Pharmacology & Pharmacy
Vol.10 No.02(2019), Article ID:90749,12 pages
10.4236/pp.2019.102007
Cytotoxic, Antimicrobial and Antioxidant Properties of Commelina diffusa Burm. F.
Mahmuda Nasrin1, Farhana Afroz2, Suriya Sharmin2, Md. Sohel Rana1, Md. Hossain Sohrab2
1Department of Pharmacy, Jahangirnagar University, Dhaka, Bangladesh
2Pharmaceutical Sciences Research Division, BCSIR Laboratories, Dhaka, Bangladesh

Copyright © 2019 by author(s) and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/



Received: January 20, 2019; Accepted: February 23, 2019; Published: February 26, 2019
ABSTRACT
Commelina diffusa Burm. F. is a herbaceous tropical plant with different traditional medicinal uses. Present study is aimed to isolate the bioactive compounds from DCM-Methanol extract of the powdered whole plant of Commelina diffusa and to investigate the cytotoxic, antimicrobial and antioxidant activities of the crude extract and its twelve vacuum liquid chromatographic fraction (CD1-12). Vacuum Liquid Chromatography (VLC) was employed to isolate bioactive metabolites. The physicochemical properties of the isolated compound were examined and the molecular structure was elucidated by NMR spectroscopy. Cytotoxic, antibacterial, antioxidant activities were evaluated following brine shrimp lethality bioassay, disc diffusion method and DPPH free radical scavenging assay respectively. The isolated compound was identified as steroid (stigmasterol) which had significant cytotoxic effect on vero cell line. The crude extract and its fractions (CD8-CD12) exhibited strong cytotoxicity in brine shrimp lethality bioassay having LC50 values 3.79, 9.19, 29.49, 16.60, 19.36, 44.58 μg/mL respectively. The crude extract showed mild to strong antibacterial activity. Fractions (CD5-CD12) showed mild to strong antibacterial activity against Escherichia coli in comparison with Kanamycin (standard). Strong antioxidant activities were found in crude extract (IC50 value 30.52 μg/ml), CD11 (IC50 value 39.27 μg/ml) and CD12 (IC50 value 19.50 μg/ml). The study suggests Commelina diffusa plant extract to have strong antioxidant and cytotoxic activity which is indicative of presence of compounds with broad spectrum of curative applications. One compound namely stigmasterol has been isolated from the plant.
Keywords:
Commelina diffusa, Stigmasterol, Brine Shrimp Lethality Bioassay, Disc Diffusion Method, Free Radical Scavenging Assay

1. Introduction
A remarkable number of potent drugs have been isolated from natural sources such as plants, which are well established in their use as traditional medicine [1]. Molecules derived from natural sources have played a dominant role in the discovery of lead compounds for various diseases because of the unmatched availability of chemical modification. Being non-narcotic with fewer side-effects, easy availability at affordable prices increases the demand for chemical modification in both developing and developed countries. So, research studies leading to extraction, isolation, identification, purification and biological study of the plant constituents have now been the major field of study. Moreover, in vitro study plays an important role for the pre-clinical studies for any activity by supporting the in vivo methods [2]. Modern pharmacopoeia estimated that at least 25% drugs derived from plants and others are synthetic analogues built on prototype compounds. Also, World Health Organization (WHO) assumed that 80% of the population relies on traditional medicines, mostly plant drugs, for their primary health care needs [3].
Commelinaceae is a family of pantropical herbaceous plants made up of about 50 genera and 27 species. In Bangladesh, it is existed in 10 genera and 27 species [4]. In this study, Commelina diffusa (Bengali name: manaina) belonging to Commelinaceae family had been chosen for phytochemical investigation. C. diffusa, a medicinal herb located in tropical and subtropical areas worldwide is used traditionally as febrifuge, diuretic agent, blood coagulant, antidote and heart tonic [5]. Other traditional uses are in urinary and respiratory tract infections, diarrhea, hemorrhoids, enteritis, and eye problems like ophthalmia, and conjunctivitis in different countries of Asia, Africa and America [6]. The leaves and stem of the plant are traditionally used for the treatment of abscess, boils, and malaria, for treatment of insect, snake and bug bites, edema, laryngitis, sore throats, acute tonsillitis, otitis media and in nose bleeding [6]. This plant is also used for groin pain, wound dressing, influenza, dermatitis, dysmenorrhea, leprosy, kidney diseases and in treating many other diseases [5]. The wound healing property of the crude extract has been attributed to its antimicrobial and antioxidant properties [7]. Previous phytochemical reports of C. diffusa suggest presence of glycoside, flavonoid, sterol, terpenoid, tannin, alkaloid, anthraquinone etc. [5] [6]. The antimicrobial, antioxidant and anti-inflammatory [6] [8] activities of the plant were also investigated with positive result.
Therefore, the aim of this study is to investigate the phytochemical content of C. diffusa grown in Bangladeshi climate by chromatographic separation of the crude extract and its fractions and evaluate their antioxidant, cytotoxic and antimicrobial activities for possible isolation of leads for drug development.
2. Materials and Methods
2.1. Instrumentation
Silica gel 60 G (Merck, Germany) was used for chromatographic techniques. NMR spectra were obtained on Bruker (Germany) DPX 400 MHz apparatus, operating at 400 MHz for 1H NMR and at 100 MHz for 13C NMR; and the spectra were referenced to the residual nondeuterated solvent signal. TLC (20 × 5 cm) was carried out on TLC Silica gel 60 F254 on aluminium plates with thickness of 0.25 mm (Merck, Germany). Spots on TLC plates were visualized under UV light at 254 and 365 nm and then by spraying the developed plates with 1% vanillin-sulfuric acid followed by heating for 5 minutes at 110˚C. The UV absorbance was recorded by a UV-Vis spectrophotometer (Analytic Jena AG, Germany). All solvents were of analytical grade and obtained from commercial sources (Sigma Aldrich, USA; Merck, Germany; Active Fine Chemicals Ltd., Dhaka, Bangladesh). Rotary vacuum evaporator (Heidolph Instruments GmbH & Co. KG, Germany) was used for evaporating solvents.
2.2. Collection of the Plant Materials
The whole plant of C. diffusa was collected from Gazipur district, Bangladesh in the month of January, 2017. The plant was identified in Bangladesh National Herbarium, Dhaka, Bangladesh where a voucher specimen (ACC. No. 38390) of the plant has been deposited.
2.3. Preparation of Extract and Fractions
The fresh whole plant (1000 g) of C. diffusa was air dried at room temperature and powdered by using a grinder. The powdered plant was macerated in DCM:Methanol (1:1) at room temperature for 7 days after which the extract was filtered and this process was repeated twice. The combined filtrate was concentrated at reduced pressure and the crude extract (36.84 g) was collected. The crude extract was subjected to VLC using solvent system of n-hexane, chloroform and methanol with increasing polarities to obtain 12 major fractions (CD1-CD12). At first VLC was eluted with n-hexane: CHCl3 gradient system in order of increasing polarities (9:1, 8:2, 7:3, 6:4, 1:1, 4:6, 2:8) to give seven fractions (CD1-CD7) followed by solvent system of CHCl3:MeOH in order of increasing polarities (9.8:0.2, 9:1, 8:2, 1:1, 0:10) to give five fractions (CD8-CD12). The use of spraying reagent revealed the presence of sterols in fraction CD7 eluted with n-hexane/CHCl3 (70 - 80 %v/v). Solvent treatment of this fraction gave a white crystal and named compound 1 (5 mg).
2.4. Bioassays
2.4.1. Anti-Proliferative Assay for the Pure Compound
Anti-proliferative activity was determined through the Trypan Blue Exclusion Method [9] against African Green Monkey Kidney cell (Vero cell) line (CLS 605372, Germany). A stock solution of the test sample was prepared in DMSO (1.0 mg/mL). The cultures of Vero cells were grown in Dulbecco’s Modified Eagles Medium (DMEM) pH 7.4 in 75 cm2 sterile flasks, supplemented with 10% (v/v) fetal bovine serum (FBS), 1% penicillin (100 U/mL)/streptomycin (0.1 mg/mL)/neomycin (0.1 mg/mL), 25 mM HEPES in 5% (v/v) CO2 at 37˚C [10]. According to the experimental design, cells were split into 3 treatment groups in triplicate which were compared with the vehicle. Vero cells were split to culture a number of 2.5 × 106 cells into T-25 flask on the day before experiment. The instantly prepared doses (1.0, 5.0, 10.0, 15.0, 20.0 µg/mL) were administered into these 1 day old cell culture flasks and incubated for 24 hour. 0.5% (v/v) Trypsin was used for cell deattachment. Cell suspension with 0.4% w/v trypan blue (1:1) were added on the surface of haemocytometer and was placed on automated cell counter (LUNA-IITM, analytikjena, South Korea) [11]. The number of unstained (viable) cells and stained (non-viable) cells were counted. Cells were calculated as percentage of dead cells by Equation (1).
, (1)
2.4.2. Cytotoxic Activity for the Crude Extract and Its Fractions
For cytotoxicity screening, dimethyl sulfoxide (DMSO) was used as a solvent and negative control while vincristine sulfate (VS) served as the positive control. In brine shrimp lethality bioassay [12] DMSO solution of the fractions were applied against 1-day old Artemia salina leaches in an in vitro assay. For the experiment, the crude and fraction test samples were dissolved in DMSO. Varying concentrations (400, 200, 100, 50, 25, 12.50, 6.25, 3.125, 1.563, 0.781 μg/mL) of the sample were obtained by serial dilution technique in premarked glass test tubes containing 5 mL of sea water with 10 shrimp naupli. The median lethal concentration (LC50) of the test samples were determined by a plot of percentage of the shrimp mortality against the logarithm of the sample concentrations.
2.4.3. Antimicrobial Activity
In vitro antimicrobial activity of the crude extract and column fractions were assessed against two Gram-positive; Staphylococcus aureus (ATCC 25923) and Bacillus megaterium (ATCC 28318) as well as two Gram-negative bacteria; Pseudomonus aeruginosa (ATCC 27833) and Escherichia coli (ATCC 28739) and two fungal strains namely, Aspergillus niger and Aspergillus flavus. The antimicrobial activity was tested by the standard disc diffusion method [13]. The samples were dissolved separately in specific volume of methylene chloride or methanol depending on their solubility. Kanamycin (30 μg/disc) and Ketoconazole (30 μg/disc) were used as standards for antibacterial and antifungal screening, respectively.
2.4.4. Antioxidant Activity
The DPPH scavenging method [14] was used to evaluate the antioxidant potential of various test samples with some modification. Different concentrations (200, 100, 50, 25, 12.5, 6.25, 3.125 and 1.562 µg/mL) of methanolic solution of the samples were obtained by serial dilution technique in test tubes where 2.0 mL of the sample solution was mixed with 2.0 mL of a DPPH-methanol solution (20 µg/mL) to obtain the above mentioned concentrations. After 30 minute reaction period at room temperature in dark the absorbance was measured against methanol as negative control. Trolox and Butylated Hydroxy Anisole (BHA) were used as positive controls. Percent inhibition of free radical (I%) was calculated by Equation (2).
, (2)
where A is the absorbance of blank (DPPH solution without sample) and B is the absorbance of sample solution (DPPH solution with sample/positive control). Concentration of the sample required for 50% inhibition (IC50) was determined by percent inhibition (I%) versus the concentration curve. Each of the samples was tested in three replication and the results were averaged.
2.5. Statistical Analysis
IC50 values for anti-proliferative assay were compared using independent student’s t-tests. For the analysis continuous variables between groups were compared with one-way analysis of variance (ANOVA) with post Hoctukey’s test using “Graphpad Instat” version 5.00 for windows, Graphpad software, San Diego, California, USA. Statistical values were significant when P < 0.001. Antimicrobial, antioxidant and cytotoxic activity were conducted in triplicate and standard deviation was calculated in Microsoft Excel.
3. Results
3.1. Spectroscopic Characterization for Compound 1
The compound 1 (Figure 1) was obtained as white powder and identified by extensive spectral analysis of NMR data as well as by comparison with previously reported values.
Compound 1: 1H NMR (400 MHz, CDCl3): δ = 3.55 (m, 1H, 3-H), 5.37 (m, 1H, 6-H), 5.18 (dd, 1H, J = 15.1, 8.5 Hz, 22-H), 5.04 (dd, 1H, J = 15.1, 8.5 Hz, 23-H), 1.03 (s, 3H, 18-H), 0.72 (s, 3H, 19-H), 0.86 (d, 26-H) and 0.87 (d, 27-H), 0.95 (d, 3H, J = 6.4 Hz, 21-H), 0.83 (t, 3H, 29-H).
13C NMR (100 MHz, CDCl3): δ = 37.3 (C-1), 28.2 (C-2), 71.8 (C-3), 42.3 (C-4), 140.8 (C-5), 121.7 (C-6), 31.9 (C-7), 33.0 (C-8), 50.2 (C-9), 36.5 (C-10), 26.2 (C-11), 39.8 (C-12), 42.3 (C-13), 56.8 (C-14), 24.3 (C-15), 29.2 (C-16), 56.1 (C-17), 12.1 (C-18), 19.4 (C-19), 40.5 (C-20), 21.2 (C-21), 138.3 (C-22), 129.3 (C-23), 51.3 (C-24), 45.9 (C-25), 19.4 (C-26), 19.8 (C-27), 24.4 (C-28), 12.2 (C-29).
3.2. Bioassays
3.2.1. Anti-Proliferative Activity by Trypan Blue Exclusion Method for the Pure Compound
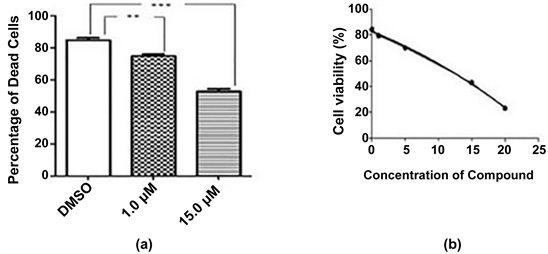
Cytotoxic activity of the pure compound (stigmasterol) was determined on African Green Monkey Kidney cell (Vero cell) line. Figure 2(a) showed increase in cytotoxic activity from 1.0 µM to 15.0 µM against vero cell line which indicated stigmasterol might have anticancer property.
Figure 1. Chemical structure of stigmasterol (1).
Figure 2. (a) Cytotoxic effects of compound on vero cell line after 24 hour incubation. Results are compared with vehicle (DMSO). The results are expressed as mean ± SEM (n = 3). Degrees of significance determined using ANOVA with Post Hoc Tukey’s test for comparison of isolated pure compound with vehicle are ***P < 0.001 and 0.01. (b) IC50 value of compound on vero cell line.
Vero cells were incubated with varying concentration of stigmasterol (0.0, 1.0, 5.0, 15.0, 20.0 µM) and 0.6% DMSO was used as control. The half maximal inhibitory value of stigmasterol was calculated by the analysis of dose response curve and the value was 15.1 µM (Figure 2(b)).
3.2.2. Cytotoxicity by Brine Shrimp Lethality Bioassay for the Crude Extract and Its Fractions
Brine shrimp lethality assay is considered as convenient probe for preliminary assessment of toxicity, detection of fungal toxins, antitumor effect and other pharmacological actions. The results of the brine shrimp lethality bioassay were shown in Figure 3. The LC50 values were calculated by the best fit line slope of the curve of % mortality against log values of test sample concentration. The LC50 value indicated the concentration by which 50% of the shrimp were dead. The crude extract should potent cytotoxicity to brine shrimp with LC50 value of 3.79 µg/mL. There was no cytotoxic effect observed within the remaining fractions CD1-7. Among the column fraction CD8 exhibited highest lethality in
Figure 3. Brine shrimp lethality bioassay of the samples with standard deviation error bar (n = 3).
Brine Shrimp Bioassay with 9.19 µg/mL. LC50 values for other fractions CD9 (29.49 µg/mL), CD10 (16.60 µg/mL), CD11 (19.36 µg/mL) were found to be significant in comparison with vincristine sulfate (VS) as positive control with (0.81 µg/mL).
3.2.3. Antimicrobial Activity Using Disc Diffusion Method
In the antimicrobial screening the crude extract showed mild to moderate activity as the zone of inhibition formed against a wide range of gram positive and gram negative bacteria (Table 1). However, fungal strains (A. niger and A. flavus) were resistant against all the samples. Among all fractions CD6, CD7 and CD8 showed strong activities against E. coli and others fractions showed mild activities compared to kanamycin (30 µg/disc). On the other hand, CD6 and CD7 exhibited strong activities against B. megaterium with zone of inhibition of approximately 20 mm.
3.2.4. Free Radical Scavenging Activity Using DPPH Method
The free radical scavenging activity was expressed by the amount of antioxidant required to decrease the initial absorbance of DPPH. Free radical scavenging activities (Figure 4) were found to be significant in crude extract (IC50 = 30.52 μg/mL), CD11 (IC50 = 39.27 μg/mL) and CD12 (IC50 = 19.50 μg/mL) compared with Trolox (IC50 = 10.93 μg/mL) or Ascorbic Acid (IC50 = 6.43 μg/mL). Additionally, CD6 and CD7 showed lowest antioxidant activity. The other column fractions and the isolated compound stigmasterol were devoid of antioxidant activity and not shown in Figure 4.
4. Discussion
Stigmasterol was obtained as needle shaped crystals with melting point 141˚C. It is not visible under UV light. Spraying the developed plate with Vanillin-H2SO4
Table 1. Results of Antimicrobial Screening of crude extract and its fractions.
*I―Values are in mean inhibition zone (mm) ± SD of three replicates; %I―% inhibition compared to the standard; “KM”―Kanamycin; “KC”―Ketoconazole; “-” indicates no sensitivity; “NA” indicates not applicable.
Figure 4. Free radical scavenging activity of the samples with standard deviation error bar (n = 3).
spray reagent, followed by heating at 110˚C for several minutes, gave purple color (Rf = 0.27, toluene/10% EtOAc). It was found to be soluble in methylene chloride and chloroform.
The 1H NMR spectrum (400 MHz, CDCl3) revealed a one proton multiplet at δ 3.55, the position and multiplicity of which was indicative of H-3 of steroidal nucleus. The typical signal for the olefinic H-6 of the steroidal skeleton was evident from one proton multiplet at δ 5.37. Two olefinic protons, H-22 and H-23 appeared as characteristic downfield signals at δ 5.18 and δ 5.04, respectively. Each of the signals was observed as double doublet, which indicated coupling with the neighbouring olefinic and methine protons. The spectrum further revealed two three-proton singlets at δ 1.03, δ 0.72 assignable for Me-18 and Me-19, respectively. In addition, two doublets at δ 0.86 and δ 0.87 could be ascribed to the two methyl groups for Me-26 and Me-27, respectively and another three-proton doublet at δ 0.95 for Me-21.On the other hand, one three-proton triplet at δ 0.83 could be assigned to Me-29.
The 13C NMR (100 MHz, CDCl3) of stigmasterol displayed 29 carbon resonances, while the DEPT 135 experiment showed signals for six methyls, nine methylenes, eleven methines. The 13C NMR spectral data also suggested that stigmasterol was a sterol with 6 methyl signals at δ = 12.1, 12.2, 19.0, 19.4, and 19.8 and oxygenated methylene signal at δ = 71.8 and 4 olefinic carbon signals at δ = 121.7, 129.3, 138.3, and 140.8. The physical and spectral data of the compound was in complete agreement to the reported data in literature value [15].
Stigmasterol may be a potential chemotherapeutic or a chemopreventive agent based on its ability to induce apoptosis or necrosis in transformed kidney cells or kidney (renal) cancer cells with relatively low toxicity to normal cells. Further studies with in vivo and clinical trials needs to be conducted to establish drugs for cancer therapy [16].
The brine shrimp lethality bioassay has been found as a safe, practical and economic method for determination of bioactivities of synthetic compound as well as plant products. This method was conducted in order to validate the ethno medical claim of C. diffusa in cancer treatment. LC50 value from regression analysis in 24 h showed that the crude extract has LC50 value of 3.79 μg/mL. Comparison of this result with the standard vincristine sulfate (0.81 μg/ml) indicated the lethality of crude extract suggesting its notable therapeutic importance against tumor cell, or as pesticides, herbicides etc. which can be proved by further specific tests. The cytotoxic effect of plant is principally contributed by the presence of phytochemical groups such as alkaloid, tannin, terpenoid, glycosides, steroids, phlobatannin in their extract. Recent studies on different solvent extracts of this plant showed the presence of secondary metabolites like tannins, terpenoids, cardiac glycosides, steroids, phlobatannins, saponins. However, alkaloids and steroids are also known to show cytotoxicity by inhibiting cellular DNA in a concentration [17].
Disc diffusion method is extensively used to explore the antimicrobial activity of natural substances and plant extracts. The presence of flavonoids and tannins in the extract thought to be responsible for activity against pathogenic microorganisms. The crude extract exhibited mild to strong antibacterial activity against bacterial strains with the diameter of inhibition zone ranging approximately from 12 - 17 mm compared to standard, kanamycin. Fractions CD6, CD7 and CD8 were found to possess strong antibacterial effect against E. coli as compared to the standard kanamycin with approximately 20-21 mm of zone of inhibition. Both CD6 and CD7 showed strong activity against B. megaterium as compared to the standard kanamycin. In this study, no fungal strains respond to crude extract and its fractions. However, activity against both Gram-positive and Gram-negative bacteria may be indicative of the presence of broad spectrum of antibacterial compounds [18] [19].
DPPH free radical assay is a rapid and reliable method for evaluating the antioxidant activity of the extracts or pure compounds. The crude extract and CD12 showed significant scavenging effect on DPPH free radical in concentration dependent manner. When compared with standard antioxidants used in the experiment, all the samples showed relatively lower DPPH free radical scavenging potential. This scavenging activity might prevent reactive radical species from damaging biomolecules such as protein, DNA and sugars in susceptible biological and food systems [20].
5. Conclusion
Nowadays natural products have been the focus of scientific studies for ailments by different disease causing agent. Based on these results it can be concluded that the crude extract and its fractions possess significant antioxidant and cytotoxic activity that after rigorous higher and specific tests can be proved to be beneficial for use in humans. The compound stigmasterol, isolated and characterized by chromatographic and spectroscopic techniques, has been reported for the first time as constituents of C. diffusa. The isolated steroid stigmasterol exhibited cytotoxicity against African Green Monkey Kidney (vero) cell line which suggested its anticancer or antitumor property. Findings of this study indicated that the plant Commelina diffusa may be targeted to develop new chemotherapeutic or chemopreventive agents. Furthermore, extracts and fractions obtained from this plant showed weak to strong antimicrobial activity against the human pathogenic bacteria. Further study on specific fractions with antioxidant and cytotoxic activity can be helpful in lead compound isolation that might be used as precursors for designing effective therapeutic drugs.
Acknowledgements
Authors are grateful to Pharmaceutical Science Research Division, BCSIR Laboratories Dhaka for providing with the necessary laboratory and instrumental facilities and also for all the chemical and reagent supports.
Conflicts of Interest
The authors declare no conflicts of interest regarding the publication of this paper.
Cite this paper
Nasrin, M., Afroz, F., Sharmin, S., Rana, Md.S. and Sohrab, Md.H. (2019) Cytotoxic, Antimicrobial and Antioxidant Properties of Commelina diffusa Burm. F. Pharmacology & Pharmacy, 10, 82-93. https://doi.org/10.4236/pp.2019.102007
References
- 1. Cragg, G.M. and Newman, D.J. (2005) International Collaboration in Drug Discovery and Development from Natural Sources. Pure and Applied Chemistry, 77, 1923-1942. https://doi.org/10.1351/pac200577111923
- 2. Meshram, G.A., Metangale, G.S., Khamkar, S. and Kulkarni, T. (2016) Isolation of Sterols from Ocimum sanctum Leaves and Its Inhibitory Action on Glucoamylase in Vitro. European Journal of Pharmaceutical and Medical Research, 3, 341-345.
- 3. Beyene, B., Beyene, B. and Deribe, H. (2016) Review on Application and Management of Medicinal Plants for the Livelihood of the Local Community. Journal of Resources Development and Management, 22, 33-39.
- 4. Rahman, A.H.M.M., Sultana, M.Z., Rani, R. and Islam, A.K.M.R. (2015) Taxonomic Studies of the Family Commelinaceae at Rajshahi, Bangladesh. International Journal of Advanced Research, 3, 978-989.
- 5. Suganya, R.A. and Jothi, G.J. (2014) Preliminary Phytochemical Screening, Antibacterial and Antioxidant Activities of C. nudiflora (Commelinaceae). International Research Journal of Pharmacy, 5, 851-855. https://doi.org/10.7897/2230-8407.0511174
- 6. Khan, M.A.A., Islam M.T. and Sadhu, S.K. (2011) Evaluation of Phytochemical and Antimicrobial Properties of Commelina diffusa Burm. f. Oriental Pharmacy and Experimental Medicine, 11, 235-241. https://doi.org/10.1007/s13596-011-0028-0
- 7. Mensah, A.Y., Houghton, P.J., Dickson, R.A., Fleischer, T.C., Heinrich, M. and Bremner, P. (2006) In Vitro Evaluation of Effects of Two Ghanaian Plants Relevant to Wound Healing. Phytotherapy Research, 20, 941-944. https://doi.org/10.1002/ptr.1978
- 8. Mensah, A.Y., Mireku, E.A., Damoah, A.O. and Amponsah, I.K. (2014) Anti-Inflammatory and Antioxidant Activities of Commelina diffusa (Commelinaceae). World Journal of Pharmaceutical Sciences, 2, 1159-1165.
- 9. Strober, W. (2001) Trypan Blue Exclusion Test of Cell Viability. Current Protocol in Immunology, 111, A.3B.1-A.3B.2. https://doi.org/10.1002/0471142735.ima03bs21
- 10. Wilson, C.H., Ali, E.S, Scrimgeour, N., Martin, A.M., Hua, J., Tallis, G.A., Rychkov, G.Y. and Barritt, G.J. (2015) Steatosis Inhibits Liver Cell Store-Operated Ca2+ Entry and Reduces ER Ca2+ through a Protein Kinase C-Dependent Mechanism. Biochemical Journal, 466, 379-390. https://doi.org/10.1042/BJ20140881
- 11. Berry, M.N., Barritt, G.J. and Edwards, A.M. (1991) Isolated Hepatocytes: Preparation, Properties and Applications. Elsevier Science, New York, 459 p.
- 12. Meyer, B.N., Ferrigni, N.R., Putnam. J.E., Jacobsen, L.B., Nichols, D.E. and McLaughlin, J.L. (1982) Brine Shrimp: A Convenient General Bioassay for Active Plant Constituents. Journal of Medical Plant Research, 45, 31-34. https://doi.org/10.1055/s-2007-971236
- 13. Bauer, A.W., Kirby, W.M., Sherris, J.C. and Turck, M. (1966) Antibiotic Susceptibility Testing by a Standardized Single Disc Method. American Journal of Clinical Pathology, 45, 493-496. https://doi.org/10.1093/ajcp/45.4_ts.493
- 14. Braca, A., Tommasi, N.D., Bari, L.D., Pizza, C., Politi, M. and Morelli, I. (2001) Antioxidant Principles from Bauhinia terapotensis. Journal of Natural Products, 64, 892-895. https://doi.org/10.1021/np0100845
- 15. Atiku, I., Musa, A.M. and Sule, M.I. (2013) Isolation of Stigmasterol from Methanolic Extract of Cissus cornifolia Baker (Planch). Nigerian Journal of Pharmceutical Sciences, 12, 1-4.
- 16. Khan, N., Afroz, F., Begum, M.N., Rony, S.R., Sharmin, S., Moni, F., Hasan, C.M., Saha, K. and Sohrab, M.H. (2018) Endophytic Fusarium solani: A Rich Source of Cytotoxic and Antimicrobial Napthaquinone and Aza-Anthraquinone Derivatives. Toxicology Reports, 5, 970-976. https://doi.org/10.1016/j.toxrep.2018.08.016
- 17. Zubair, M.S., Anam, S. and Lallo, S. (2016) Cytotoxic Activity and Phytochemical Standardization of Lunasia amara Blanco Wood Extract. Asian Pacific Journal of Tropical Biomedicine, 6, 962-966. https://doi.org/10.1016/j.apjtb.2016.04.014
- 18. Malanovic, N. and Lohner, K. (2016) Antimicrobial Peptides Targeting Gram-Positive Bacteria. Pharmaceuticals, 9, 1-33. https://doi.org/10.3390/ph9030059
- 19. Srinivasan, D., Perumalsamy, L.P., Nathan, S. and Sures, T. (2001) Antimicrobial Activity of Certain Indian Medicinal Plants Used in Folkloric Medicine. Journal of Ethnopharmacology, 49, 217-222. https://doi.org/10.1016/S0378-8741(00)00345-7
- 20. Tchimene, M.K., Nwaehujor, C.O., Moses Ezenwali, M., Okoli, C.C. and Iwu, M.M. (2016) Free Radical Scavenging Activity of Lupeol Isolated from the Methanol Leaf Extract of Crateva adansonii Oliv (Capparidaceae). International Journal of Pharmacognosy and Phytochemical Research, 8, 419-426.