Pharmacology & Pharmacy
Vol.4 No.3(2013), Article ID:32322,6 pages DOI:10.4236/pp.2013.43040
Research of Quality Standards for Stachydrine Hydrochloride in Chinese Medicine TJF Granule
![]()
1Faculty of Sciences, School of Biomedical Sciences, University of Nottingham (Malaysia Campus), Semenyih, Malaysia; 2National Engineering Research Center for Traditional Chinese Medicine, Shanghai, China.
Email: khyx2lyo@nottingham.edu.my
Copyright © 2013 Yao Lu et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received March 18th, 2013; revised May 4th, 2013; accepted May 14th, 2013
Keywords: TJF Granule; Quality Standards; Traditional Chinese Medicine; Evaporative Light Scattering Detector (ELSD); High-Performance Liquid Chromatographic (HPLC) Method; Stachydrine Hydrochloride; Pharmaceutical Analysis; Determination
ABSTRACT
A high-performance liquid chromatographic method was applied to the determination of stachydrine hydrochloride concentration in TJF granule (Chinese name: Tiao-Jing-Fang), using a mobile phase of methanol-acetonitrile (50:50, v/v) by the Agilent Kro-masi NH2 column (250 mm × 4.6 mm, 5 μm, S/N: 22N25110). Detection wavelength was 201 nm. The result revealed good linearity of stachydrine hydrochloride and was obtained within the range of 0.20 - 1.98 μg/mL (R = 0.9995). The average recovery was 97.01%; the relative standard deviation (RSD) was 0.19%. To the best of our knowledge, this is the first report dedicated to the determination of stachydrine hydrochloride by the evaporative light scattering detector-high-performance liquid chromatographic (ELSD-HPLC) method.
1. Introduction
Stachydrine Hydrochloride (Figure 1) is one of the major active constituent of Herba Leonuri (Chinese herbal name: Yi-Mu-Cao) [1]. The crude extract of Herba Leonuri has been used in traditional Chinese medicine to treat irregular menses and other gynecologic diseases [2-9].
Since any natural product considered for its therapeutic value must be evaluated for consistency, a proper identification of the herbal ingredients in each preparation or medical product is an obvious requirement. Concentrated pharmaceutical herbal products and the decoction herbal products have been widely adopted for clinical use in most Asian countries like: China, Malaysia, Mongolia, Japan, Korea, and even in certain countries of the whole world.
Herbal products offer an alternative to Western medicines and are often considered to be non-toxic by the general public. A major criticism of herbal products is that they fail to meet modern quality assurance standards established for conventional medicines. Thus, quantitative determination of the active or marker components in medicinal herbs is a compulsory element for quality control. Several high-performance liquid chromatographic (HPLC) methods have been developed for the determination of stachydrine hydrochloride in herbs using silica columns [10,12] or reversed-phase C18 columns [11,12]. The aim of this current study is to develop a simple, reliable routine analytical method for the quality control of herbal medicine preparations by determining the stachydrine hydrochloride concentration in Herba Leonuri, and a traditional herbal preparation named TJF (Chinese name: Tiao-Jing-Fang) granule.
2. Experimental
2.1. Chemicals & Reagents
Stachydrine hydrochloride standard substance (The National Institute for Food and Drug Control, batch number: 110712-200709), TJF Granule (batch number: 091201, 091202, 091203), methanol (Merck, chromatographically pure), acetonitrile (ACN) (Merck, chromatographically pure), ultrapure water (UPW), ethanol (Merck, analytically pure (AR)), N-butanol (Merck, analytically pure (AR)), hydrochloric acid (HCl) (Merck, analytically pure (AR)), bismuth subnitrate (Merck, analytically pure (AR)), glacial acetic acid (Merck, analytically pure (AR)),
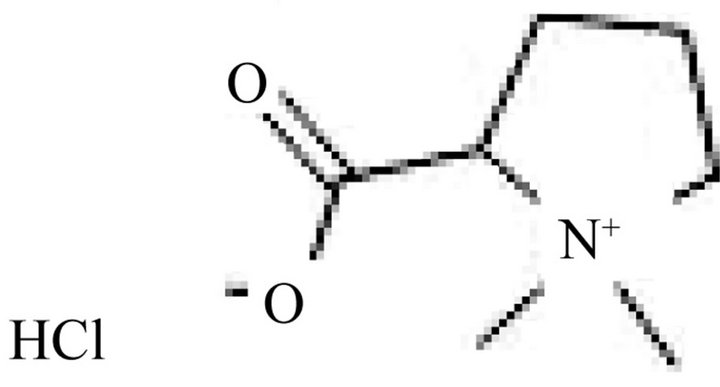
Figure 1. Chemical structure of stachydrine hydrochloride.
potassium iodide (Merck, analytically pure (AR)).
The traditional Chinese herbal preparation TJF Granule containing: Herba Leonuri (18 g), Rhizoma Chuanxiong (9 g), Rhizoma Cyperi (9 g), Semen Vaccariae (9 g), Caulis Spatholobi (9 g), and Fructus Lipuidambaris (9 g) was kindly provided by Dr. Wang Ling Tai from Shanghai Shu Guang Hospital.
2.2. Chromatography
Agilent 1100 High Performance Liquid Chromatograph (HPLC), includes: Alltech 2000 Evaporative Light Scattering Detector (ELSD), Agilent 1100 ChemStation, Alltech 1100 Full Wavelength Ultraviolet (UV) Detector, Agilent 1100 Online Vacuum Degasser, Agilent 1100 manual injector; Agilent Kro-masi NH2 column (250 mm × 4.6 mm, 5 μm, S/N: 22N25110); Si mplicity Superpure Water System (Milipore); Analytical Balance (METTLER XS105); 50 μl Microsyringe (Hamilton).
The mobile phase consisted of methanol-acetonitrile (50:50, v/v), its flow-rate was 1 ml /min. The mobile phase was filtered through a Millipore 0.45 μm filter and degassed prior to use. The detection wavelength was set at 201 nm.
2.3. Method Validation and Result
2.3.1. Herba Leonuri Identification by TLC
Preparation of reference solution: To get stachydrine hydrochloride standard substance, add ethanol and make a solution into the concentration of 2 mg/1 mL.
Preparation of TJF Granule sample solution: To get 6 g TJF Granule, add ethanol 30 mL, extracted 60 minutes twice by hot reflux extraction method, filters and combine the filtrate, concentrates evaporation to dryness, dissolves the residue in 0.5 mL ethanol for analysis by TLC as TJF Granule sample solution.
Preparation of negative control product solution: To get 6 g Negative control product granule (TJF Granule without Herba Leonuri), add ethanol 30 mL, extracted 60 minutes twice by hot reflux extraction method, filters and combine the filtrate, concentrates evaporation to dryness, dissolves the residue in 0.5 mL ethanol for analysis by TLC as negative control product solution.
Preparation of chromogenic agent (diluted iodized bismuth potassium solution): To get 0.85 g Bismuth subnitrate, adds 10 ml glacial acetic acid and dissolves into 40 ml Distilled water—that is the former solution. Take 5 ml former solution before use, add 5 ml potassium iodide solution (4 → 10) and 20 ml glacial acetic acid, dilute into 100 ml solution by Distilled water. (China Pharmacopoeia Committee, 2005).
According to the thin-layer chromatography (TLC) test in appendix VIB of the first section of Chinese Pharmacopoeia 2005 Edition, accurate absorb 20 μL negative reference substance solution and TJF Granule sample solution respectively, respectively point in the same silica gel plate, developing with the developing solvent N-butanol-Hydrochloric acid-Distilled water (4:1:0.5), developing distance is about 8 cm, remove and dry, spraying with the test solution of diluted iodized bismuth potassium solution, heating in 105˚C until characteristic spots appear clearly.
Result: In the identification test there’s the same colour speck in the preparation Chromatography while there’s no the same colour speck in the control sample. The result is shown in Figure 2.
Developing solvent: N-butanol-Hydrochloric acidDistilled water (4:1:0.5).
Chromogenic agent: Diluted iodized bismuth potassium solution.
1) Negative control product solution (TJF Granule without Herba Leonuri);
2) Stachydrine hydrochloride standard substance;
3)-5) TJF Granule sample (batch number: 091201, 091202, 091203).
2.3.2. Determination of Stachydrine Hydrochloride by HPLC
1) Chromatography condition and System suitability test The determination was performed by ELSD-HPLC, Agilent Kro-masi NH2 column (250 mm × 4.6 mm, 5 μm, S/N: 22N25110) was used with methanol-acetonitrile (50:50) as mobile phase. Detection wavelength was 201 nm.
Number of theoretical plates calculated by chromatographic peak of stachydrine hydrochloride would be no

Figure 2. Identification of Herba Leonuri.
less than 2000.
2) Solution Preparation Preparation of reference solution: To get moderate Stachydrine hydrochloride standard substance (The National Institute for Food and Drug Control, batch number: 110712-200709), weighting accurately, dry in 105˚C for 3 hours, weighting accurately, add ethanol to make a solution with a concentration of 0.2 mg/1 mL.
Preparation of sample solution: to get TJF Granule 3 g, weighting accurately, adding ethanol 30 mL, heating to reflux for 2 times, I hour for each time, filtering after being cooled, merging of filtrates, concentrating the mixed filtrate to a moderate amount, adding ethanol to dissolve, moving into a 5 ml volumetric flask, dilute with ethanol to volume, and mix, filter, take the subsequent filtrate as the sample solution.
Preparation of negative control product solution: To weight all the ingredients of TJF Granule except the Herba Leonuri, preparing the negative reference substance solution by the same way of TJF Granule sample solution preparation.
3) Determination method Respectively accurate absorb reference substance solution 10 μL, 20 μL; TJF Granule sample solution 10 μL; and negative reference substance solution 10 μL; inject into the high performance liquid chromatograph according to the chromatographic condition which above-mentioned and test.
4) Linear relationship inspection To get moderate stachydrine hydrochloride standard substance (The National Institute for Food and Drug Control, batch number: 110712-200709), weighting accurately, adding ethanol to make a solution with concentration of 0.2 mg/mL. Respectively accurate absorb above-mentioned solution 1000 μL, 500 μL, 125 μL, 63 μL and 32 μL, move them into 5 mL volumetric flasks respectively, dilute with ethanol to volume and mix, prepare these solutions as the series of standard substance solutions.
Series of standard substance solutions are injected respectively, and analysis according to the chromatographic condition based on the “Determination of Stachydrine Hydrochloride by HPLC” item, record peak area, calculate regressively through sample concentration (μg/mL) as the abscissa, and logarithm of peak area as the ordinate. Result displays a good linear relation is shown within the detection range of stachydrine hydrochloride. Linear equation is: y = 0.041x + 2.882, R = 0.9995; linear range is: 0.20 - 1.98 μg/mL. Since the standard curve is not pass the origin, external standard dual point method is adopted to determinate the content of Stachydrine hydrochloride standard substance in products. The result is shown in Figure 3 and Table 1.
5) Accuracy test
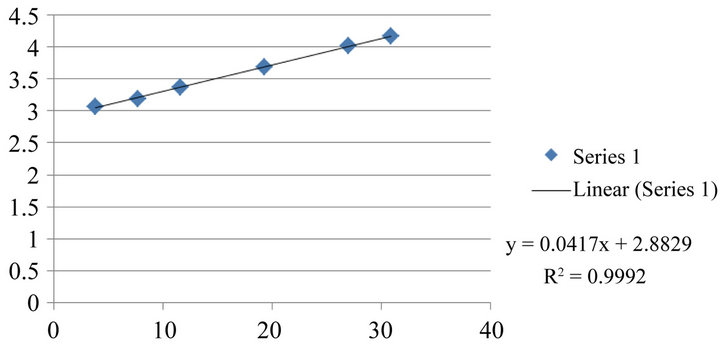
Figure 3. Standard curve of stachydrine hhydrochloride.
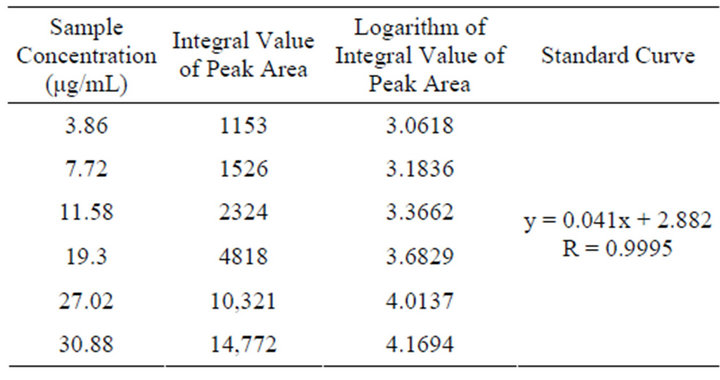
Table 1. Linear relation result of stachydrine hydrochloride.
Reference substance solution has been absorbed accurately, solutions are injected 5 times continuously. The intra-day coefficient of variation (CV) is calculated by testing the relative standard deviation (RSD) for each chromatographic peak’s logarithm of peak area (lgA). The result is shown in Table 2.
6) Stability Test To get TJF Granule sample solution from same batch number, solutions are injected in 0 hour, 2 hours, 4 hours, 6 hours, 8 hours, 12 hours and 24 hours respectively. Test result shows that TJF Granule sample solution is stable within 24 hours. The result is shown in Table 3.
7) Repeatability Test TJF Granule has been weighted accurately, preparing the sample solution by the same way of TJF Granule sample solution preparation to test the repeatability of determination method. RSD is 0.66%, which shows a good repeatability. The result is shown in Table 4.
8) Recovery Test To get 0.5 g TJF Granule which content has been calculated as a sample, weighting 9 samples; accurately absorb moderate reference substance solution; preparing the sample solution by the same way of TJF Granule sample solution preparation; calculate the recovery of Stachydrine hydrochloride. The result is shown in Table 5.
9) TJF Granule Determination To get TJF Granule 5 g, weighting accurately, adding ethanol 40 mL, heating to reflux for 2 times, 1 hour for each time, filtering after being cooled, merging of

Table 2. The result of accuracy test.
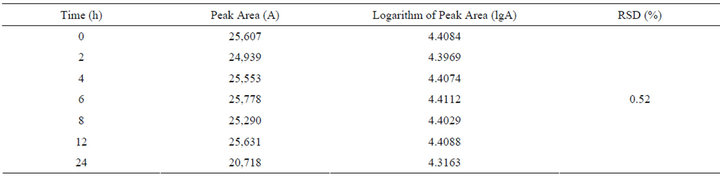
Table 3. The result of stability test.
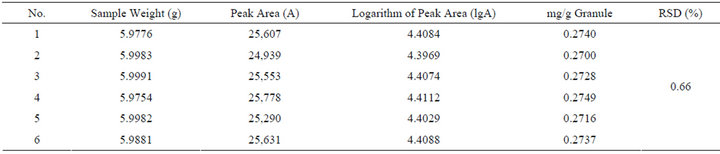
Table 4. The result of repeatability test.
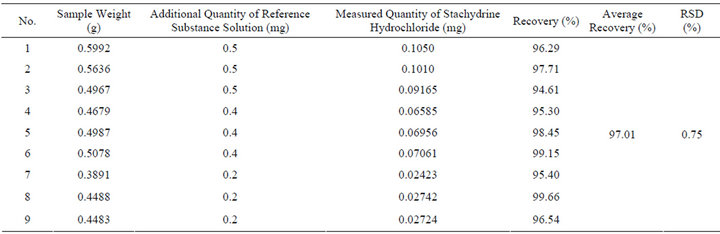
Table 5. The result of recovery test.
filtrates, concentrating the mixed filtrate to a moderate amount, adding ethanol to dissolve, move into a 5 mL volumetric flask, dilute with ethanol to volume, and mix, filter, take the subsequent filtrate 1.0 mL, and pass take the subsequent filtrate through the 0.45 μm Millipore filter as the TJF Granule sample solution.
Accurately absorbs 20 μL TJF Granule sample solution, inject into the high performance liquid chromatograph according to the chromatographic condition which above-mentioned and test, record the peak area of each chromatographic peak, each sample is paralleled tested twice, calculate the content according to the external standard dual point method and select the average. The result is shown in Table 6 and Figures 4-6.
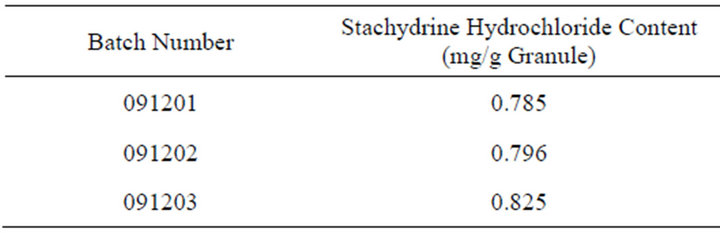
Table 6. Determination of stachydrine hydrochloride content of TJF granule.
According to the determination results of Stachydrine hydrochloride for different batch number of TJF Granule, select the average number with a 20% decrease of the Stachydrine hydrochloride content in 3 batch numbers of TJF Granule, temporary define no less than 3.80 mg Stachydrine hydrochloride should be tested for each bag of TJF Granule.
2.4. Experimental Result of Stachydrine Hydrochloride in TJF granule
RESULT: Good linearities of stachydrine hydrochloride and was obtained within the range of 0.20 - 1.98 μg/mL (R = 0.9995; the average recoveries were 97.01%; RSD were 0.19%.
3. Discussion and Conclusion
DISCUSSION: Determination method of stachydrine hydrochloride: scanning result of stachydrine hydrochloride standard substance tested by the ultraviolet spectrophotometer displays the wavelength of maximum absorption is 201 nm, which is less than the cut-off wavelength of most mobile phases like methanol. Hence the ultraviolet detector is not suitable for the test, while the
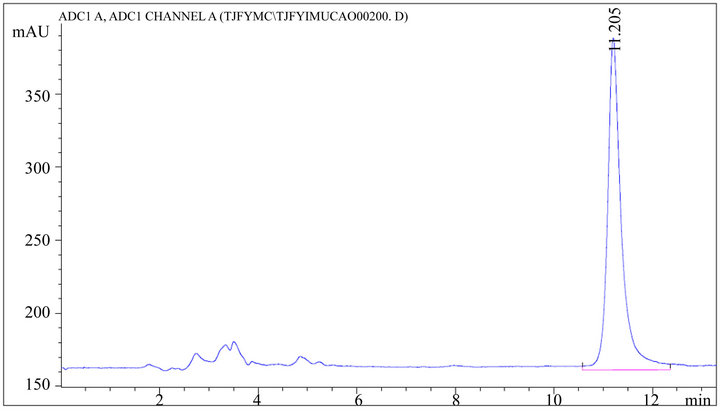
Figure 4. HPLC chromatogram of stachydrine hydrochloride standard substance.
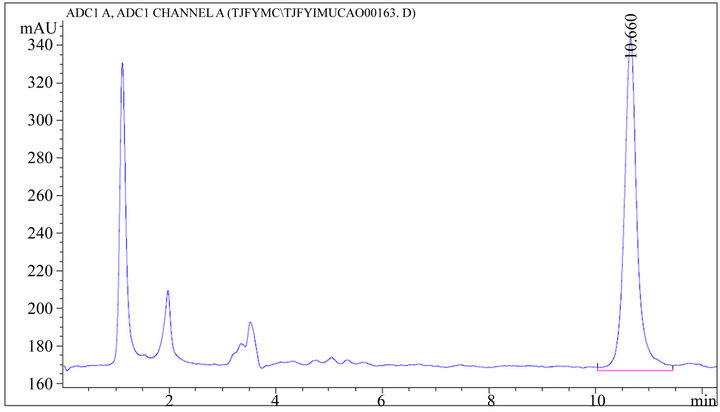
Figure 5. HPLC chromatogram of TJF granule sample.
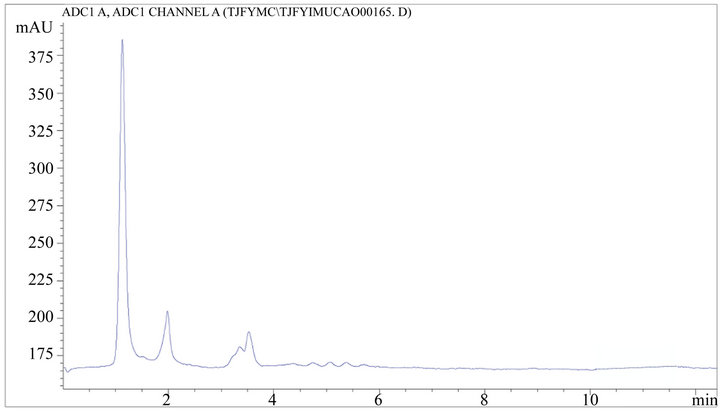
Figure 6. HPLC chromatogram of TJF granule negative reference substance.
evaporative light scattering detector (ELSD) should be adopted for determination.
In the first section of Chinese Pharmacopoeia 2005 Edition, determination method of Extractum Leonuri Liquidum is thin-layer chromatography scarmlng method (TLCS method). The ELSD-HPLC method is more convenient on operation and takes less time. Through the experiment, when the C18 reversed phase column is used on determination, chromatographic peak of Stachydrine hydrochloride has a severe zone tailing phenomenon, therefore, the NH2 column is adopted instead of the C18 reversed phase column.
The developing distance of Herba Leonuri Identification by TLC according to the first section of Chinese Pharmacopoeia 2005 Edition is not satisfied, the detection condition is not complete yet, and needs screening conditions continuously.
CONCLUSION: This method can be used for quality control of TJF granule.
REFERENCES
- China Pharmacopoeia Committee, “Section One,” In: China Pharmacopoeia Committee, Ed., Chinese Pharmacopoeia 2005 English Edition, Chemical Industry Press (CIP), Beijing, 2005, pp. 203-205.
- Z. S. Huang, “Formula of Promoting Blood Circulation for Removing Blood Stasis,” In: Z. S. Huang, Ed., Chinese Materia Medica, People’s Medical Publication, Beijing, 2007, pp. 303-317.
- Z. Z. Tao, “Blood-Activating Formula,” In: Z. Z. Tao, Ed., Chinese Materia Medica, People’s Medical Publications, Beijing, 2006, pp. 346-387.
- F. L. Yin, “Menstruation Disorder,” In: F. L. Yin, Ed., Internal Medicine, Shanghai Science and Technology Publications, Shanghai, 2001, pp. 113-117.
- C. H, Zeng and W. H. Dai, “Emmeniopathy,” In: C. H, Zeng and W. H. Dai, Eds., Internal Medicine, People’s Medical Publications, Beijing, 2003, pp. 208-212.
- Y. H. Q. Ou, “Menstruation Diseases,” In: Y. H. Q. Ou, Ed., Gynecology of Traditional Chinese Medicine, People’s Medical Publications, Beijing, 2006, pp. 52-96.
- X. J. Chen, “Emmeniopathy,” In: X. J. Chen, Ed., Internal Medicine of Traditional Chinese Medicine, Shanghai Science and Technology Publication, Shanghai, 2002, pp. 268-271.
- Y. K. Wang and F. C. Wang, “Menstruation Diseases,” In: Y. K. Wang and F. C. Wang, Eds., Gynecology of Traditional Chinese Medicine, Chinese Press of Traditional Chinese Medicine, Beijing, 2009, pp. 31-78.
- Y. Z. Zhang, “Menstruation Disorder,” In: Y. Z. Zhang, Ed., Traditional Chinese Gynecology, Chinese Press of Traditional Chinese Medicine, Beijing, 2005, pp. 25-63.
- X. M. Liang, Y. Wang and X. M. Zhang, “Determination of Stachydrine Hydrochloride in Herba Leonuri Granules by TLC,” Chinese Traditional Patent Medicine, Vol. 7, No. 8, 2006, pp. 1073-10744.
- J. L. Ruan, “Research Developments of Herba Leonuri on the Chemical, Pharmacological and Clinical Researches,” Journal of Chinese Herbal Medicine, Vol. 11, No. 6, 2003, pp. 15-19.
- X. Y. Meng and J. Q. Yang, “Research Developments of Herba Leonuri,” Heilongjiang Medical Journal, Vol. 5, No. 1, 2007, pp. 467-468.

