Pharmacology & Pharmacy
Vol.06 No.02(2015), Article ID:53906,8 pages
10.4236/pp.2015.62007
Antiproliferative Activity of Acerogenin C, a Macrocyclicdiarylheptanoid, on PDGF-Induced Human Aortic Smooth Muscle Cells Proliferation
Byung-Yoon Cha1, Wen Lei Shi2, Kotaro Watanabe3, Takayuki Yonezawa1, Toshiaki Teruya1, Kiyotake Suenaga3, Yuichi Ishikawa3, Shigeru Nishiyama3, Kazuo Nagai1,2, Je-Tae Woo1,2
1Research Institute for Biological Functions, Chubu University, Matsumoto, Japan
2Department of Biological Chemistry, Chubu University, Matsumoto, Japan
3Department of Chemistry, Faculty of Science and Technology, Keio University, Yokohama, Japan
Email: bycha@isc.chubu.ac.jp
Copyright © 2015 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 22 September 2014; accepted 6 February 2015; published 10 February 2015
ABSTRACT
Platelet-derived growth factor (PDGF)-BB is one of the most potent factors in the development and progression of various vascular disorders, such as atherosclerosis and restenosis. PDGF is a major stimulant for vascular smooth muscle cells (VSMCs) proliferation via the mitogenesis signaling pathway. In the present study, we investigated the effect of acerogenin C, a macrocyclicdiarylheptanoid, on PDGF-BB-stimulated human aortic smooth muscle cells (HASMCs) proliferation. Acerogenin C significantly inhibited PDGF (20 ng/mL)-BB-induced [3H]-thymidine incorporation into DNA at concentrations of 0.1, 1 and 10 mM without any cytotoxicity. Acerogenin C also blocked PDGF-BB-stimulated phosphorylation of PLCγ1 and Akt but had no effect on extracellular signal- regulated kinase 1/2 (ERK1/2) and PDGF beta-receptor (Rb) activation. In addition, acerogenin C (0.1 - 10 μM) induced cell-cycle arrest in the G1 phase, which was associated with the down-regu- lation of cyclins and the up-regulation of p27kip1. These results suggest that acerogenin C blocks PDGF-BB-stimulated HASMCs proliferation via G0/G1 arrest in association with the induction of p27kip1 and the suppression of PLCγ1 and phosphatidylinositol 3-kinase (PI3-K)/Akt signaling pathways. Furthermore, acerogenin C may be used for prevention and treatment of atherosclerosis during restenosis after coronary angioplasty.
Keywords:
HASMCs, Acerogenin C, PDGF-BB, p27kip1, Cell Cycle

1. Introduction
Whereas PDGF is expressed at low levels in arteries in healthy adults, its expression is increased in conjunction with the inflammatory-fibroproliferative response that characterizes atherosclerosis [1] . Thus, studies of balloon catheter-injured arterial tissue [2] -[4] , naturally occurring atherosclerosis [5] -[8] , coronary arteries after percutaneous transluminal coronary angioplasty [9] , and experimentally induced atherosclerosis [10] [11] revealed increased expression of PDGF and PDGF receptors in these lesions.
It is well-known that binding of signal transduction molecules to different phosphorylated tyrosine residues in PDGF-Rb and PDGF-BB triggers the PI3-K/PKB (Akt) and PLCγ1 pathways in addition to the ERK pathway [12] . PI3-K mediates many different cellular responses, including actin reorganization, chemotaxis, cell growth and the serine/threonine kinase Akt/PKB for the antiapoptotic effect [13] -[15] . Interestingly, full activation of PLCγ is dependent on PI3-K; the PI(3,4,5)P3 formed by PI3-K binds the PH domain of PLCγ and may anchor the enzyme at the membrane [16] . Mitogenic growth factors such as PDGF-BB share a final common signaling pathway in the cell cycle. Quiescent (G0) cells enter a gap period (G1), during which the factors necessary for DNA replication in the subsequent synthetic (S) phase are assembled. After DNA replication is completed, the cells enter another gap phase (G2) in preparation for mitosis (M). Restriction points at the G1-S and G2-M interphases ensure orderly cell cycle progression [17] .
In the arterial media, VSMCs are normally quiescent, proliferate at low indices (<0.05%), and remain in the G0/G1 phases of the cell cycle [18] . After vessel injury, vascular smooth muscle cells migrate into the intimal layer of the arterial wall, where they leave their quiescent state and reenter the cell cycle [1] . In many cells, transit through G1 of the cell cycle and entry into the S phase require the binding and activation of cyclin/CDK com- plexes, predominantly cyclin D1/CDK4 and cyclin E/CDK2 [19] [20] . The kinase activities of the cyclin/CDK complexes are negatively regulated by CDK inhibitors, such as p27kip1 [21] [22] .
Acerogenin is a diarylheptanoid whose characteristic structural feature is the presence of two hydroxylated aromatic rings tethered by a linear seven-carbon chain. Acerogenin C (Figure 1) was isolated from stems of Boswellia ovalifoliolata BAL. & HENRY (Burseraceae) [23] , while acerogenin C has been synthesized by Gonzalez G. I. et al. [24] . This diarylheptanoid exhibits a broad range of potent biological activities that include anti-inflammatory, antihepatotoxic, antifungal, antibacterial and related effects [23] [25] .
However, the mechanism by which acerogenin C affects VSMCs function is still largely unknown. The present study aimed to investigate, for the first time, the inhibitory effects of acerogenin C on PDGF-induced proliferation and signaling transduction pathways in HASMCs.
2. Materials and Methods
2.1. Materials and Reagents
PDGF-Rβ, Akt, PLCγ1, ERK1/2, cyclin D1, CDK4, cyclin E, CDK2, p27kip1 and α-actin antibodies were purchased from Cell Signaling Technology Inc. (Beverly, MA, USA). Acerogenin C was a gift of Dr. Nishiyama (Department of Chemistry, Faculty of Science and Technology, Keio University; 3-14-1 Hiyoshi, Kohoku-ku, Yokohama 223-8522, Japan).
2.2. Cell Cultures
HASMCs were purchased from Cascade Biologics, Inc. (Portland, OR, USA). HASMCs were grown in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% FBS, 100 IU/mL penicillin and 100 mg/mL
Figure 1. Chemical structures of acerogenin C.
streptomycin at 37˚C in a humidified 95% air/5% CO2 atmosphere. Cells were used at passages three through eight. For all experiments, HASMCs were grown to 80% - 90% confluence and quiescence was induced by starvation for at least 24 h.
2.3. [3H]-Thymidine Incorporation Assay
HASMCs proliferation was determined by [3H]-thymidine incorporation. HASMCs were incubated for 20 h with or without PDGF-BB (20 ng/mL) and various concentrations of acerogenins and then pulse-labeled with 1 mCi/mL of [3H]-thymidine for 4 h. Cells were harvested using a Universal Harvester (Perkin Elmer, Waltham, MA, USA) and then transferred to a GF/C filter (Perkin Elmer). The filter was dried and counted in scintillation fluid using a Microplate Scintillation and Luminescence Counter (Topcount NXT, Perkin Elmer).
2.4. Cell Viability
Cell viability was determined using the trypan blue dye exclusion method. Cells were incubated for 24 h with or without PDGF-BB (20 ng/mL) and various concentrations of acerogenin C and were then harvested from the dishes using a 0.1% w/v trypsin solution. Cell viability was examined by the trypan blue dye exclusion test. The number of viable cells was estimated by microscopic cell counting using a hemocytometer.
2.5. SDS-PAGE and Immunoblotting
Western blotting for protein analysis was performed as described previously [26] . Cells were harvested in lysis buffer containing 1 mM sodium vanadate, 1 mM phenylmethylsulfonyl fluoride, 5 mg/mL aprotinin and 5 mg/mL leupeptin. The protein concentration was determined by the Bradford assay (Bio-Rad, Hercules, CA, USA). Lysates corresponding to equal amounts of proteins were boiled in Laemmli sample buffer and the supernatants were loaded onto gels for SDS-PAGE. Proteins were transferred onto PVDF membranes and probed with the following primary antibodies: anti-phospho-PDGF-Rb, anti-PDGF-Rb, anti-phospho-PLCγ1 (Tyr783), anti- PLCγ1 anti-phospho-ERK1/2, anti-ERK, anti-phospho-Akt (Thr308) and anti-Akt. Appropriate horseradish peroxidase-coupled secondary antibodies were used at 1:10,000. Immunoreactive bands were visualized using enhanced chemiluminescence (ECL; Amersham Pharmacia Biotech, UK). The detected proteins were normalized to α-actin or the respective total protein, as appropriate. The intensities of bands were quantified using Sicon- Image for Windows (Scion Corporation, Frederick, MA, USA).
2.6. Cell Cycle Analysis
HASMCs were seeded in 6-well culture plates and grown in DMEM medium with growth supplement at 37°C in a humidified 95% air/5% CO2 atmosphere. Cells were grown to 80% confluence and made quiescent by starvation for at least 24 h. Cells were incubated for 24 h with or without PDGF-BB (20 ng/mL) and various concentrations of acerogenin C. Cells were harvested, fixed in 70% ethanol for 12 h, and stored at -20°C. Cells were then washed twice with ice-cold PBS and incubated with 100 mg/mL RNase and 50 mg/mL propidium iodide and cell-cycle phase analysis was performed by flow cytometry using a Cytomics FC500 and CXP Software Ver. 2 software (Beckman Coulter, JAPAN).
2.7. Statistical Analysis
Experimental results are expressed as means ± S.E.M. One-way analysis of variance (ANOVA), followed by Dunnett’s test, was used for multiple comparisons. P values of <0.05 and <0.01 were considered statistically significant.
3. Results
3.1. Effect of Acerogenins on PDGF-Induced HASMCs Proliferation and DNA Synthesis
In the [3H]-thymidine incorporation assay (Figure 2(a)), stimulation with PDGF-BB (20 ng/mL) increased cell proliferation by about 10-fold. Acerogenin C (0.1 to 10 mM) inhibited PDGF-induced cell proliferation in a concentration-dependent manner with about 90% inhibition observed at 10 mM. When quiescent cells were
treated with acerogenin C (0.1 to 10 mM) for 24 h in the absence of PDGF-BB, no significant difference was observed in the extent of [3H]-thymidine incorporation, suggesting that acerogenins are not cytotoxic at the concentrations tested. In particular, the lack of cytotoxicity of acerogenin C at the concentrations used in these experiments was confirmed by the trypan blue exclusion assay (Figure 2(b)). The number of cells was significantly increased after 20 ng/mL PDGF-BB stimulation (28.3 ± 0.3 ´ 104 cells/well) compared with the non- stimulated group (13.1 ± 0.1 ´ 104 cells/well) and the increased cells were significantly reduced to 21.2 ± 0.2, 15.3 ± 0.3 and 14.2 ± 0.2 ´ 104 cells/well at concentrations of 0.1, 1 and 10 mM, respectively (Figure 2(b)).
3.2. Effect of Acerogenin C on PDGF-Induced PDGF-Rβ, PCLγ1, Akt and ERK 1/2 Activation
The HASMCs were pre-incubated in the presence or absence of various concentrations of acerogenin C in a serum-free medium for 24 h and then stimulated for 10 min with 20 ng/mL PDGF-BB. As shown in Figure 3, PDGF-BB markedly increased phosphorylation levels on the PDGF-Rb but acerogenin C treatment had no significant effect. To determine the effects of acerogenin C on the downstream intracellular signal transduction pathway of PDGF-BB, we determined the phosphorylation of PLCγ1, Akt and ERK1/2. PDGF-BB treatment clearly increased phosphorylation levels on the PLCγ1, Akt and ERK1/2. Acerogenin C blocked the phosphorylation of PLCγ1 and Akt in a concentration-dependent manner but had no effect on ERK 1/2 phosphorylation.
3.3. Effect of Acerogenin C on Cell Cycle Progression in HASMCs
Flow cytometric analysis was performed to determine whether acerogenin C-induced cell growth inhibition was due to an arrest in a specific point of the cell cycle. As shown in Figure 4, pre-incubation of HASMCs in a serum-free medium for 24 h resulted in approximately 91.8 ± 2.5% synchronization of the cell cycle in the G0/G1 phase. During cell cycle analysis, stimulation with PDGF-BB (20 ng/mL) increased the percentage of cells in the S phase from 5.6 ± 1.3% to 27.5 ± 2.1%. Acerogenin C (0.1 to 10 mM) significantly blocked cell cycle progression in a concentration-dependent manner. The percentage of cells in the S phase was significantly reduced to 12.6 ± 1.2% (P < 0.05, n = 3), 7.4 ± 1.4% (P < 0.05, n = 3) and 5.4 ± 1.4% (P < 0.05, n = 3) at concentrations of 0.1, 1 and 10 mM acerogenin C, respectively (Figure 4). These results suggest that acerogenin C may act against DNA synthesis in the early events of the cell cycle.
3.4. Effect of Acerogenin C on Cell Cycle Regulatory Protein Expression
Using immunoblot analysis, we analyzed the protein expressions of the cyclins and CDKs, which are known to
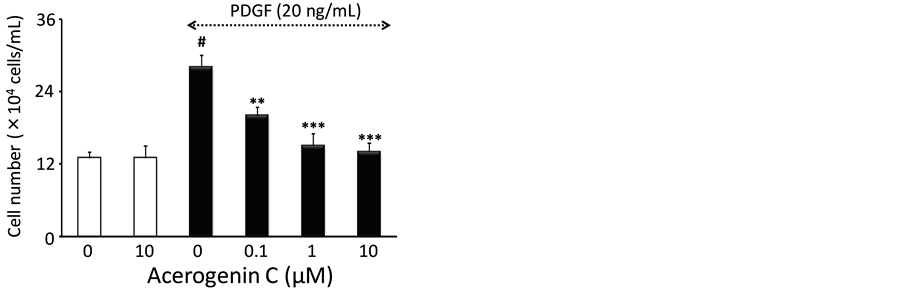
Figure 2. The effect of acerogenin on PDGF-BB-stimulated HASMCs proliferation. Cells were incubated for 20 h with or without PDGF-BB (20 ng/mL) and various concentrations of acerogenin C, and then pulse-labeled with [3H]-thymidine for 4 h (a). The cells were pre-incubated for 24 h with or without PDGF-BB and indicated concentrations of acerogenin C. The cells were trypsinized and then counted using a hemocytometer (b). Results are means ± S.E.M. from three independent experiments. #P < 0.005 compared with control; **P < 0.01 and ***P < 0.001 compared with PDGF-stimulation.
Figure 3. The effect of acerogenin C on PDGF-stimulated PDGF-Rb, ERK 1/2, PLCγ1, and Akt phosphorylation in HASMCs. The cells were incubated for 10 min with or without PDGF-BB and various concentrations of acerogenin C in 6-well culture plates. Cells were lysed and lysates were immunoblotted with antibodies. Total protein was used for respective normalization. After densitometric quantification, data are representative of at least three independent experiments with similar results.
be regulated by p27kip1, following treatment with acerogenin C. As shown in Figure 5, acerogenin C decreased the protein expression of cyclin E and CDK2, as well as cyclin D1 and CDK4, in a concentration-dependent manner. In addition, the expression of p27kip1, one of the cyclin-dependent kinase inhibitors, was down regulated by PDGF-BB treatment. In contrast, the p27kip1 protein level was significantly increased by acerogenin C treatment in a concentration-dependent manner.
4. Discussion
In the present study, we found that acerogenin C inhibited DNA synthesis in response to PDGF-BB (Figure 2(a)). As a result, acerogeninC showed a concentration-dependent inhibition of the incorporation of [3H]-thy- midine into HASMCs. Furthermore, the antiproliferative effect of acerogenin C on HASMCs was not due to cellular cytotoxicity, as demonstrated by the cell counting (Figure 2(b)) and MTT assays (data not shown).
To understand the mechanism of down-regulation of PDGF-BB-stimulated HASMCs proliferation, we examined whether the effect of acerogenin C is mediated by down-regulation of the intracellular signaling pathways.
The PDGF-BB-stimulated mitogenesis signaling pathway has been well characterized. Binding of PDGF-BB to PDGF-Rb can activate three major signal transduction pathways, PI3-K/Akt, PLCγ1 and ERK1/2, by activating Raf-1 [12] . As shown Figure 3, acerogenin C had no effect on the PDGF-BB-induced phosphorylation of PDGF-Rb at various concentrations. This result suggests that the inhibition of acerogenin C on HASMCs proliferation does not occur at the receptor level. Thus, we did Western blotting to understand the effect of acerogenin C on the downstream signal transduction, such as the PI3-K/Akt, PLCγ1 and ERK1/2 signaling pathways. As shown in Figure 3, we observed that acerogenin C specifically inhibited PDGF-stimulated PLCγ1 and Akt activation but not PDGFR and ERK1/2 MAP kinase activation in HASMCs. These results are similar to a previous study, in which JM91 inhibited PDGF-induced PI3-K/Akt and ERK1/2 MAP kinase activation but not PDGF Rb in HASMCs [12] . In the present study, PDGFRb, PLCγ1, ERK1/2 and Akt were used as a control for protein loading. However, the total amount of Akt rapidly decreased upon its activation. PDGF caused a rapid decrease in the Akt protein levels, concomitant with Akt activation. PDGF causes the regulated proteolytic down- regulation of Akt, which is dependent on PI3-K and proteasome activities. The proteasome-dependent down- regulation of Akt might be a fundamental mechanism that regulates the activity and function of Akt in VSMCs [27] . Interestingly, full activation of PLCγ is dependent on PI3-K; the PI(3,4,5)P3 formed by PI3-K binds the PH domain of PLCγ and may anchor the enzyme at the membrane [16] . PLCγ appears not to be of primary impor-


Figure 4. The effect of acerogenin C on PDGF-BB-stimulated cell cycle progression in HASMCs. The cells were incubated for 24 h with or without PDGF-BB and various concentrations of acerogenin C in 6-well culture plates. The cells were trypsinized and then analyzed by flow cytometry. Each item is derived from a representative experiment where data from at least 10,000 events were obtained. Data are representative of at least three independent experiments with similar results.
tance for the stimulation of cell growth and motility in most cell types. However, in certain cell types, PLCγ affects these responses [28] . Members of the PI3-K family that bind to and are activated by tyrosine kinase receptors consist of a regulatory subunit, p85, and a catalytic subunit, p110. Their preferred substrate is phosphatidylinositol 4,5-bisphosphate [PI(4,5)P2], which is phosphorylated to phosphatidylinositol 3,4,5-trisphosphate [PI(3,4,5)P3]. Phosphatidylinositol 39-kinase plays a central role in intracellular signal transduction; it can be activated by several different signals, it has a number of downstream effector molecules, and it mediates many different cellular responses, including actin reorganization, chemotaxis, cell growth and antiapoptosis [15] .
In addition, cell cycle analysis was performed to investigate the antiproliferative effect of acerogenin C. As shown in Figure 4, acerogenin C inhibits PDGF-BB-stimulated HASMCs proliferation via G0/G1 arrest. Several reports suggest that the G1 phase is a major point of control for cell proliferation in mammalian cells [29] . Many studies have attributed the regulation of G1 cell-cycle arrest to a number of cellular proteins, including the CDK inhibitor, p27kip1 [22] . Our data demonstrates a significant up-regulation of p27kip1. However, under similar experimental conditions, the expression levels of another cyclin-dependent kinase inhibitor, p21waf1 protein, was not changed (data not shown), suggesting that p21waf1 is unlikely to be involved in the cell-cycle arrest induced
Figure 5. The effect of acerogenin C on PDGF-stimulated expression of cell cycle regulatory proteins in HASMCs. The cells were incubated for 24 h with or without PDGF-BB and various concentrations of acerogenin C in 6-well culture plates. The cells were lysed and proteins were analyzed by SDS-PAGE and immunoblotting. α-actin was used for normalization. Data are representative of at least three independent experiments with similar results.
by acerogenin C. We assessed the effect of acerogenin C treatment on the cyclins and CDKs operative in the G1-phase of the cell cycle, such as cyclin D1, CDK4, cyclin E and CDK2. Acerogenin C inhibited the expression of cyclin D1, CDK4, cyclin E and CDK2 in a concentration-dependent manner. Our results indicate that cell cycle arrest in the G1-phase might be due to the down-regulation of CDKs/cyclins complex expression.
5. Conclusion
We showed that acerogenin C inhibited PDGF-BB-induced HASMCs proliferation via G0/G1 arrest, in association with the down-regulation of the expression of cyclin D1, CDK4, cyclin E and CDK2 and the up-regulation of p27kip1. Therefore, acerogenin C may be useful for prevention and treatment of vascular diseases, such as restenosis after coronary angioplasty.
Acknowledgements
This study was performed at a laboratory supported by an endowment from Erina Co., Inc.
References
- Ross, R. (1993) The Pathogenesis of Atherosclerosis: A Perspective for the 1990s. Nature, 362, 801-809. http://dx.doi.org/10.1038/362801a0
- Kanzaki, T., Shinomiya, M., Ueda, S., Morisaki, N., Saito, Y. and Yoshida, S. (1994) Enhanced Arterial Intimal Thickening after Balloon Catheter Injury in Diabetic Animals Accompanied by PDGF Beta-Receptor Overexpression of Aortic Media. European Journal of Clinical Investigation, 24, 377-381. http://dx.doi.org/10.1111/j.1365-2362.1994.tb02179.x
- Majesky, M.W., Reidy, M.A., Bowen-Pope, D.F., Hart, C.E., Wilcox, J.N. and Schwartz, S.M. (1990) PDGF Ligand and Receptor Gene Expression during Repair of Arterial Injury. Journal of Cell Biology, 111, 2149-2158. http://dx.doi.org/10.1083/jcb.111.5.2149
- Uchida, K., Sasahara, M., Morigami, N., Hazama, F. and Kinoshita, M. (1996) Expression of Platelet-Derived Growth Factor B-Chain in Neointimal Smooth Muscle Cells of Balloon Injured Rabbit Femoral Arteries. Atherosclerosis, 124, 9-23. http://dx.doi.org/10.1016/0021-9150(95)05742-0
- Libby, P., Salomon, R.N., Payne, D.D., Schoen, F.J. and Pober, J.S. (1989) Functions of Vascular Wall Cells Related to Development of Transplantation-Associated Coronary Arteriosclerosis. Transplantation Proceedings, 21, 3677- 3684.
- Libby, P., Warner, S.J., Salomon, R.N. and Birinyi, L.K. (1988) Production of Platelet-Derived Growth Factor-Like Mitogen by Smooth-Muscle Cells from Human Atheroma. New England Journal of Medicine, 318, 1493-1498. http://dx.doi.org/10.1056/NEJM198806093182303
- Rubin, K., Tingström, A., Hansson, G.K., Larsson, E., Rönnstrand, L., Klareskog, L., et al. (1988) Induction of B-Type Receptors for Platelet-Derived Growth Factor in Vascular Inflammation: Possible Implications for Development of Vascular Proliferative Lesions. Lancet, 1, 1353-1356. http://dx.doi.org/10.1016/S0140-6736(88)92177-0
- Wilcox, J.N., Smith, K.M., Williams, L.T., Schwartz, S.M. and Gordon, D. (1988) Platelet-Derived Growth Factor mRNA Detection in Human Atherosclerotic Plaques by in Situ Hybridization. Journal of Clinical Investigation, 82, 1134-1143. http://dx.doi.org/10.1172/JCI113671
- Ueda, M., Becker, A.E., Kasayuki, N., Kojima, A., Morita, Y. and Tanaka, S. (1996) In Situ Detection of Platelet-Derived Growth Factor-A and -B Chain mRNA in Human Coronary Arteries after Percutaneous Transluminal Coronary Angioplasty. American Journal of Pathology, 149, 831-843.
- Golden, M.A., Au, Y.P., Kirkman, T.R., Wilcox, J.N., Raines, E.W., Ross, R., et al. (1991) Platelet-Derived Growth Factor Activity and mRNA Expression in Healing Vascular Grafts in Baboons. Association in Vivo of Platelet-Derived Growth Factor mRNA and Protein with Cellular Proliferation. Journal of Clinical Investigation, 87, 406-414. http://dx.doi.org/10.1172/JCI115011
- Ross, R., Masuda, J., Raines, E.W., Gown, A.M., Katsuda, S., Sasahara, M., et al. (1990) Localization of PDGF-B Protein in Macrophages in All Phases of Atherogenesis. Science, 248, 1009-1012. http://dx.doi.org/10.1126/science.2343305
- Seo, J.M., Jin, Y.R., Ryu, C.K., Kim, T.J., Han, X.H., Hong, J.T., et al. (2008) JM91, a Newly Synthesized Indoledione Derivative, Inhibits Rat Aortic Vascular Smooth Muscle Cells Proliferation and Cell Cycle Progression through Inhibition of ERK1/2 and Akt Activations. Biochemical Pharmacology, 75, 1331-1340. http://dx.doi.org/10.1016/j.bcp.2007.11.013
- Dudek, H., Datta, S.R., Franke, T.F., Birnbaum, M.J., Yao, R., Cooper, G.M., et al. (1997) Regulation of Neuronal Survival by the Serine-Threonine Protein Kinase Akt. Science, 275, 661-665. http://dx.doi.org/10.1126/science.275.5300.661
- Kauffmann-Zeh, A., Rodriguez-Viciana, P., Ulrich, E., Gilbert, C., Coffer, P., Downward, J., et al. (1997) Suppression of c-Myc-Induced Apoptosis by Ras Signalling through PI(3)K and PKB. Nature, 385, 544-548. http://dx.doi.org/10.1038/385544a0
- Vanhaesebroeck, B., Leevers, S.J., Panayotou, G. and Waterfield, M.D. (1997) Phosphoinositide 3-Kinases: A Conserved Family of Signal Transducers. Trends in Biochemical Sciences, 22, 267-272. http://dx.doi.org/10.1016/S0968-0004(97)01061-X
- Falasca, M., Logan, S.K., Lehto, V.P., Baccante, G., Lemmon, M.A. and Schlessinger, J. (1998) Activation of Phospholipase Cγ by PI 3-Kinase-Induced PH Domain-Mediated Membrane Targeting. EMBO Journal, 17, 414-422. http://dx.doi.org/10.1093/emboj/17.2.414
- Elledge, S.J. (1996) Cell Cycle Checkpoints: Preventing an Identity Crisis. Science, 274, 1664-1672. http://dx.doi.org/10.1126/science.274.5293.1664
- Gordon, D., Reidy, M.A., Benditt, E.P. and Schwartz, S.M. (1990) Cell Proliferation in Human Coronary Arteries. Proceedings of the National Academy of Sciences of the United States of America, 87, 4600-4604. http://dx.doi.org/10.1073/pnas.87.12.4600
- Sherr, C.J. (1994) G1 Phase Progression: Cycling on Cue. Cell, 79, 551-555. http://dx.doi.org/10.1016/0092-8674(94)90540-1
- Sherr, C.J. (1996) Cancer Cell Cycles. Science, 274, 1672-1677. http://dx.doi.org/10.1126/science.274.5293.1672
- Xiong, Y., Hannon, G.J., Zhang, H., Casso, D., Kobayashi, R. and Beach, D. (1993) p21 Is a Universal Inhibitor of Cyclin Kinases. Nature, 366, 701-704. http://dx.doi.org/10.1038/366701a0
- Toyoshima, H. and Hunter, T. (1994) p27, a Novel Inhibitor of G1 Cyclin-Cdk Protein Kinase Activity, Is Related to p21. Cell, 78, 67-74. http://dx.doi.org/10.1016/0092-8674(94)90573-8
- Akihisa, T., Taguchi, Y., Yasukawa, K., Tokuda, H., Akazawa, H., Suzuki, T., et al. (2006) Acerogenin M, a Cyclic Diarylheptanoid, and Other Phenolic Compounds from Acer nikoense and Their Anti-Inflammatory and Anti-Tumor- Promoting Effects. Chemical and Pharmaceutical Bulletin, 54, 735-739. http://dx.doi.org/10.1248/cpb.54.735
- Gonzalez, G.I. and Zhu, J. (1997) First Total Synthesis of Acerogenin C and Aceroside IV. Journal of Organic Chemistry, 62, 7544-7545. http://dx.doi.org/10.1021/jo9714324
- Keseru, G.M. and Nogradi, M. (1995) The Chemistry of Natural Diarylheptanoids. In: Rahman, A.U., Ed., Studies in Natural Products Chemistry, Vol. 17, Elsevier, Amsterdam, 357-394.
- Cha, B.Y., Shi, W.L., Yonezawa, T., Teruya, T., Nagai, K. and Woo, J.T. (2009) An Inhibitory Effect of Chrysoeriol on Platelet-Derived Growth Factor (PDGF)-Induced Proliferation and PDGF Receptor Signaling in Human Aortic Smooth Muscle Cells. Journal of Pharmacological Sciences, 110, 105-110. http://dx.doi.org/10.1254/jphs.08282FP
- Adachi, M., Katsumura, K.R., Fujii, K., Kobayashi, S., Aoki, H. and Matsuzaki, M. (2003) Proteasome-Dependent Decrease in Akt by Growth Factors in Vascular Smooth Muscle Cells. FEBS Letters, 554, 77-80. http://dx.doi.org/10.1016/S0014-5793(03)01109-8
- Heldin, C.H. and Westermark, B. (1999) Mechanism of Action and in Vivo Role of Platelet-Derived Growth Factor. Physiological Reviews, 79, 1283-1316.
- Fang, F. and Newport, J.W. (1991) Evidence That the G1-S and G2-M Transitions Are Controlled by Different cdc2 Proteins in Higher Eukaryotes. Cell, 66, 731-742. http://dx.doi.org/10.1016/0092-8674(91)90117-H











