Centre National pour la Recherche Scientifique et Technique (CNRST), Division, Unités d’Appui Technique à la Recherche Scientifique, Angle Allal Fassi/FAR, Hay Riad, Rabat, Morocco
3MAScIR Foundation, Institute of Nanomaterials & Nanotechnology, L’Ecole Normale Supérieure de l’Enseignement Technique, Rabat, Morocco
Email: *m.zahouily@mascir.com
Received April 20, 2012; revised May 30, 2012; accepted June 17, 2012
Keywords: Catalyst; Free Solvent; Nanostructured Pyrophosphate; Protection of Carbonyl Compounds; Iodine
ABSTRACT
A new solvent free method for protection of carbonyl compounds as their thioacetals has been accomplished through the use of iodine supported on nanostructured pyrophosphate. Advantages of the methodology include very short reaction time, the requirement for minimum amounts of catalyst, the remarkably simple experimental procedure, and no necessity for solvents or inert atmospheres, excellent yields and recyclability of the catalyst used. An efficient method for the chemoselective thioacetalization of ketones in the presence of aldehydes using I2/nanostructured pyrophosphate is also reported in this article. The nanostructured pyrophosphate was characterized by scanning electron microscopy, X-ray diffraction, infrared spectroscopy, Transmission electron microscopy and thermal gravimetric analysis, respectively.
1. Introduction
Designing of new specific catalysts and exploring their catalytic activity has caused profound effects in optimizing the efficiency of a wide range of organic synthesis. Development of such catalysts has resulted in more economical and environmentally friendly chemistry through replacing nonselective, unstable, or toxic catalysts [1,2]. In the last few years, literature has highlighted the importance of nanosized materials in several scientific and technological areas, and many research councils have intensified investments in nanotechnology for the coming years [3].
The protection of carbonyl functionality as a dithioacetal or a thioketal is important in the multistep total synthesis of complex natural and non-natural products [4,5]. Among carbonyl protecting groups, 1,3-dithiolanes, 1,3-oxathiolanes, and 1,3-dithianes are important as they are inherently stable under both mildly acidic and basic conditions [6]. In addition, these are also utilized as masked acyl anions [7] or masked methylene functional groups [8] in carbon-carbon bond forming reactions. This ‘umpolung’ or inversion of polarity is an integral step of many multistep organic syntheses. In this view, there have been continued improvements in the methods of preparation of thioacetals. Generally, they are prepared by condensation of carbonyl compounds with thiols in the presence of strong acid catalysts such as such as AlCl3 [9], LnCl3 [10], ZnCl2 [11], TiCl4 [12], WCl6 [13], InCl3 [14], In(OTf)3 [15], Sc(OTf)3 [16,17], Bi(NO3)3 [18] and VO(OTf)2 [19] A number of milder procedures employing lithium salts [20-24], NiCl2 [25], CoCl2 [26], NBS [27], and I2 [28] have also been reported for this purpose. Although some of these methods have been carried out under mild reaction conditions, most of them require [29] reflux temperature [30], long reaction times and use expensive and not readily available reagents [31]. They also suffer from a tedious work-up procedure [32] and require the use of stoichiometric reagents [11]. Some of the methods mentioned above are incompatible with other protecting groups and fail to protect deactivated aromatic substrates [33].
Recently, a number of solid supported reagents have also been used for thioacetalization of various types of carbonyl compounds, e.g. ZrCl4-SiO2 [33], SOCl2-SiO2 [34], CoBr2-SiO2 [35], TaCl5-SiO2 [36], Cu(OTf)2-SiO2 [37], NaHSO4-SiO2 [38], I2/natural phosphate [39], ZnCl2/natural phosphate [40] and Si(CH2)2SO3H-SiO2 [41].
Finally, the main disadvantage of almost all the existing methods is that the catalysts are destroyed in the work-up procedure and cannot be recovered or reused. Therefore, there is further scope in exploring mild, chemoselective and efficient methods for thioacetalization of carbonyl compounds.
Furthermore, many studies have been published describing the synthesis and use of the pyrophosphate (Na2CaP2O7) in various fields, particularly in heterogeneous catalysis [42-45]. Bennazha et al. were able to synthesize and characterize a series of pyrophosphates by adopting a dry technique [46]. Recently, we have developed a method to tailor the nanostructure of this pyrophosphate by focusing on reactant crashing and the speed of calcination. This nanostructured phosphate has been used with great success to catalyze the synthesis of 2-amino-chromenes [47]. As a part of our continuing interest in the development of new synthetic methodologies; an efficient method for the chemoselective thioacetalization of aliphatic and both activated and deactivated aromatic carbonyl compounds using iodine supported on nanostructured pyrophosphate (N-Na2CaP2O7) catalyst at room temperature under solvent-free is reported here (Scheme 1).
2. Experimental
2.1. General Remarks
All commercial reagents were purchased from Aldrich Chemical Company and were used without further purification. 1H and 13C NMR spectra were recorder at 400 and 100 MHZ, respectively, on a Bruker DRX-400 spectrometer in CDCl3, using CDCl3 as internal standard. IR spectra were obtained on a FTIR (Bruker Vector 22 spectrometer) and reported in wave numbers (cm–1). Thermogravimetric analyses (TGA) were conducted under air using a TA Instruments Q 500 apparatus with 10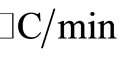 ramp between 25 and 1000˚C. X-ray diffraction (XRD) patterns of the catalyst were obtained at room
ramp between 25 and 1000˚C. X-ray diffraction (XRD) patterns of the catalyst were obtained at room

Scheme 1. Selective protection of carbonyl compounds I2/nanostructured pyrophosphate.
temperature on a Bruker AXS D-8 diffractometer using Cu-Ka radiation in Bragg-Brentano geometry (q-2q). Scanning electron microscopy (SEM) pictures were recorded on a FEI Quanta 200 microscope after carbon metallization. The TEM micrographs were obtained on a Tecnai G2 microscope at 120 kV. The specific surface areas were determined from the nitrogen adsorption/desorption isotherms (at –196˚C) measured with a Quantachrome Autosorb-1 automatic analyzer, using the BET equation at 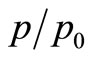 = 0.98. Melting points were determined using a Stuart SN5228 apparatus. Melting points were determined with a ‘Thomas Hoover’ melting (capillary method) apparatus and are uncorrected.
= 0.98. Melting points were determined using a Stuart SN5228 apparatus. Melting points were determined with a ‘Thomas Hoover’ melting (capillary method) apparatus and are uncorrected.
2.2. Preparation of the Catalyst and Structural Characteristics
The synthesis of Na2CaP2O7 was carried out by a dry technique. The pyrophosphate was prepared as a white powder starting from Na2CO3, CaCO3 and NH4H2PO4 of high purity grade and in a 1:1:2 molar ratios, respectively (Scheme 2). These materials were ground together in an agate mortar and progressively heated to 723 K in a porcelain crucible. In order to obtain a homogeneous single phase product, additional grinding and progressive heating steps were carried out. The size of the final product does not only depend on the quality of the starting products but also on the precision of the weighing, grinding and grinding-heating from 373 to 723 K.
Thermogravimetric analysis (TGA) of a mixture of these three reagents showed that the thermal behavior of this blend was suitable to form the desired pyrophosphate (Figure 1). Three major mass losses were observed and corresponded to the dehydration reaction, the loss of NH3 and CO2 as well as the crystallization of this material. X-ray diffraction (XRD) of Na2CaP2O7 showed that this system crystallized in the triclinic system with the space group P1bar (Figure 2). The lattice parameters of the prepared Na2CaP2O7 were in excellent agreement with standard data: a = 5.361 Å, b = 7.029 Å and c = 8.743 Å, with the characteristic peaks of this pyrophosphate all present [Green].

Scheme 2. Synthesis of nanostructured pyrophosphate (N-Na2CaP2O7).
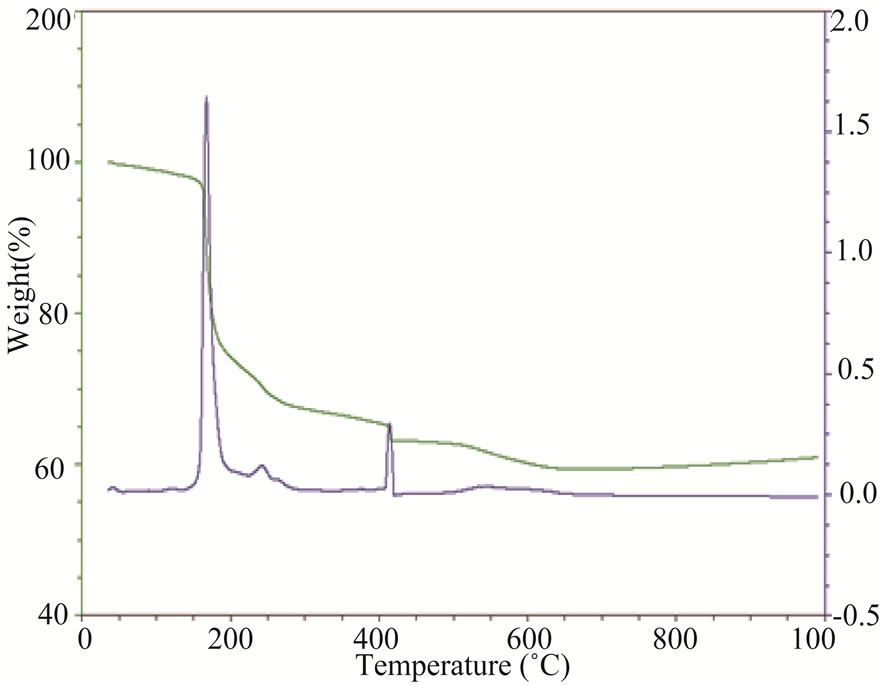
Figure 1. Thermal gravimetric analysis (TGA) of Na2CaP2O7.
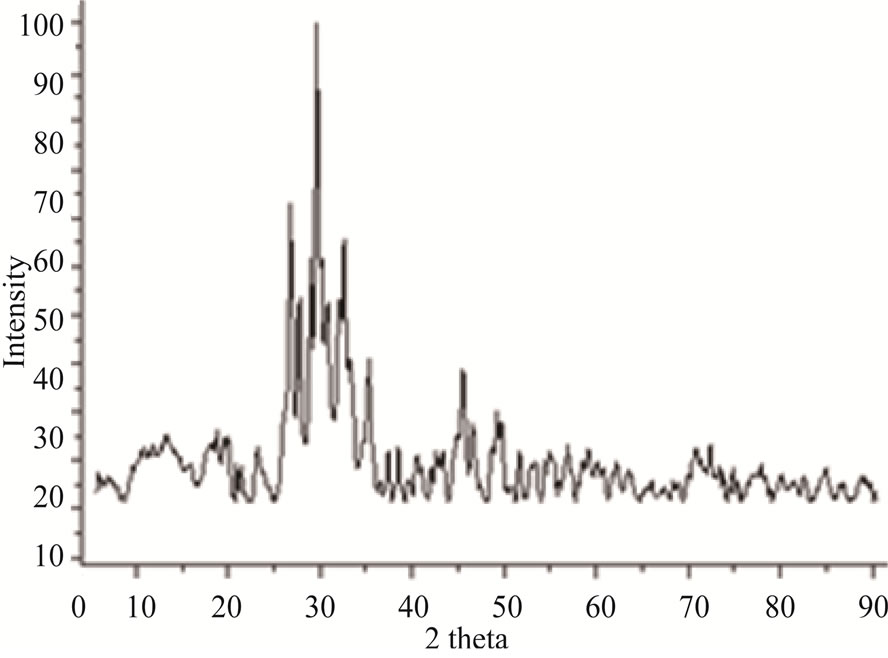
Figure 2. XRD pattern of Na2CaP2O7.
The FTIR spectrum of N-Na2CaP2O7 is presented on the Figure 3. The existence of P2O7 units was confirmed by the symmetrical vibration bands of P-O-P at 720 cm–1 as well as the anti-symmetric vibration bands at 893 cm–1. The related vibrations of the PO4 groups were shared between two fields: a field of symmetrical vibrations at 996 cm–1 and 1031 cm–1 and another that ranged from 1130 to 1278 cm–1 (Figure 3). Scanning Electronic Microscopy (SEM) was used to study the morphology of the surface of N-Na2CaP2O7 (Figure 4). This micrograph shows a homogeneous microstructure that consisted of layers of various sizes and forms.
The shape, size, and morphology of N-Na2CaP2O7 particles were revealed by Transmission electron microscopy “TEM” (Figure 5). The micrograph shows nanoparticles that are rod-like which agglomerate to form superstructures with different aspect ratios of grain crystals (i.e. length/diameter). N-Na2CaP2O7 is characterized by an average grain diameter and length of 15 nm and 40 nm, respectively.
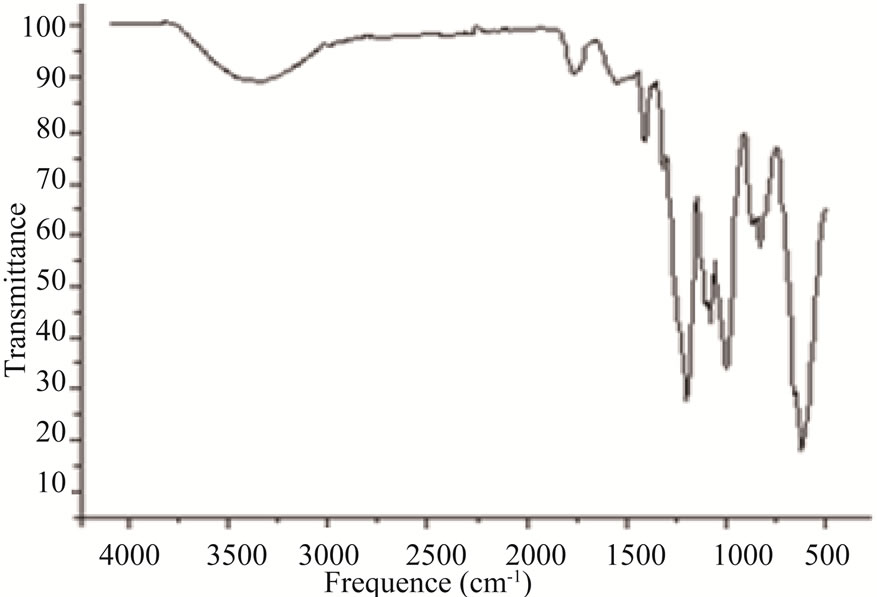
Figure 3. FTIR spectrum of Na2CaP2O7.
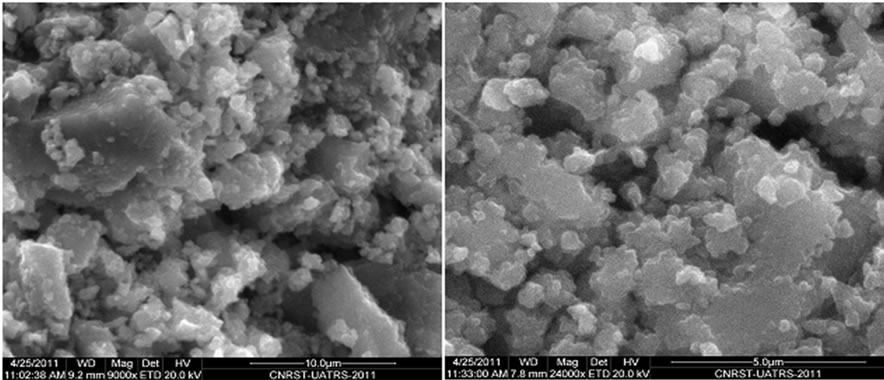
Figure 4. SEM images of Na2CaP2O7 nanopowder.
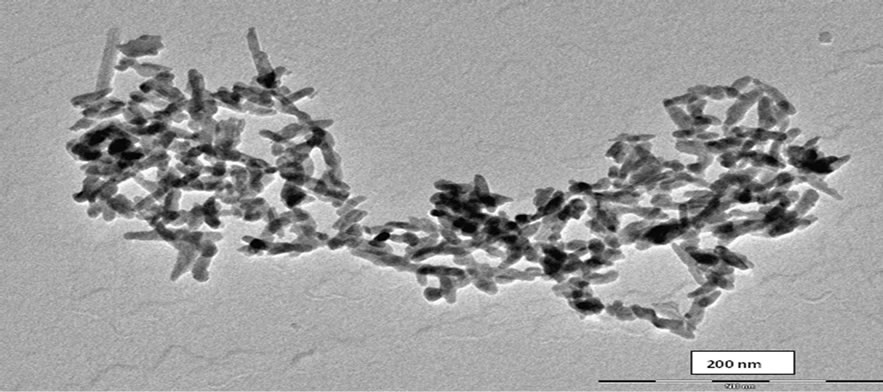
Figure 5. TEM of Na2CaP2O7.
2.3. General Procedure for the Thioacetalization of Carbonyl Compounds
To a freshly prepared catalyst (0.2 g, 0.2 mmol of iodine) under stirring, a mixture of carbonyl compound 1 (2 mmol) and monothiol or dithiol (4.2 or 2.1 mmol, respectively) was added and the mixture was stirred at room temperature and the progress was monitored by TLC using hexane: dichloromethane as an eluent. Following completion of the reaction, the crude reaction mixture was filtered and the catalyst washed with dichloromethane. After concentration of the filtrate under reduced pressure the residue was subjected to chromatography (eluent: 95% hexane and 5% ethyl acetate) leading to the respective thioacetal (2, 3 or 4, Scheme 1). The product structure was analysed by 1H, 13C NMR, IR spectrometry and melting points. When NP and I2 are mixed one pot in the reaction mixture without previous absorption the thioacetal (2, 3 or 4) was obtained in slightly lower yields.
2.4. Typical Procedure for the Recycling of the N-Na2CaP2O7 (Catalyst)
After completion of the reaction, the reaction mixture was filtered and the catalyst washed with dichloromethane. The N-Na2CaP2O7 was washed with acetone 3 - 4 times and drying at 423 K in vacuum or calcined at 723 K to confirm the complete removal of any residual material. The same process was repeated after each reaction cycle to isolate and reuse the N-Na2CaP2O7 as catalyst.
3. Results and Discussion
The scope and failure of the catalyst nanostructured pyrophosphate (N-Na2CaP2O7) as a heterogeneous catalyst in dithioacetalization of a variety of carbonyl compounds under solvent-free examined. Benzaldehyde, thiophenol and iodine (Scheme 1) were chosen as model substrates to determine suitable conditions. The optimum weight of N-Na2CaP2O7 is 200 mg. In a blank reaction (20 min), N-Na2CaP2O7 alone or 0.1 mmol alone the thioacetal 4a was obtained with low yields 13% or 31%, respectively. The N-Na2CaP2O7 was the preferred choice as a catalyst and support to keep the reaction medium under mild and neutral conditions.
Interestingly, the experimental procedure for thioacetalization is remarkably simple and does not require the use of solvents or inert atmospheres. A catalytic quantity of I2 supported on nanostructured pyrophosphate was added to the carbonyl compound, to this was added the required monothiols or dithiols (2.1 or 1.1 equiv., respectively) and the mixture was stirred at room temperature. The generality of this process has been proved in a wide range of aromatic, aliphatic and, α,β-unsaturated carbonyl compounds. Accordingly, dithioacetals, 1,3-dithiolanes and 1,3-dithianes have been obtained by the reaction of thiophenol, 1,2-ethanedithiol and 1,3-propanedithiol, respectively, in the presence of catalytic amounts of I2/N-Na2CaP2O7 (Scheme 1); the results are illustrated in Table 1.
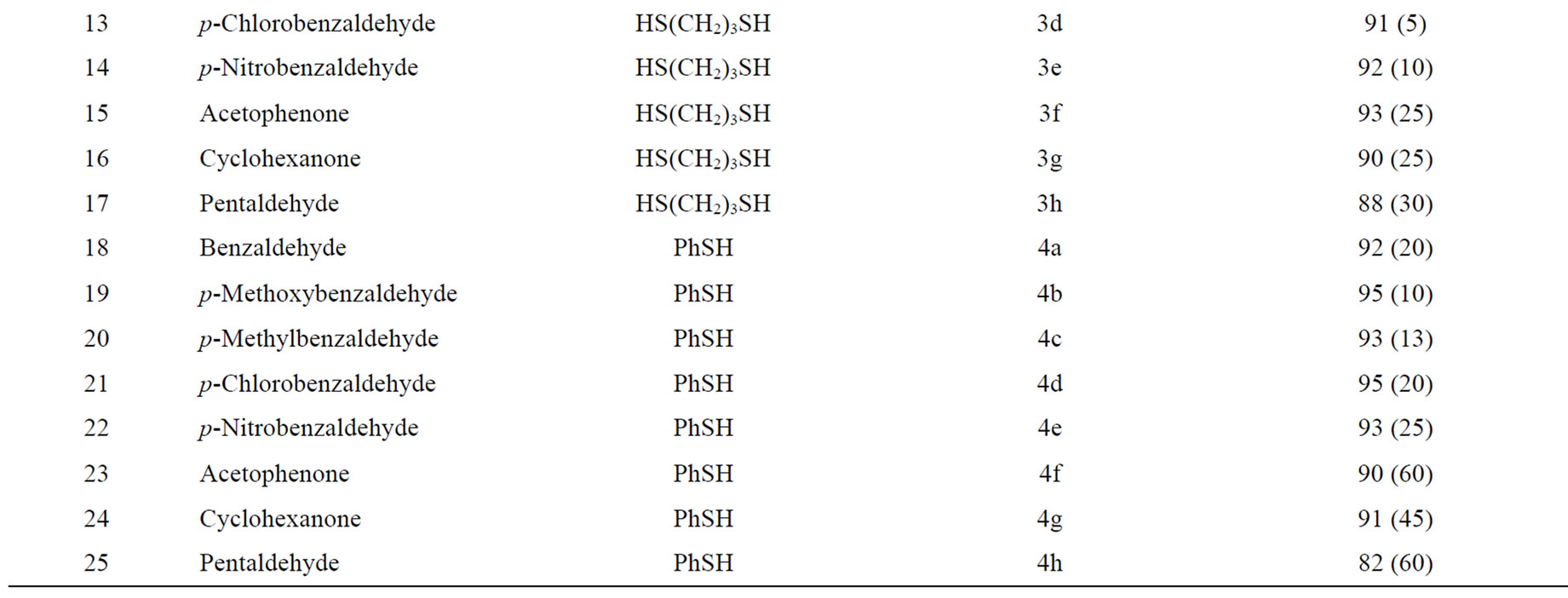
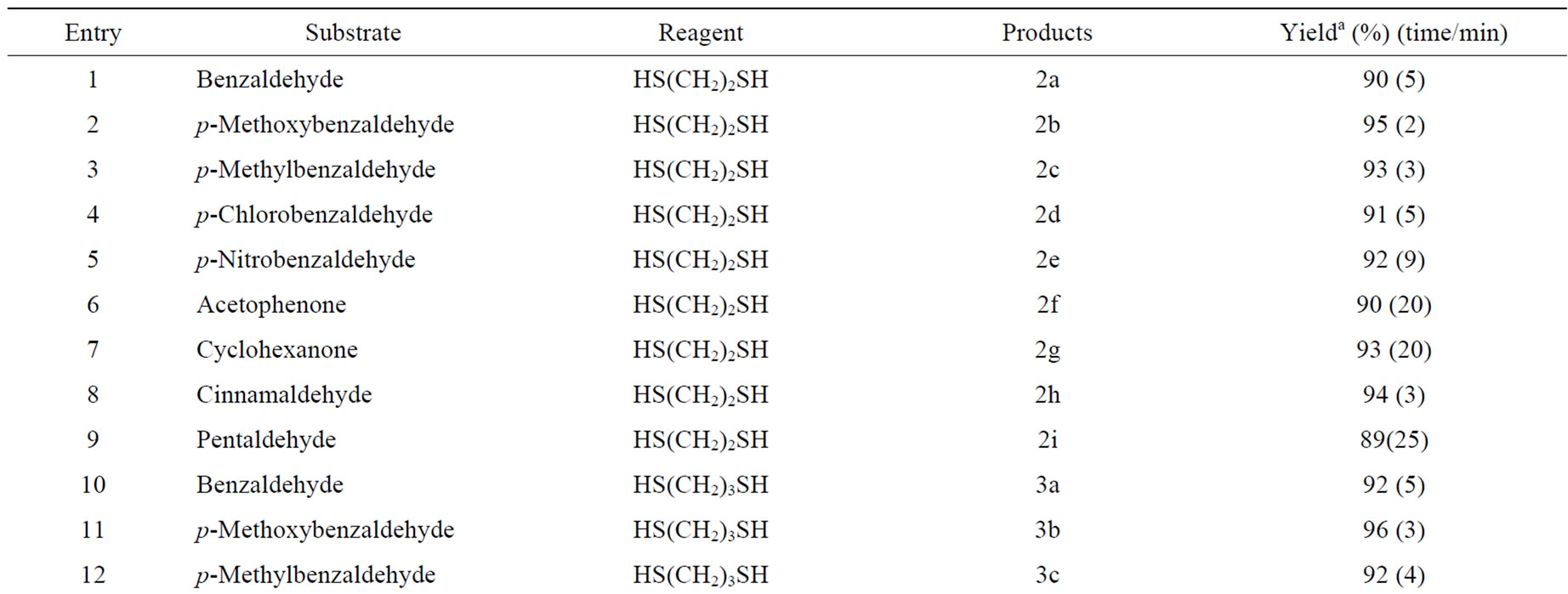
Table 1. I2 supported on nanostructured pyrophosphate (I2/N-Na2CaP2O7) catalysed selective dithioacetalization of carbonyl compoundsa.
We observed that the reaction of aldehydes takes place rapidly in the presence of I2/N-Na2CaP2O7 when compared to that of ketones (see Table 1). This method is suitable for aldehydes and ketones both aromatic and aliphatic as well as, α,β-unsaturated carbonyl compounds. It is free from the problem of Michael addition encountered in some case of, α,β-unsaturated carbonyl systems (Table 1, Entry 8) with some reagents [48].
It is clear from the mechanism (Figure 6), that the corresponding methyl ketones show a lower yield as compared to the aldehydes (Table 1, Entry-1 and 6; 10 and 15; 18 and 23 can be chosen as representative examples of aromatic and aliphatic carbonyl compounds for proving this point). This might have been because of steric factors (the extra bulk imposed by the methyl group on the attaching thiol nucleophile), or electronic factors (the electron-donating inductive effect of methyl group renders the carbonyl carbon less electrophilic) or a combination of both.
Amongst the aromatic carbonyl compounds, the reaction mechanism suggests that the electron donating substituents would activate the benzene ring and thus increase the yield of the products. On the other hand, the electron withdrawing substituents (Table 1, Entry-2 and 5, 11 and 14) would show a slight decrease in the yield of the corresponding 1,3-dithiolanes.
The difference in reactivity of the I2/N-Na2CaP2O7 catalyst towards aldehydes and ketones gave us impetus to study chemoselective reactions. With this objective, as a representative example we carried out some experiments with equimolar mixtures of an aldehyde and a ketone (Scheme 3). It was observed that in this mixture, the corresponding aldehyde formed the 1, 3-dithiolane whilst the ketone was almost completely recovered.
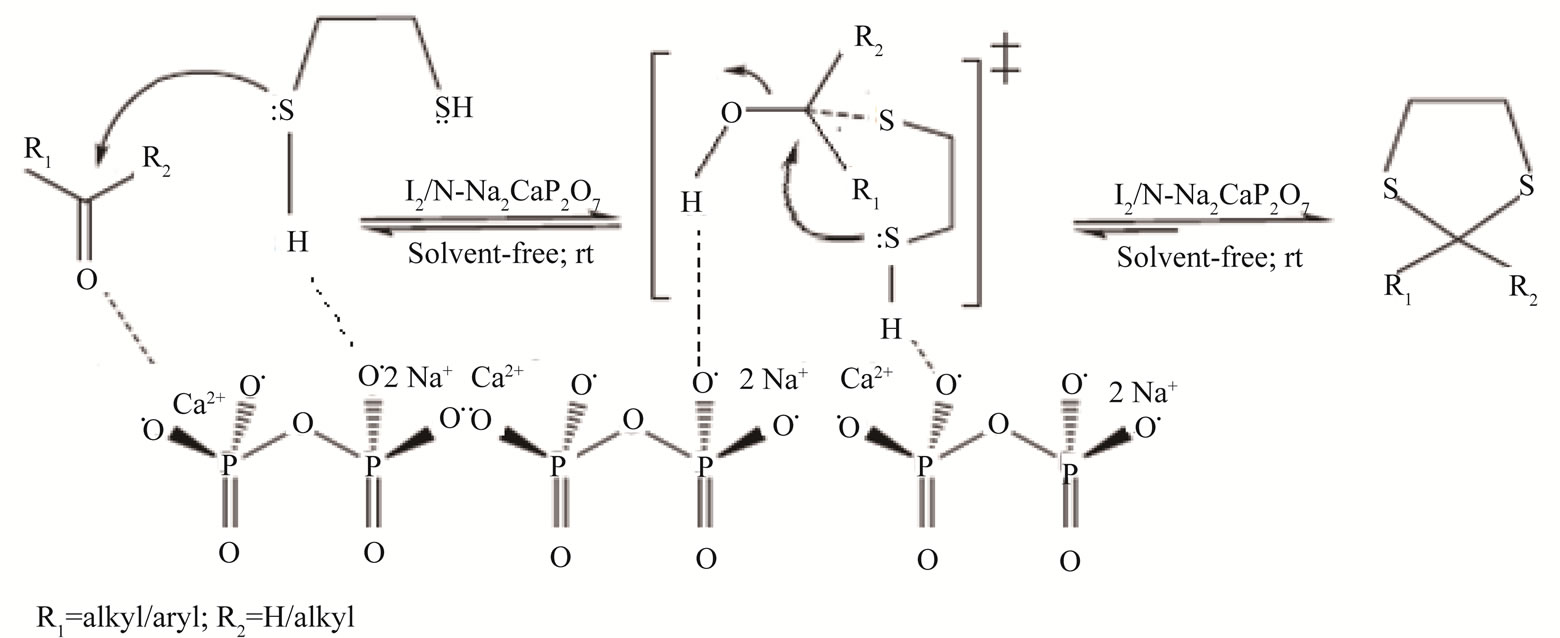
Figure 6. Proposed mechanism for the protection of carbonyl compound using ethane dithiol over I2 supported on nanostructured pyrophosphate (I2/N-Na2CaP2O7) catalyst.
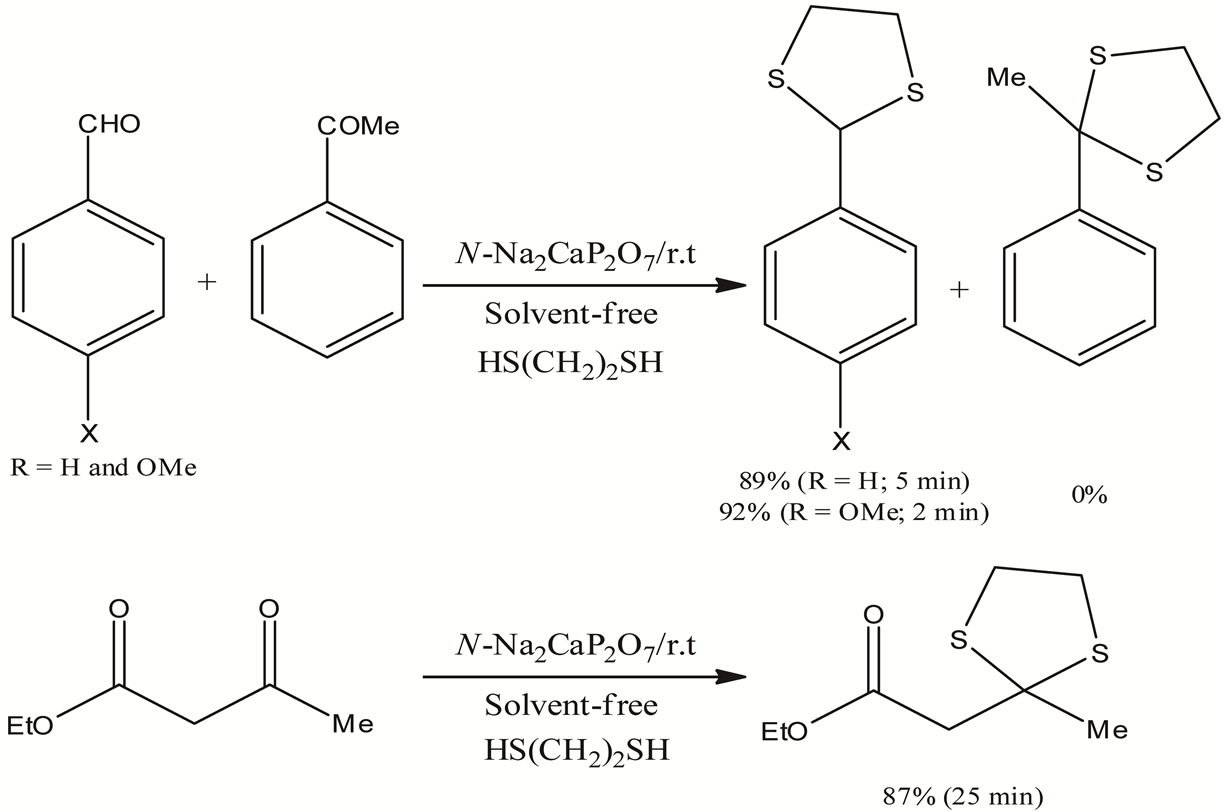
Scheme 3. Chemioselective protection in presence I2/Nanostructured Pyrophosphate catalyst.
This method has also been extended to the intramolecular chemoselectivity between keto and ester functionalities (Scheme 3). Only keto functionality was thioacetalized by this method in a short period (25 min) with a high yield (87%). When compared with the existing method, the present method was found to be superior in many respects.
In all these cases, the aldehyde reacted in almost quantitative yields while the ketone remained unreacted. These reactions are kinetically controlled. The reaction time is only 10 min and thus equilibrium is not allowed to set in. The final step of the reaction mechanism is irreversible as the water which is eliminated separates out from the organic phase into an aqueous phase. In this way, our claim of having developed a gentle methodology for the chemoselective protection of aldehydes using 1,3-dithiolanes in the presence of ketones is justified.
The N-Na2CaP2O7 could be recycled by separating them from the reaction mixture by simple filtration. They could be used as a catalyst for the same reaction again and the change in their catalytic activity was studied. The relation between the number of cycles of the reaction and the catalytic activity in terms of yield is presented in the Figure 7. The catalyst can be recovered and reused at least six times without appreciable loss of activity.
It was observed that with the increasing number of cycles of the reaction, the catalytic activity of the N-Na2CaP2O7 slightly decreased. This might have been due to the slow poisoning of N-Na2CaP2O7.
4. Conclusion
In conclusion a new solvent free method for protection of carbonyl compounds as their thioacetals has been accomplished through the use of iodine supported on nanos
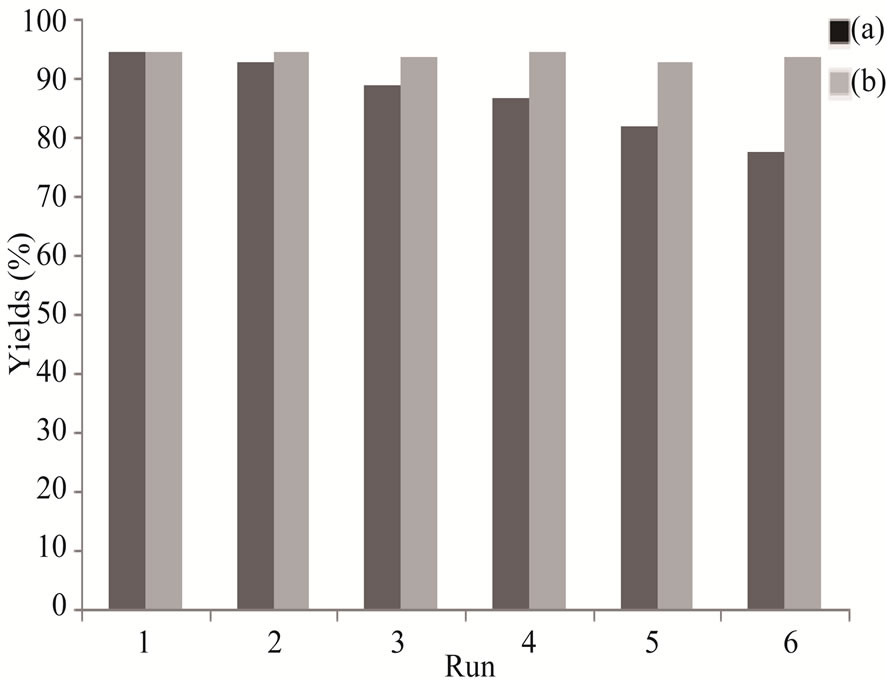
Figure 7. Recycling of the N-Na2CaP2O7 catalyst in the synthesis 2a. (a) Catalyst recoverable dried at 423 K for 1 h; (b) Catalyst recoverable, washed with acetone, dried and calcined at 723 K for 15 - 30 min.
tructured pyrophosphate. Advantages of the methodology include very short reaction time, the requirement for minimum amounts of catalyst, the remarkably simple experimental procedure, and no necessity for solvents or inert atmospheres, excellent yields and recyclability of the catalyst used. Furthermore, the relatively slow reaction rate of ketones allows chemoselective protection of aldehydes in the presence of ketones, making this an important tool in the synthetic organic chemistry.
5. Acknowledgements
The authors thank Centre National de la RechercheScientifiqueet Technique (CNRST) for funding this project under the RS program. We also thank the technical and administrative support teams of the MAScIR Foundation.
REFERENCES
- R. Singh, R. M. Kissling, M. A. Letellier and S. P. Nolan, “Transesterification/Acylation of Secondary Alcohols Mediated by N-heterocyclic Carbene Catalysts,” Journal of Organic Chemistry, Vol. 69, No. 1, 2004, pp. 209-212. doi:10.1021/jo035431p
- P. T. Anastas, L. B. Bartlett, M. M. Kirchhof and T. C. Williamson, “The Role of Catalysis in the Design, Development, and Implementation of Green Chemistry,” Catalysis Today, Vol. 55, No. 1-2, 2000, pp. 11-22. doi:10.1016/S0920-5861(99)00222-9
- P. Claus, A. Bruckner, C. Mohr and H. Hofmeister, “Supported Gold Nanoparticles from Quantum Dot to Mesoscopic Size Scale: Effect of Electronic and Structural Properties on Catalytic Hydrogenation of Conjugated Functional Groups,” Journal of the American Chemical of Society, Vol. 122, No. 46, 2000, pp. 11430- 11439. doi:10.1021/ja0012974
- T. W. Greene and P. G. M. Wuts, “Protective Groups in Organic Synthesis,” Third Edition, Wiley, New York, 1999. doi:10.1002/0471220574
- P. J. Kocienski, “Protecting Groups, Stuttgart,” Thieme, 1994.
- E. L. Eliel and S. M. Natschke, “Asymmetric Syntheses Based on 1,3-Oxathianes. 1. Scope of the Reaction,” Journal of the American Chemical of Society, Vol. 106, No. 10, 1984, pp. 2937-2942.
- B. T. Grobel and D. Seebach, “Reactivity of Carbonyl-Compounds through Sulfur-Containing Reagents,” Synthesis, Vol. 6, 1977, pp. 357-402. doi:10.1055/s-1977-24412
- G. R. Pettit and E. E. V. Tamelen, “Desulfurization with Raney Nickel,” Organic Reactions, Vol. 12, 1962, pp. 356-529.
- B. S. Ong, “A Simple and Efficient Method of Thioacetal —And Ketalization,” Tetrahedron Letters, Vol. 21, No. 51, 1980, pp. 4225-4228. doi:10.1016/S0040-4039(00)92868-5
- L. Garlaschelli and G. Vidari, “Anhydrous Lanthanum Trichloride, a Mild and Convenient Reagent for Thioacetalization,” Tetrahedron Letters, Vol. 31, No. 40, 1990, pp. 5815-5816. doi:10.1016/S0040-4039(00)97967-X
- D. A. Evans, L. K. Truesdule, K. G. Grimm and S. L. Nesbitt, “Thiosilanes, a Promising Class of Reagents for Selective Carbonyl Protection,” Journal of the American Chemical of Society, Vol. 99, No. 15, 1977, pp. 5009- 5017. doi:10.1021/ja00457a020
- V. Kumar and S. Dev, “Titanium Tetrachloride, an Efficient and Convenient Reagent for Thioacetalization1,1,” Tetrahedron Letters, Vol. 24, No. 12, 1983, pp. 1289- 1292. doi:10.1016/S0040-4039(00)81637-8
- H. Firouzabadi, N. Iranpoor and B. Karimi, “Tungsten Hexachloride (WCl6) as an Efficient Catalyst for Chemoselective Dithioacetalization of Carbonyl Compounds and Transthioacetalization of Acetals,” Synlett, Vol. 7, 1998, pp. 739-740. doi:10.1055/s-1998-1756
- S. Muthusamy, S. Arulanada Babu and C. Gunanathan, “Indium(III) Chloride as an Efficient, Convenient Catalyst for Thioacetalization and Its Chemoselectivity,” Tetrahedron Letters, Vol. 42, No. 3, 2001, pp. 359-362. doi:10.1016/S0040-4039(00)01966-3
- S. Muthusamy, S. Arulananda Babu and C. Gunanathan, “Indium Triflate: A Mild Lewis Acid Catalyst for Thioacetalization and Transthioacetalization,” Tetrahedron, Vol. 58, No. 39, 2002, pp. 7897-7901. doi:10.1016/S0040-4020(02)00897-9
- A. Kamal and G. Chouhan, “Scandium Triflate as a Recyclable Catalyst for Chemoselective Thioacetalization,” Tetrahedron Letters, Vol. 43, No. 7, 2002, pp. 1347-1350. doi:10.1016/S0040-4039(01)02378-4
- A. Kamal and G. Chouhan, “Chemoselective Thioacetalization and Transthioacetalization of Carbonyl Compounds Catalyzed by Immobilized Scandium(III) Triflate in Ionic Liquids,” Tetrahedron Letters, Vol. 44, No. 16, 2003, pp. 3337-3340. doi:10.1016/S0040-4039(03)00580-X
- N. Komatsu, M. Uda and H. Suzuki, “Bismuth(III) Halides and Sulfate as Highly Efficient Catalyst for the Sulfenylation of Carbonyl and Related Compounds,” Synlett, Vol. 9, 1995, pp. 984-986. doi:10.1055/s-1995-5137
- S. K. De, “Vanadyl Triflate as an Efficient and Recyclable Catalyst for Chemoselective Thioacetalization of Aldehydes,” Journal of Molecular Catalysis A: Chemical, Vol. 226, No. 1, 2005, pp. 77-79. doi:10.1016/j.molcata.2004.09.044
- H. Firouzabadi, N. Iranpoor and B. Karimi, “Lithium Bromide-Catalyzed Highly Chemoselective and Efficient Dithioacetalization of α,β-Unsaturated and Aromatic Aldehydes under Solvent-Free Conditions,” Synthesis, Vol. 22, 1999, pp. 58-60. doi:10.1055/s-1999-3679
- V. G. Saraswathy, V. Geetha and S. Sankararaman, “Chemoselective Protection of Aldehydes as Dithioacetals in Lithium Perchlorate-Diethyl Ether Medium. Evidence for the Formation of Oxocarbenium Ion Intermediate from Acetals,” Journal of Organic Chemistry, Vol. 59, No. 16, 1994, pp. 4665-4670. doi:10.1021/jo00095a049
- L. F. Tietze, B. Weigand and C. A. Wulff, “Mild and Efficient Method for the Preparation of 1,3-Dithianes from Aldehydes and Ketones,” Synthesis, Vol. 2000, No. 1, 2000, pp. 69-71. doi:10.1055/s-2000-6226
- J. S. Yadav, B. V. S. Reddy and S. K. Pandey, “LiBF4 Catalyzed Chemoselective Conversion of Aldehydes to 1,3-Oxathiolanes and 1,3-Dithianes,” Synlett, Vol. 2, No. 1, 2001, pp. 238-239.
- H. Firouzabadi, B. Karimi and S. Eslami, “Lithium Trifluoromethanesulfonate (LiOTf) as a Highly Efficient Catalyst for Chemoselective Dithioacetalization of Carbonyl Compounds under Neutral and Solvent-Free Conditions,” Tetrahedron Letters, Vol. 40, No. 21, 1999, pp. 4055-4058. doi:10.1016/S0040-4039(99)00647-4
- T. H. Khan, E. Mondal, D. R. Sahu and S. Islam, “Nickel(II) Chloride as an Efficient and Useful Catalyst for Chemoselective Thioacetalization of Aldehydes,” Tetrahedron Letters, Vol. 44, No. 5, 2003, pp. 919-922. doi:10.1016/S0040-4039(02)02771-5
- S. K. De, “Cobalt(II) Chloride Catalyzed Chemoselective Thioacetalization of Aldehydes,” Tetrahedron Letters, Vol. 45, No. 5, 2004, pp. 1035-1036. doi:10.1016/j.tetlet.2003.11.082
- A. Kamal and G. Chouhan, “Mild and Efficient Chemoselective Protection of Aldehydes as Dithioacetals Employing N-Bromosuccinimide,” Synlett, Vol. 3, 2002, pp. 474-476. doi:10.1055/s-2002-20469
- S. Samajdar, M. K. Basu, F. F. Becker and B. K Banik, “A New Molecular Iodine-Catalyzed Thioketalization of Carbonyl Compounds: Selectivity and Scope,” Tetrahedron Letters, Vol. 42, No. 27, 2001, pp. 4425-4427. doi:10.1016/S0040-4039(01)00752-3
- Y. Kamitori, M. Hojo, R. Masuda, T. Kimura and T. Yoshida “Selective Protection of Carbonyl Compounds. Silica Gel Treated with Thionyl Chloride as an Effective Catalyst for Thioacetalization,” Journal of Organic. Chemistry, Vol. 51, No. 9, 1986, pp. 1427-1431. doi:10.1021/jo00359a009
- C. Djerassi and M. Gorman, “Studies in Organic Sulfur Compounds. Cyclic Ethylene and Trimethylene Hemithioketals,” Journal of the American Chemical of Society, Vol. 75, No. 13, 1953, pp. 3704-3708. doi:10.1021/ja01111a029
- N. M. Leonard, M. C. Oswald, D. A. Friederg, B. A. Nattier, R. C. Smith and R. S. Mohan, “A Simple and Versatile Method for the Synthesis of Acetals from Aldehydes and Ketones Using Bismuth Triflate,” Journal of Organic Chemistry, Vol. 67, No. 7, 2002, pp. 5202-5207. doi:10.1021/jo0258249
- B. S. Ong, “A Simple and Efficient Method of Thioacetal and Ketalization,” Tetrahedron Letters, Vol. 21, No. 44, 1980, pp. 4225-4228. doi:10.1016/S0040-4039(00)92868-5
- H. K. Pantey and S. Margan, “Zirconium(IV) ChlorideSilica Catalysed Thioacetalisation of Carbonyl Compounds,” Tetrahedron Letters, Vol. 37, 1996, pp. 4621- 4622. doi:10.1016/0040-4039(96)00892-1
- Y. Kamitori, M. Hojo, R. Masuda, T. Kimura and T. Yoshida, “Selective Protection of Carbonyl Compounds. Silica Gel Treated with Thionyl Chloride as an Effective Catalyst for Thioacetalization,” Journal of Organic Chemistry, Vol. 51, No. 9, 1986, pp. 1427-1431. doi:10.1021/jo00359a009
- H. K. Patney, “Anhydrous Cobalt(II) Bromide Dispersed on Silica Gel: A Mild and Efficient Reagent for Thioacetalisation of Carbonyl,” Tetrahedron Letters, Vol. 35, No. 31, 1994, pp. 5717-5718. doi:10.1016/S0040-4039(00)77287-X
- S. Chandrasekhar, M. Takhi, Y. R. Reddy, S. Mohapatra, C. R. Rao and K. V. Reddy, “TaCl5-Silicagel and TaCl5 as New Lewis Acid Systems for Selective Tetrahydropyranylation of Alcohols and Thioacetalisation, Trimerisation and Aldolisation of Aldehydes,” Tetrahedron, Vol. 53, No. 44, 1997, pp. 14997-15004. doi:10.1016/S0040-4020(97)01051-X
- R. V. Anand, P. Sarvanan and V. K. Sigh, “Solvent Free Thioacetalization of Carbonyl Compounds Catalyzed by Cu(OTf)2-SiO2,” Synlett, Vol. 4, 1999, pp. 415-416. doi:10.1055/s-1999-2635
- B. Das, R. Ramu, M. R. Reddy and G. Mahender, “Simple, Mild and Efficient Thioacetalization and Transthioacetalization of Carbonyl Compounds and Deprotection of Thioacetals: Unique Role of Thiols in the Selectivity of Thioacetalization,” Synthesis, Vol. 280, No. 1-2, 2005, pp. 250-254. doi:10.1055/s-2004-834934
- M. Zahouily, A. Mezdar, J. Rakik, A. Elmakssoudi, A. Rayadh and S. Sebti, “A Mild and Efficient Method for the Protection of Carbonyl Compounds as Dithioacetals, Dithiolanes and Dithianes Catalysed by Iodine Supported on Natural Phosphate,” Journal of Molecular Catalysis A: Chemical, Vol. 233, No. 1-2, 2005, pp. 233, 43-47.
- M. Zahouily, A. Mezdar, J. Rakik, A. Elmakssoudi, B. Mounir, A. Rayadh, S. Sebti and H. B. Lazrek, “Solvent Free Lewis Acid Catalyzed Vinylogous Condensation,” ARKIVOC, Vol. 2, 2006, pp. 31-40.
- B. Karimi and M. Khalkhali, “Silica Functionalized Sulfonic Acid as a Recyclable Interphase Catalyst for Chemoselective Thioacetalization of Carbonyl Compounds in Water,” Journal of Molecular Catalysis A: Chemical, Vol. 271, No. 1-2, 2007, pp. 75-79. doi:10.1016/j.molcata.2007.02.018
- A. Elmakssoudi, M. Zahouily, A. Mezdar, A. Rayadh and S. Sebti, “Na2CaP2O7 a New Catalyst for the Synthesis of α-Amino Phosphonates under Solvent-Free Conditions at Room Temperature,” Comptes Rendus Chimie, Vol. 8, No. 11-12, 2005, pp. 1954-1959. doi:10.1016/j.crci.2005.05.006
- J. Bennazha, M. Zahouily, S. Sebti, A. Boukhari and E. M. Holt, “Na2CaP2O7, a New Catalyst for Knoevenagel Reaction,” Catalysis Communications, Vol. 2, No. 3-4, 2001, pp. 101-104. doi:10.1016/S1566-7367(01)00015-2
- M. Zahouily, M. Salah, J. Bennazha, A. Rayadh and S. Sebti, “Na2CaP2O7, a New Catalyst for the Synthesis of Unsaturated Arylsulfones,” Tetrahedron Letters, Vol. 44, No. 16, 2003, pp. 3255-3257. doi:10.1016/S0040-4039(03)00630-0
- M. Zahouily, Y. Abrouki and A. Rayadh, “Na2CaP2O7, a new Catalyst for Michael Addition,” Tetrahedron Letters, Vol. 43, No. 43, 2002, pp. 7729-7730. doi:10.1016/S0040-4039(02)01846-4
- J. Bennazha, A. Boukhari and E. M. Holt, “Synthesis and Crystal Structure of Na2CaP2O7,” Solid State Sciences, Vol. 1, No. 6, 1999, pp. 373-380. doi:10.1016/S1293-2558(00)80091-6
- A. Solhy, A. Elmakssoudi, R. Tahir, M. Karkouri, M. Larzek, M. Bousmina and M. Zahouily, “Clean Chemical Synthesis of 2-Amino-chromenes in Water Catalyzed by Nanostructured Diphosphate Na2CaP2O7,” Green Chemistry, Vol. 12, 2010, pp. 2261-2267. doi:10.1039/c0gc00387e
- E. J. Corey and K. Shimoji, “Magnesium and Zinc-Catalyzed Thioketalization,” Tetrahedron Letters, Vol. 24, No. 2, 1983, pp. 169-172. doi:10.1016/S0040-4039(00)81357-X
NOTES
*Corresponding author.

