Neuroscience & Medicine
Vol.3 No.2(2012), Article ID:19604,11 pages DOI:10.4236/nm.2012.32018
Single Dose Effects of Pascoflair® on Current Source Density (CSD) of Human EEG*
![]()
1Justus-Liebig-University c/o NeuroCode AG, Wetzlar, Germany; 2NeuroCode AG, Wetzlar, Germany; 3Pascoe pharmazeutische Präparate GmbH, Giessen, Germany.
Email: {#w.dimpfel, info}@neurocode-ag.com, gabriele.weiss@pascoe.de
Received February 10th, 2012; revised March 19th, 2012; accepted April 20th, 2012
Keywords: Passion Flower; EEG; Clinical Study; Source Density; Discriminant Analysis
ABSTRACT
Pascoflair® 425 mg is a herbal drug based on Passiflora incarnata dry extract and is registered in different countries for the treatment of nervous restlessness and anxiety and also as an aid to sleep. The study was initiated for the quantitative assessment of the effect of this preparation on brain electric activity and cognition in human volunteers. Quantitative electroencephalographic current source density (CSD) from 16 healthy male and female human volunteers (average age 49 years) was used in a randomized, placebo-controlled crossover study. Data were taken 0.5, 1.5, 3 and 4 hours after administration of the preparations under the conditions of 6 minutes eyes open, 5 minutes d2 concentration test, mathematical calculation test and memory test respectively. During mental load, changes in spectral band power were used to analyse drug-induced effects. All variables were fed into a linear discriminant analysis (LDA) for comparison with other drug profiles. Spectral power in the delta and theta range was significantly attenuated at 3 and 4 hours after administration in comparison with the time-dependent increase normally observed due to circadian rhythm. Discriminant analysis revealed a difference to placebo for all recordings as early as 30 minutes after intake of 3 coated tablets of Pascoflair® 425 mg. Using LDA data location within the poly-dimensional space, verum was projected into the area of the effects of Gingko/Ginseng as reference drugs tested earlier under identical conditions. Psychometric performance was not disrupted. Pascoflair® 425 mg can be regarded as a well characterized plant-derived drug with anxiolytic and calming properties without negative sedative and cognition-attenuating side effects. Current results document the effectiveness of the preparation as early as after 30 minutes. In addition, they indicate persistence of good mental performance for hours. Trial registration: the study has been registered at ClinicalTrials.gov under NCT01047605.
1. Introduction
Many extracts from different species of the genus passionflower have been tested pharmacologically in animals, mainly looking for anxiolytic and calming effects [1-3]. Due to a large number of potentially active ingredients such as C-glycosylflavonoids [4], a single mechanism of action cannot be expected. However, there is some evidence that the neurotransmitter GABA (γ-amino butyric acid) could be involved in the action [5], especially since binding studies attributed the affinity of Passiflora incarnata L. to GABA A and GABA B receptors. Passiflora incarnata L. could be identified as an antagonist of the GABA B receptor. In contrast the ethanoland benzodiazepine site of GABA A receptors were not affected by this extract [6].
In addition, there have been reported differences in anxiolytic and sedative properties with respect to extract origin or dosage. For example, anxiolytic effects in mice have been recorded in the presence of extracts manufactured from Passiflora edulis “edulis”, whereas sedative effects were seen in the presence of extracts made from Passiflora edulis “flavicarpa” [7]. On the other hand, anxiolytic and sedative effects have been reported to be dose-dependent [8]. This means that each preparation to be marketed has to be tested on its own for proof of efficacy at the recommended dosage.
The aim of the present investigation is the clinical testing of Pascoflair® 425 mg, registered in different countries for the treatment of nervous restlessness and anxiety and also as an aid to sleep (in cases of restlessness or insomnia due to mental stress).
This drug contains a special dry extract from Passiflora incarnata. Two major questions were addressed. The first is the question as to whether a single intake was able to show an effect during the next four hours after adiminstration of the preparation. For this purpose the most sensitive methodology of recording electric brain activity from the human scalp was used. Secondly, mental performance was assessed concomitantly with the EEG recording, since experiments in rats had shown that Passiflora extracts induced anxiolytic effects without disrupting memory processes [9]. Finally, a comparison with the electric profile of other drugs acting on the human brain should give further indications for its clinical use.
2. Methods
2.1. Subjects
Sixteen healthy volunteers (8 males, (48.7 ± 7) years old and 8 females, (47 ± 8) years old) were recruited by advertisement and participated in this study. They underwent a medical examination before entering the trial. Subjects reporting neurological disturbances of the central nervous system were excluded from the study. Subjects with a history of drug or ethanol abuse or participation in another study within the last six months were also excluded. It was ensured that they were not on alcohol. On the day of examination, no beverages containing caffeine were allowed within the last 12 hours preceding the EEG recording. The study was carried out according to the Helsinki 2000 declaration [10] on human rights, and was approved by the local ethics committee of the Hessen State Medical Association (Frankfurt, Germany) and government authorities (BfArM, Bonn, Germany). All subjects received detailed information about the aims of the study and gave their written informed consent to participate. Each subject was randomly allocated either to the functionally active preparation or to placebo within a crossover design with an interval between experimental of at least 1 week.
2.2. EEG Recording
Subjects sat alone in a quiet separate room in a comfortable easy chair. The light was dimmed. Baseline recording of 6 minutes under the condition of eyes open was followed by performance of three cognitive tests: a concentration test (d2-test) [11], a mathematical calculation test (according to [12]) and a memory test, consisting of the presentation of a row of letters and numbers that appeared on a screen for 4 seconds followed by 10 seconds of black screen. This was followed by a multiple choice test with four possible answers.
All recordings were repeated 0.5, 1.5, 3 and 4 hours after the administration of three tablets following the reference pre-drug period (overview in Figure 1). Between assessments, subjects spent their time in the facility’s break room. All experiments took place at the same time of the day (starting at 8 a.m.).
The EEG was recorded bipolary from 17 surface electrodes according to the international 10/20 system with Cz as physical reference electrode for calculation of the common average reference [13] (Computer aided topographical electroencephalometry: CATEEM®—from Mewicon GmbH, 4164Schwarzenberg, Austria) using an electrocap. The raw signals were amplified, digitalized (2048 Hz/12 bit) and transmitted to the computer via fibre optical devices. The automatic artefact rejection of the CATEEM®-System, which eradicates EEG-alterations caused by eye blinks, swallowing, respiration etc. during the recording was visually controlled and individually adjusted by the investigator. Electrocardiogram (ECG) and electrooculogram (EOG) were recorded on one channel each in order to facilitate detection of those signals superposed onto the EEG. The artefact rejection set-up was observed for about 5 minutes prior to the start of the recording to ensure that all artefacts were correctly recognized and eliminated from further evaluation. For safety purposes, the original raw data were saved on optical disk and separate hard disks in order to allow reevaluation of the artefact rejection mode if necessary. In these cases, the experimental session was re-examined off-line with a newly adapted rejection mode. The amount of rejected data was determined automatically and given as a percentage of total recording time. In addition, the entire recording and the computer-based automatic artefact rejection were continuously supervised and adjusted by a trained technician [14]. Logarithm of data was taken for discriminant analysis in order to approach normal distribution of values, which underwent frequency analysis for quantitative evaluation as first proposed nearly 80 years ago [15].
In this study, the EEG was computed not in the potential mode measured as voltage, but in a surface charge mode obtained by Laplacian estimates also known as current source density (CSD) analysis [16]. The charge is the 2nd derivative of the potential and gives the spatial curvature of the potential. All calculations are based on the standard set-up of the 10/20 system of recording. Under the condition of using a homogenous, steadily
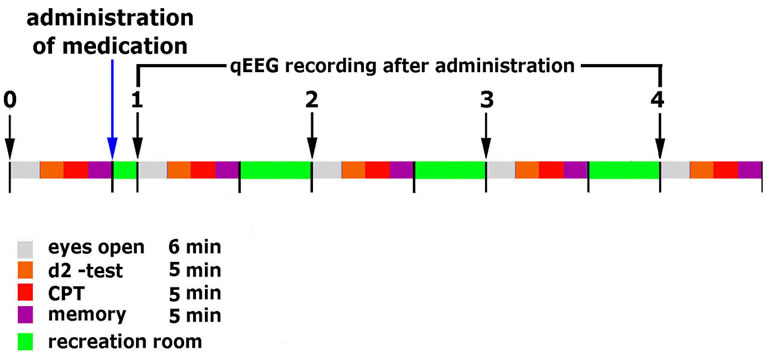
Figure 1. Timeline of experimental setup.
conducting medium, the surface charge mode provides the source density of the electrical flow on the cortex surface. Whereas the EEG in the potential mode tends to produce a more extensive and diffuse picture of changes, the Laplacian estimate acts as a spatial filter emphasizing local sources over distant sources. Others [17] were able to demonstrate that spectral parameters obtained from the CSD showed higher correlations with computer tomography data than those calculated from the potential mode of the EEG. We therefore used this methodology in order to describe the focal changes in brain activity. The signals from all 17 electrode positions underwent the Fast Fourier Transformation (FFT) based on 4-second sweeps of data epochs (Hanning window). Data were analysed from 0.86 to 35 Hz using the CATEEM® software. In this software, the resulting frequency spectra are divided into six frequency bands: delta (1.25 - 4.50 Hz), theta (4.75 - 6.75 Hz), alpha1 (7.00 - 9.50 Hz), alpha2 (9.75 - 12.50 Hz), beta1 (12.75 - 18.50 Hz) and beta2 (18.75 - 35.00 Hz). This frequency analysis is based on absolute spectral power values. Band powers are first compared between placebo and verum during relaxation, and the results of verum and placebo then compared under different mental loads.
2.3. Statistics
EEG data from the first recording session before drug intake are given as absolute figures (μV2). By setting the absolute electric power of this first pre-drug recording to 100%, changes produced by the preparations are given as percentage of this pre-drug condition. Except for generating brain maps, only those particular electrode positions were used for the numeric comparison of verum to placebo during mental load, which have been found to change during the particular mental challenge. For explorative statistical evaluation, the nonparametric sign test was used. For mathematical classification of drug effects, linear discriminant analysis according to Fischer was used. Results from the first three discriminant functions were projected into space (x, y and z coordinates), whereas results from the fourth to sixth discriminant functions were coded into red, green and blue respectively. Values on the 4th to 6th linear discriminant functions respectively determine the amount of red, green and blue in additive colour mixtures analogous to the RGB mode of TV. Reference preparations used for comparison were tested in our laboratory earlier under identical conditions. A linear projection was defined by taking these reference data. Data sets from other preparations to be analysed are processed according to the originally defined mapping function of reference compounds.
Psychometric performance was evaluated by the mathematical product of “quantity × quality”, where quantity was defined as the number of correct answers and quality as the number of correct answers divided by the number of tasks tackled.
2.4. Preparation
Pascoflair® 425 mg is a coated tablet containing dry extract (5 - 7:1) of Passiflora incarnata L. (Passionflower) 425 mg, extractant: ethanol 50% (V/V). Pascoflair® 425 mg is registered in different countries for the treatment of nervous restlessness and anxiety and also as an aid to sleep (in cases of restlessness or insomnia due to mental stress).
Pascoflair® 425 mg is marketed by Pascoe pharmazeutische Präparate GmbH, Giessen, Germany. The verum preparation consisted of the maximum daily dosage of 3 coated tablets of Pascoflair® 425 mg.
3. Results
3.1. Analysis of Single Frequency Ranges during Relaxation
Source density analysis of the primary recording of the reference EEG before drug administration revealed similar absolute spectral power values for the day of placebo administration (Pl) compared with the day of active drug administration (Pas) for all electrode positions as documented in Table 1. These data were taken as the baseline; all other data after drug intake refer to this reference recording of the particular experimental day and are expressed as % of these pre-drug data. After ingestion of 3 coated tablets of Pascoflair® 425 mg, some differences were recognized with respect to spectral power values after Fast Fourier Transformation in comparison to the pre-drug values, when the median was calculated for all electrode positions. The greatest differences between verum and placebo were seen with respect of the diurnal increases of delta and additionally to a lesser degree of theta spectral power. In the presence of verum, the increase in delta power as normally observed was completely attenuated as documented in Figure 2. This difference was highly statistically significant (p < 0.02) at 3 and 4 hours after administration. Theta spectral power also was attenuated at 3 and 4 hours after ingestion. Statistical significance, however, was less pronounced at 3 hours (p < 0.08). In addition, there was some increase of alpha2 spectral power which did not reach statistical significance.
3.2. Analysis of Spectral Power during Mental Performance
Changes of electric power within all frequency bands at single electrode positions during mental performance are shown in Figure 3. Obviously, fronto-temporal beta power
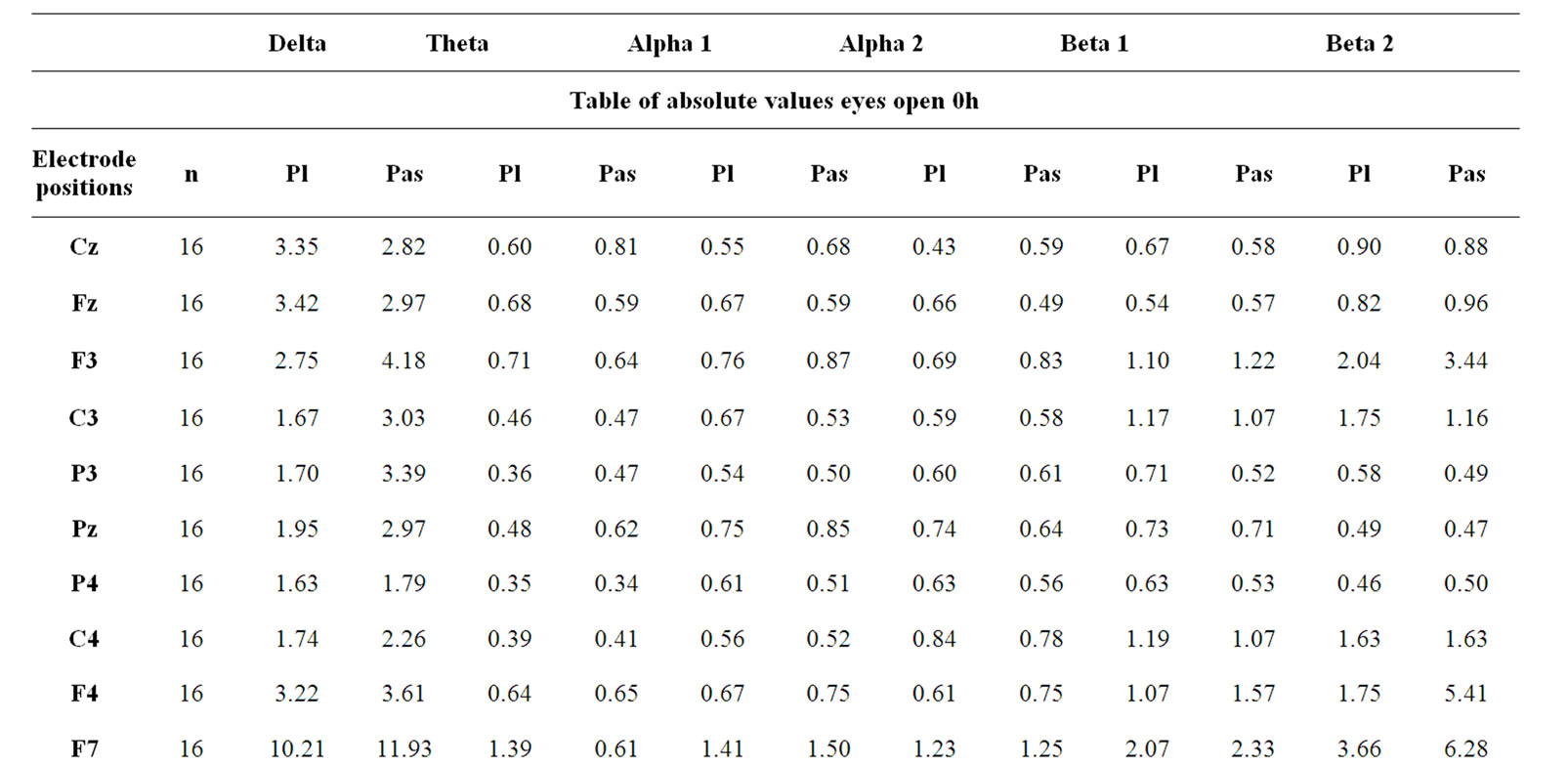

Table 1. Absolute EEG spectral power.
increased to some degree during all three mental challenges. The greatest differences between placebo and Pascoflair® 425 mg were observed during the performance of the memory test. Here not only beta but also delta and theta activity was enhanced. There are some more statistically significant changes to spectral power with regard to single frequency changes in comparison to placebo, but statistical analysis of the different locations is not Bonferroni-corrected for multiple comparisons. With regard to median values of all electrode positions, EEG analysis revealed a statistically significant attenuation of delta and theta power. During performance of the d2-test, significant attenuation of delta power took place 3 and 4 hours after ingestion (Figure 4). Attenuation of theta power was significant at 1.5 and 4 hours after ingestion, alpha2 power at 3 hours. During the concentration-performance test (CPT), delta and to some degree also theta power was attenuated with regard to the medians of all electrode positions. This effect was strongest at 3 and 4 hours after intake of the active preparation as shown in Figure 5. Attenuation of the other frequencies was not statistically significant (not shown). However, there was a highly significant enhancement of alpha2 power after only 30 minutes (p < 0.02). During performance of the memory test, less theta and alpha1 spectral power was produced in the presence of verum in comparison to placebo with regard to the median of all electrode positions. Statistical significance was reached for theta at 4 hours after intake and for alpha1 after 3 hours (not shown). Changes with respect to the other frequencies were not consistent and only reached statistical significance for beta1 at 3 hours after administration (not shown).
Performance of psychometric tasks did not reveal any
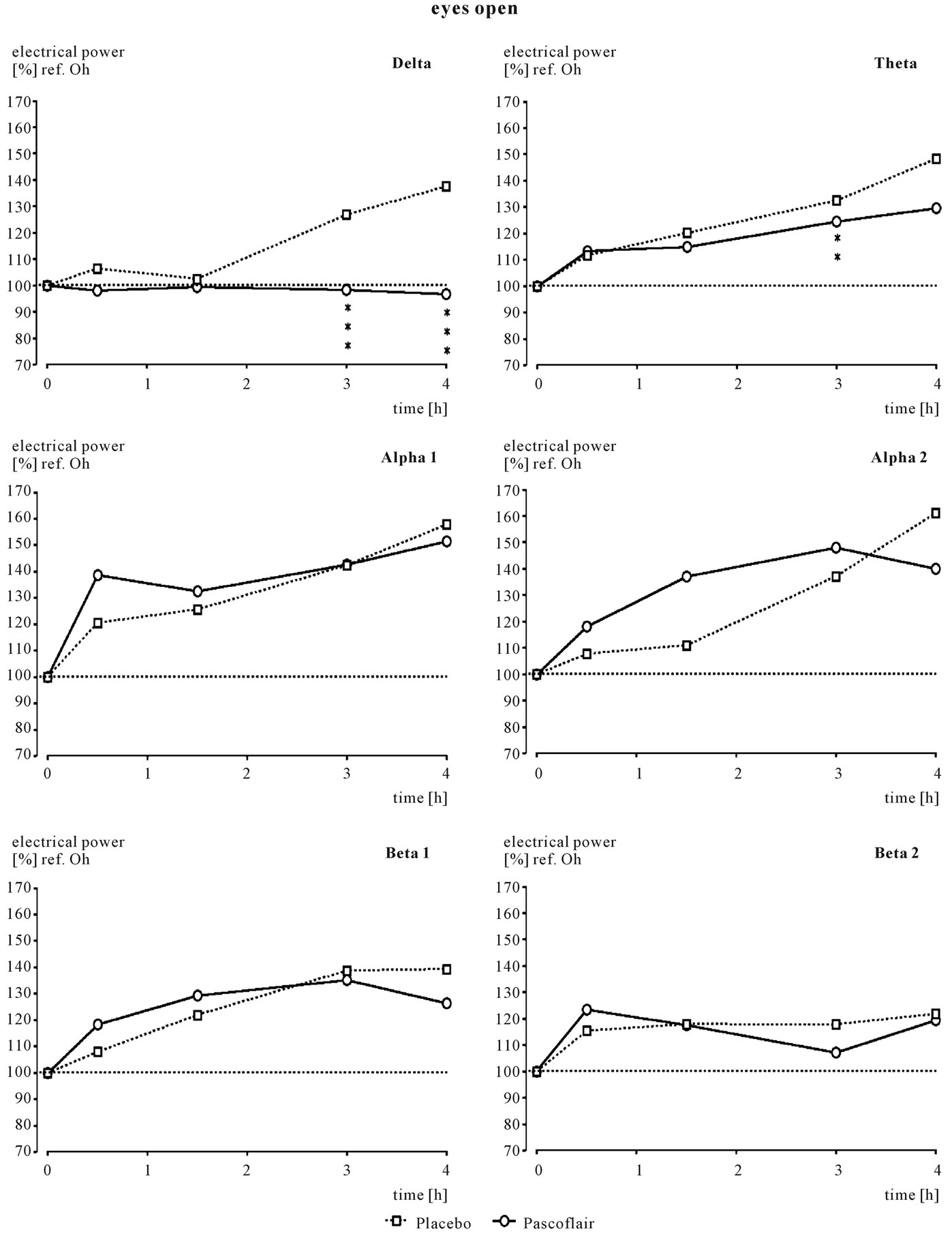
Figure 2. Time course of spectral frequency changes in comparison to pre-drug values, which are set to 100%. Comparison of verum and placebo. Statistics: **correspond to p < 0.8; ***correspond to p < 0.02.
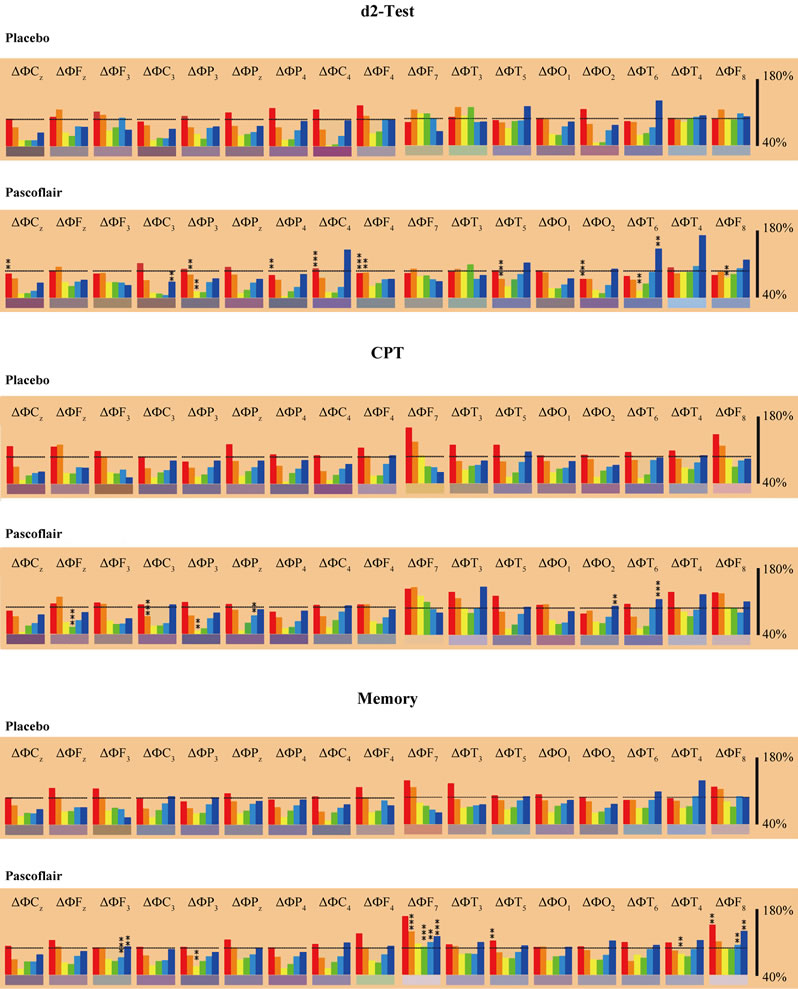
Figure 3. Spectral frequency changes at 17 electrode positions during mental performance (CPT = concentration performance test with calculations). Red: delta, orange: theta, yellow: alpha1, green: alpha2, light blue: beta1 and dark blue: beta2. F = frontal, C = central, P = parietal, T = temporal, 0 = occipital. Even numbers represent the right hemisphere, uneven number represent the left hemisphere. Statistics: **correspond to p < 0.8; ***correspond to p < 0.02. Data are documented for 3 h after intake.
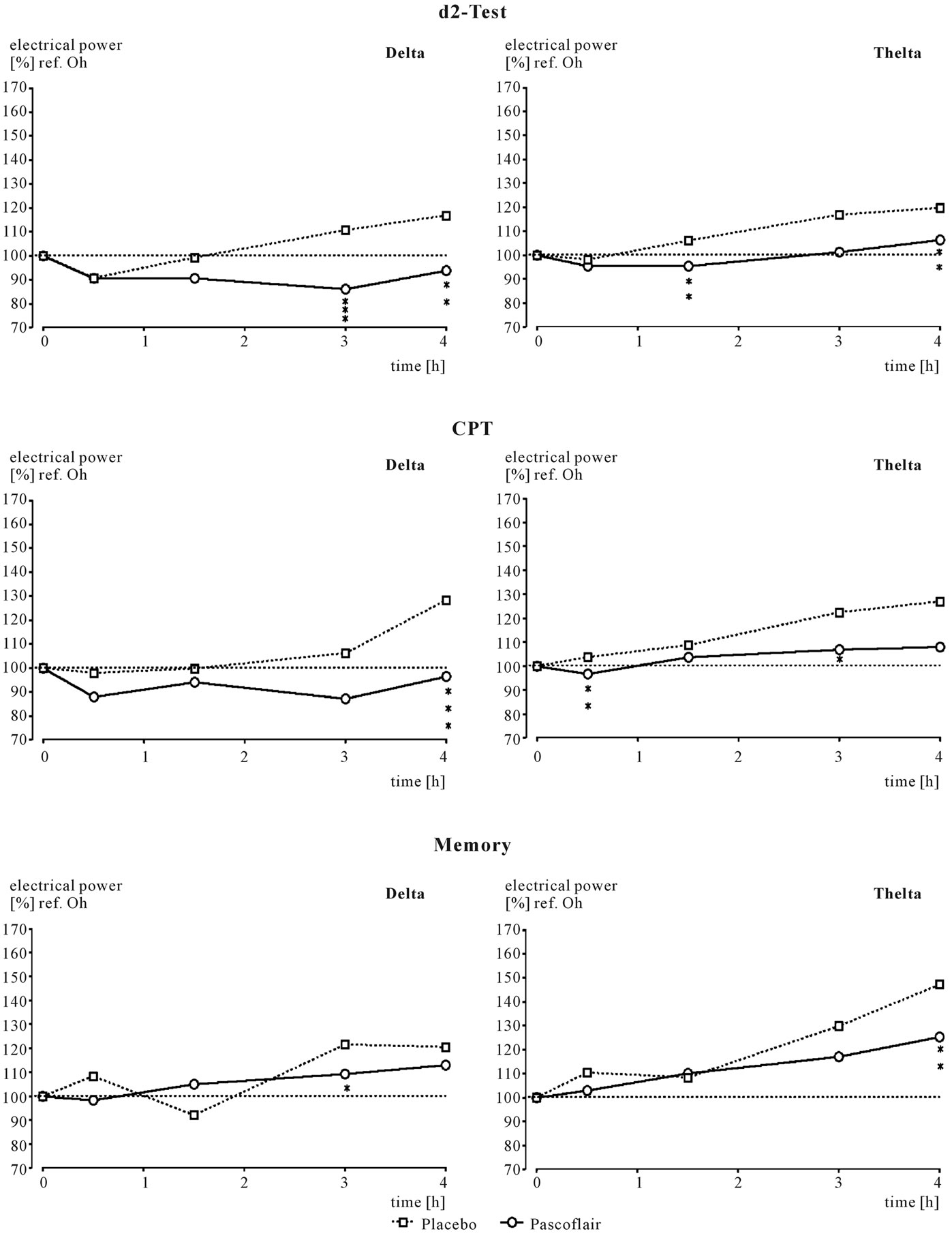
Figure 4. Time course of spectral frequency changes in comparison to pre-drug values, which are set to 100%. Comparison of verum and placebo. Statistics: **correspond to p < 0.8; ***correspond to p < 0.02.

Figure 5. Depiction of the result of linear discriminant analysis of EEG source density data for all four recording periods after administration of verum and placebo. Result from the first three discriminant functions is displayed by means of space coordinates x, y and z. Values of the 4th to 6th linear discriminant functions respectively determine the amount of red, green and blue in additive colour mixtures analogous to the RGB mode of TV.
difference between placebo and verum (Table 2).
4. Discussion
This experimental series showed that the preparation Pascoflair® 425 mg already modulated electric brain activity after a single acute intake compared to placebo. Spectral power in the delta and theta range was attenuated in comparison with the time-dependent increase normally observed due to circadian rhythm. The median value calculated from all electrode positions indicated a statistically significant attenuation of spectral power 3 and 4 hours after administration. The meaning of this preparation-dependent effect is not absolutely clear, especially since it was observed under nearly every recording condition. It can possibly be interpreted as a kind of stabilization of electric activity which might make brain activity more resistant to external disturbances. Focal pathological increases of delta and theta power are regularly observed in patients suffering from epilepsy. In fact, there is one report in the literature describing anticonvulsive effects of the aerial parts of Passiflora incarnata extract in mice [18]. It is possible that the use of Pascoflair® 425 mg might eventually be used in an addon therapy for epileptic patients. But this interpretation must remain speculative until supported by data from further clinical tests.
Since it is quite difficult to evaluate the data from 17 electrode positions in the presence of 6 frequency ranges (a total of 102 parameters), the mathematical tool of discriminant analysis was used to describe the action of the active drug compared to placebo for the recording condition “eyes open”. This method permits the time-dependent evaluation of all changes with respect to the predrug condition. As can be seen in Figure 5, all placebo values (labelled PL05 to PL4)—representing circadian rhythm dependent changes—are clustered. Depiction of the result of the effect of verum at 0.5, 1.5, 3 and 4 hours after administration in comparison to the effect of other synthetic reference drugs or plant-derived preparations tested earlier revealed clear effects for the active drug after only 30 minutes (labelled Pas05, Pas15, Pas3 and Pas4 in Figure 5). This early effect of Pascoflair® 425 mg is in line with a report on another Passiflora incarnata


Table 2. Result of psychometric procedures during d2-test.
extract tested in 60 ambulatory surgery patients. In a placebo-controlled, double-blind study, preoperative anxiety scores after only 30 minutes were significantly lower in the Passiflora group than in the control group [19].
In the past, discriminant analysis of spectral power was successfully used to differentiate drug profiles from each other in rats according to their clinical indication [20]. Drugs with a similar clinical indication clustered together whereas drugs with different indications were widely diverse in space and colour. The same reasoning can be followed in showing that Pascoflair® 425 mg took position near some other drugs with well-known clinical indications. One of the closest neighbours in space (representing the result of the first to third discriminant function)—but with a different colour due to the result of the fourth to sixth discriminant function—is L-Theanine. Interestingly, L-Theanine is regarded as a compound with relaxing properties and is reported to reduce psychologycal and physiological stress responses [21]. At the same time, possible neuroprotective and cognitive enhancing properties are also reported [22]. Another neighbour not too far away is a preparation consisting of Gingko and Ginseng extract [23].
Interestingly, there was obviously no negative influence on mental activity, since psychometric performance remained stable throughout the whole experimental period. On the contrary, during performance of the memory test, increases of delta, theta and beta power at frontal electrode positions indicative of good mental fitness were observed. This frontal increase of delta and theta power during mental load was not observed in demented people [24] and subjects suffering from mild cognitive impairment [25]. Presumably, the great scatter of psychometric results from only 16 volunteers prevented more insight into effects on memory function in the presence of Pascoflair® 425 mg.
5. Conclusion
In summary, Pascoflair® 425 mg was shown to produce a significant change to the pattern of electric activity in the brain as a surrogate parameter for its central efficacy. The increase of delta, theta and beta power at frontal electrode positions during performance of the memory test is indicative of the preservation of good mental fitness. Spectral changes of electric activity resemble those seen in the presence of L-Theanine and Ginkgo/Ginseng. The results from this study document the effectiveness of the preparation after only 30 minutes. In contrast with reports in the literature on sedative and cognition attenuating effects of various synthetic preparations such as benzodiazepines, this study did not reveal any such negative side effects for Pascoflair® 425 mg.
6. Acknowledgements
Petra Werling is gratefully acknowledged for taking the EEG recordings. We thank Leonie Schombert for her help in documenting the results. We appreciate the help of Bianka Krick in critically reviewing the manuscript. Ingrid Keplinger-Dimpfel performed the logistics and quality control of the study.
7. Authors’ Contributions
W.D. provided the electrophysiological technology, supervised the performance of the experiments, gave interpretation of the results and wrote the manuscript. K.K. performed the medical examinations and evaluated unexpected events. G.W. initiated the study and made major contributions to the design. She also provided important information on the pharmacology of the preparation’s constituents.
REFERENCES
- P. C. De Castro, A. Hoshino, J. C. da Silva and F. R. Mendes, “Possible Anxiolytic Effect of Two Extracts of Passiflora Quadrangularis L. in Experimental Models,” Phytotherapy Research, Vol. 21, No. 5, 2007, pp. 481- 484. doi:10.1002/ptr.2079
- O. Grundmann, C. Wählig, C. Staiger and V. Butterweck, “Anxiolytic Effects of a Passionflower (Passiflora incarnate L.) Extract in the Elevated Plus Maze in Mice,” Die Pharmazie, Vol. 64, No. 1, 2009, pp. 63-64.
- C. Sampath, M. Holbik, L. Krenn and V. Butterweck, “Anxiolytic Effects of Fractions Obtained from Passiflora incarnate L. in the Elevated Plus Maze in Mice,” Phytotherapy Research, Vol. 25, No. 6, 2010, pp. 789- 795. doi:10.1002/ptr.3332
- L. M. Sena, S. M. Zucolotto, F. H. Reginatto, E. P. Schenkel and T. C. De Lima, “Neuropharmacolgical Activity of the Pericarp of Passiflora edulis flavicarpa Degener: Putative Involvement of C-gylcosylflavonoids,” Experimental Biology and Medicine, Vol. 234, No. 8, 2009, pp. 967- 975. doi:10.3181/0902-RM-84
- O. Grundmann, J. Wang, G. P. McGregor and V. Butterweck, “Anxiolytic Activity of a Phytochemically Characterized Passiflora incarnate Extract is Mediated Via the GABAergic System,” Planta Medica, Vol. 74, No. 15, 2008, pp. 1769-1773. doi:10.1055/s-0028-1088322
- K. Appel, T. Rose, B. Fiebich, T. Kammler, C. Hoffmann and G. Weiss, “Modulation of the γ-Amino butyric Acid (GABA) Aystem by Passiflora incarnate L.,” Phytotherapy Research, Vol. 25, No. 6, 2010, pp. 838-843.
- H. Li, P. Zhou, Y. Shen, L. Li and D. Zhao, “Comparative Studies on Anxiolytic Activities and Flavonoid Compositions of Passiflora edulis ‘edulis’ and Passiflora edulis ‘flavicarpa’,” Journal of Ethnopharmacology, Vol. 133, No. 3, 2011, pp. 1085-1090. doi:10.1016/j.jep.2010.11.039
- J. Deng, Y. Zhou, M. Bai, H. Li and L. Li, “Anxiolytic and Sedative Activities of Passiflora edulis f. flavicarpa,” Journal of Ethnopharmacology, Vol. 128, No. 1, 2010, pp. 148-153. doi:10.1016/j.jep.2009.12.043
- P. R. Barbosa, S. S. Valvassori, C. L. Bordignon Jr., V. D. Kappel, M. R. Martins, E. C. Gavioli, J. Quevedo and F. H. Regianatto, “The Aqueous Extracts of Passiflora Alata and Passiflora edulis Reduce Anxiety-related Behaviors without Affecting Memory Process in Rats,” Journal of Medicinal Food, Vol. 11, No. 2, 2008, pp. 282-288.
- World Medical Association, “Ethical Principles for Medical Research Involving Human Subjects,” Cambridge University Press, Cambridge, 2000.
- d2-Test, “Aufmerksamkeits-Belastungs-Test, Rolf Brickenkamp,” Hogrefe Verlag, Göttingen, 1994.
- H. Düker and G. A. Lienert, “Der Konzentrations-Leistungstest (KLT),” Hogrefe Verlag, Göttingen, 1965.
- D. Lehmann, “Principles of Spatial Analysis,” In: A. S. Gevins and A. Remond, eds., Handbook of electroencephalography and Clinical Neurophysiology: Methods of Analysis of Brain and Magnetic Signals, Elsevier, Amsterdam, 1987, pp. 309-354.
- F. Schober and W. Dimpfel, “Relation between Psychometric Tests and Quantitative Topographic EEG in Pharmacology,” International Journal of Clinical Pharmacology, Therapy and Toxicology, Vol. 30, No. 11, 1992, pp. 428-430.
- G. Dietsch and H. Berger, “Fourier Analyse von Elektroenzephalogrammen des Menschen,” Pflüger’s Arch, Vol. 230, 1932, pp. 106-112.
- T. Harmony, A. Fernandez-Bouzas, E. Marosi, T. Fernandez, J. Bernal, M. Rodriguez, A. Reyes, J. Silva, M. Alonso and G. Casian, “Correlation between Computed Tomography and Voltage and Current Source Density Spectral Parameters in Patient with Brain Lesions,” Electroencephalography and Clinical Neurophysiology, Vol. 87, No. 4, 1993, pp. 196-205. doi:10.1016/0013-4694(93)90019-R
- P. Benfield, R. C. Heel and S. P. Lewis, “Fluoxetine: A Review of its Pharmacodynamics and Pharmacokinetic Properties, and Therapeutic Efficacy in Depressive Illness,” Drugs, Vol. 32, No. 6, 1986, pp. 481-508.
- M. Nassiri-Asl, S. Shariati-Rad and F. Zamansoltani, “Anticonvulsive Effects of Aerial Parts of Passiflora Incarnate Extract in Mice: Involvement of Benzodiazepine and Opioid Receptors,” BMC Complementary and Alternative Medicine, Vol. 7, No. 26, 2007, pp. 26-32. doi:10.1186/1472-6882-7-26
- A. Movafegh, R. Alizadeh, F. Hajimohamadi, F. Esfehani and M. Nejatgar, “Preoperative Oral PassifloraIincarnate Reduces Anxiety in Ambulatory Surgery Patients: A Double Blind, Placebo-controlled Study,” Anesthesia & Analgesia, Vol. 106, No. 6, 2008, pp. 1728-1732.
- W. Dimpfel, “Preclinical Data Base of Pharmaco-Specific Rat EEG Fingerprints (Tele-Stereo-EEG),” European Journal of Medical Research, Vol. 8, No. 5, 2003, pp. 199-207.
- K. Kimura, M. Ozeki, L. R. Juneja and A. Ohira, “LTheanine Reduces Psychological and Physiological Stress Responses,” Biological Psychology, Vol. 74, No. 1, 2007, pp. 39-45. doi:10.1016/j.biopsycho.2006.06.006
- P. J. Nathan, K. Lu, M. Gray and C. Oliver, “The Neuropharmacology of L-Theanine (N-ethyl-L-glutamine): A Possible Neuroprotective and Cognitive Enhancing Agent,” Journal of Herbal Pharmacotherapy, Vol. 6, No. 2, 2006, pp. 21-30.
- W. Dimpfel, A. Kler, E. Kriesl and R. Lehnfeld, “Neurophysiological Characterization of Functionally Active Drink Containing Extracts of Ginkgo and Ginseng by Source Density Analysis of the Human EEG,” Nutritional Neuroscience, Vol. 9, No. 5-6, 2006, pp. 213-224.
- R. Schellenberg, A. Todorova, W. Dimpfel and F. Schober, “Pathophysiology and psychopharmacology of dementia—a New Study Design. I. Diagnosis Comprising Subjective and Objective Criteria,” Neuropsychobiology, Vol. 32, No. 2, 1995, pp. 81-97
- L. S. Prichep, E. R. John, S. H. Ferris, L. Rausch, Z. Fang, R. Cancro, C. Torossian and B. Reisberg, “Prediction of Longitudinal Cognitive Decline in Normal Elderly with Subjective Complaints Using Electrophysiological Imaging,” Neurobiology of Aging, Vol. 27, No. 3, 2006, pp. 471-481. doi:10.1016/j.neurobiolaging.2005.07.021
NOTES
*Competing interests: The authors declare that they have no competing interests.
#Corresponding author.

