Neuroscience & Medicine
Vol.2 No.4(2011), Article ID:8885,7 pages DOI:10.4236/nm.2011.24040
Biomarkers and Depressive Symptoms in a Sample of Cognitively Intact and Alzheimer’s Disease Elderly Males
![]()
1Department of Psychiatry and Neuroscience, University of North Texas Health Sciences Center, Fort Worth, USA; 2Institute for Aging and Alzheimer’s Disease Research, University of North Texas Health Sciences Center, Fort Worth, USA; 3Texas Tech University Health Sciences Center, Laura W. Bush Institute for Women’s Health & Department of Family and Community Medicine, Lubbock, USA; 4Department of Pharmacology and Neuroscience, University of North Texas Health Sciences Center, Fort Worth, USA; 5F. Marie Hall Institute for Rural & Community Health, Texas Tech Health Sciences Center, Lubbock, USA; 6Department of Neurology, Texas Tech University Health Sciences Center, Lubbock, USA.
E-mail: *james.hall@unthsc.edu
Received October 6th, 2011; revised November 18th, 2011; accepted November 26th, 2011.
Keywords: Depression, Biomarkers, Alzheimer’s
ABSTRACT
Serum-based biomarkers and GDS-30 score and subscales of depressive symptoms were examined in a cross-sectional sample of 81 elderly men drawn from the TARCC cohort. Measurements included neuropsychological assessment and serum. Thirty three patients met consensus diagnosis for probable AD and forty eight were cognitively intact. Although initial regression analysis of all subjects showed significant relationships between depression and specific biomarkers, analyses based on diagnosis indicated that none of the biomarkers were significantly associated with depression among the controls. Among AD males MIF was significantly associated with total GDS scores and subscales of dysphoria, meaninglessness, and cognitive impairment. TNF-α was significantly associated with the apathy in AD males. Higher levels of MIF were associated with less depression in AD men. TNF-α was positively associated with degree of apathy. This study suggests the importance of cognitive status, gender and subtypes of depression when investigating biomarkers and depression in the elderly.
1. Introduction
Depression occurs among the elderly population at the rate of 10% - 15% [1] with lifetime prevalence of over 40% among men and women over 70 years of age [2]. Lifetime prevalence is even higher among geriatric female samples in prospective studies (43%, [2]), men however tended not to report impairment in mixed-gender late-life depression studies [3]. Additionally, late-life depression has been detected in both cognitively intact individuals and patients with Alzheimer’s disease (AD) and has significant negative impact by promoting disability, increased mortality, reduced cognitive capacity and quality of life, and even suicide.
Similar to late-life depression, cognitive impairment also has a high prevalence rate among geriatric populations. Starkstein and colleagues [4] suggested that a slighter higher number of AD patients experience symptoms of depression compared to non-demented patients. Numerous studies have focused on identifying biomarkers associated with depression and/or AD (review; [5]). These efforts have failed to produce a clear-cut picture of the relationship between biomarkers, depression and AD.
1.1. Current Understanding of Biomarkers, Late-Life Depression, and Alzheimer’s Disease
Late-life depression has been associated with increased levels of a variety of proinflammatory cytokines [6]. Current research on biomarkers in late-life depression among cognitively intact individuals has identified a number of potential biomarkers ([7]): Interleukin-6 (IL-6, [8]); IL-10 [9,10]; IL-1 [11]; C-reactive protein (CRP; [12]);tumor necrosis factor (TNF)-α [13]; and Macrophage migration inhibitory factor (MIF) [14,15]. Additionally, brain-derived neurotropic factor (BDNF), known to promote the development of serotonergic neurons with mood-related effects [16], has been found to significantly impact expression of depression in mild AD patients [17]. Other biomarkers such as ICAM-1 have been more frequently associated with depressive symptoms in vascular models of late-life depression [18-20]. Apolipoprotein E [APOE ε4 allele] status has been associated with level of cognitive impairment in elderly patients [21] with affective states being related to cognition only among noncarriers of the APOE4 allele [22].
1.2. Current Limitations
The biomarkers for late-life depression discussed above have been implicated in both healthy controls and patient samples, but results remain inconsistent. The majority of studies have been limited to evaluating depression in either AD or cognitively intact elders; examinations of markers for late life depression in both normal controls and AD patients are lacking. Significantly, prior studies have focused on relationships between cerebrospinal fluid or serum biomarkers and depression in elderly women alone [23] or in mixed gender samples [14,16,21, 24-27], but not yet in men alone. In addition, prior studies have used global depression scores rather than examined specific subcategories or clusters of symptoms. Past research in our laboratory has demonstrated significant value in the use of symptom clusters along with global depression scores [17,28].
1.3. Current Purpose
To address the lack of data on the relationship of biomarkers to depression in elderly demented and non-demented men, the present study seeks to 1) evaluate serum-based biomarkers in men; 2) examine biomarker differences in cognitively intact and AD men; 3) assess both global depression scores and subscale scores in each group, 4) identify markers that are associated with level of cognitive function and depressive symptoms.
2. Methods
2.1. Sample
The sample was drawn from men enrolled in the Longitudinal Research Cohort of the Texas Alzheimer’s Research and Care Consortium (TARCC) who met the diagnostic criteria for mild AD or were evaluated as cognitively normal and had complete biomarker and depression data. To reduce the effect of stage of decline only AD men with a Clinical Dementia Rating scale global score of ≤1.0 were included in the sample. Eighty-one men met the criteria with 33 diagnosed with mild AD and 48 evaluated as cognitively intact (normal controls; NC). The methodology of the TARCC project has been described in detail elsewhere [29]. Briefly, the TARCC project is a longitudinal multi-site study of a cohort of AD patients and normal controls where each participant undergoes an annual evaluation that includes a medical examination, interview, neuropsychological testing, and blood draw. AD patients met consensus diagnosis for probable AD based on NINCDS-ADRDA criteria. The TARCC project received Institutional Review Board approval and all participants and/or caregivers provided written informed consent.
2.2. Assessment
The Geriatric Depression Scale (GDS 30) was administered as part of the TARCC neuropsychology core battery. In addition to the GDS, the battery consists of common instruments administered as part of the established Alzheimer’s disease clinical/research platforms at each participating institution and includes digit span (WAIS-R, WAIS-III, WMS-R), Trail Making Test, WMS Logical Memory and Visual Reproduction (WMS-R and WMS-III), Boston Naming Test (30- and 60-item versions), verbal fluency (FAS), Clock Drawing Test, the American National Adult Reading Test (AMNART), Mini-Mental State Examination (MMSE), and ratings on the Clinical Dementia Rating scale (CDR).
2.3. Description of Subscales on the Geriatric Depression Scale
The Geriatric Depression Scale (GDS-30; [30]) was the first depression scale designed specifically for older populations [30] and has been widely used primarily as a screening instrument in clinical and research settings. A recent factor analytic study [31] identified a four-factor solution underlying the scale: dysphoria; meaninglessness; apathy; and cognitive impairment. The dysphoria factor contains 11 items primarily associated with a sad mood. The meaninglessness factor consists of seven items that reflect an appraisal of the meaning (or lack thereof) in one’s life. The apathy factor is made up of six items that reflect a lack of motivation or initiative. The cognitive impairment factor consists of six items that reflect difficulty and concern with cognitive processes.
2.4. Assays
Non-fasting blood samples were collected in serumseparating tubes during clinical evaluations, allowed to clot at room temperature for 30 minutes, centrifuged, aliquoted, and stored at –80˚C in plastic vials. Batched specimens from either baseline or year-one follow-up exams were sent frozen to Rules Based Medicine (RBM, www.rulesbasedmedicine.com, Austin, TX) where they were thawed for assay without additional freeze-thaw cycles using the RBM multiplexed immunoassay human Multi-Analyte Profile (humanMAP). Individual proteins were quantified with immunoassays on colored microspheres. Information regarding the least detectable dose (LDD), inter-run coefficient of variation, dynamic range, overall spiked standard recovery, and cross-reactivity with other humanMAP analytes can be readily obtained from RBM. IL-6 levels were measured, but were below the detectable range and were not included in the analysis.
2.5. Data Analysis
Analyses of the data were performed using SPSS version 17.0. For total GDS score and each of the four GDS subscale scores One-way ANOVA compared cognitively intact and AD male (Table 1). Stepwise regression modeling was used to evaluate the link between total GDS score and each subscale (dysphoria, apathy, meaninglessness, and cognitive impairment) by level of cognitive impairment (AD or cognitively intact). Items on the GDS 30 were scored (0 = absence and 1 = presence of symptom) and then added for composite subscale scores. Independent variables for the regression analysis included biomarkers (CRP, IL-10, IL-1α, TNF-α, ICAM-1, BDNF, and MIF). All analyses were conducted using log transformed values of the biomarkers. ApoE4 status was coded (carrier or non-carrier). Covariates were age and years of formal education. Significance level was set at 0.05.
3. Results
3.1. Sample
Demographic data are presented in Table 2. Normal controls were on average 71.10 years of age (SD = 8.5) and 76.15 (SD = 8.56) for the AD group. The control sample had an average of 16.77 (3.391) years of education and the AD sample 13.58 (3.562) years. There was a significant difference on both age (p = 0.001) and education (p = 0.001) with the AD sample being both older and less educated. The majority of participants were Caucasian (98%) with black or African American (1.5%) the next largest group.
3.2. Significant Biomarkers in Elderly Men
Independent t-tests of total GDS scores revealed no significant differences between cases vs. controls on levels of CRP, IL-10, TNF-α, ICAM-1, BDNF, or MIF, although there was a significant difference between the groups on IL-1α with significantly higher levels found for controls. The results of the regression analysis (Table 3) for the total sample regardless of cognitive status revealed that MIF and level of TNF-α were associated with total GDS scores and also individual subscales. Higher MIF levels were inversely related to total GDS (t = –2.64, p < 0.01; R2 = 0.056), level of Dysphoria (t = –4.81, p < 0.001; R2 = 0.227), Meaninglessness (t = –3.13, p < 0.01; R2 = 0.110), and perceived level of Cognitive Impairment (t = –2.71, p < 0.01; R2 = 0.085). Level of TNF-α was predictive of Apathy (t = 2.23, p < 0.05; R2 = 0.059).
Regression analysis conducted separately for cases and controls revealed that none of the biomarkers were useful in predicting depressive symptoms in cognitively intact men. However for AD males (Table 4), MIF was significantly associated with total GDS scores (t = –2.731, p < 0.021; R2 = 0.118), and the subscale scores of Dysphoria (t = –5.68, p < 0.000; R2 = 0.510), Meaninglessness (t = –2.77, p = 0.009; R2 = 0.198), and Cognitive Impairment (t = –2.317, p = 0.027; R2 = 0.148). Level of Cognitive Impairment was also related to MIF and CRP, (t = –3.031, p = 0.005; R2 = 0.260). TNF-α was significantly associated with the Apathy (t = 2.08, p = 0.046; R2 = 0.123) subscale for AD males.
4. Discussion
The current study is the first to examine the relationship between serum-based biomarkers and symptoms of depression in cognitively normal and AD males. Biomarkers were not significant predictors of depressive symptoms in cognitively intact males. This may partly account for the dearth of literature in this population, as negative findings typically remain unpublished. In a combined male sample (cognitively intact and AD), MIF and TNF-α were associated with depressive symptoms. More importantly, among the AD group, MIF was a significant predictor of total depression score and all subscales except apathy. While other biomarkers such as ICAM have been linked with symptoms of apathy, MIF has not been useful in predicting apathy in the past. In fact, in a mixed sample report, with a small sample size of (n = 18 patients and 38 normal controls), MIF did not contribute any variance in predicting late-life depression [14]. While the role of MIF is unknown in the disease progression of patients with AD and late-life depression, it has been implicated in depression in a recent animal study [15], suggesting that MIF plays an inverse role in relation to depression. Likewise, the current findings suggest that increased MIF levels are negatively associated with depressive symptoms. It is important to note that MIF levels have also been associated with risk for AD [32] which could account for the finding of a relationship between MIF and depressive symptoms in AD males. However there was no significant difference in MIF levels between AD and control males (p = 0.792). Additionally, post hoc analysis of the relationship between

Table 1. GDS subscale means and standard deviations.
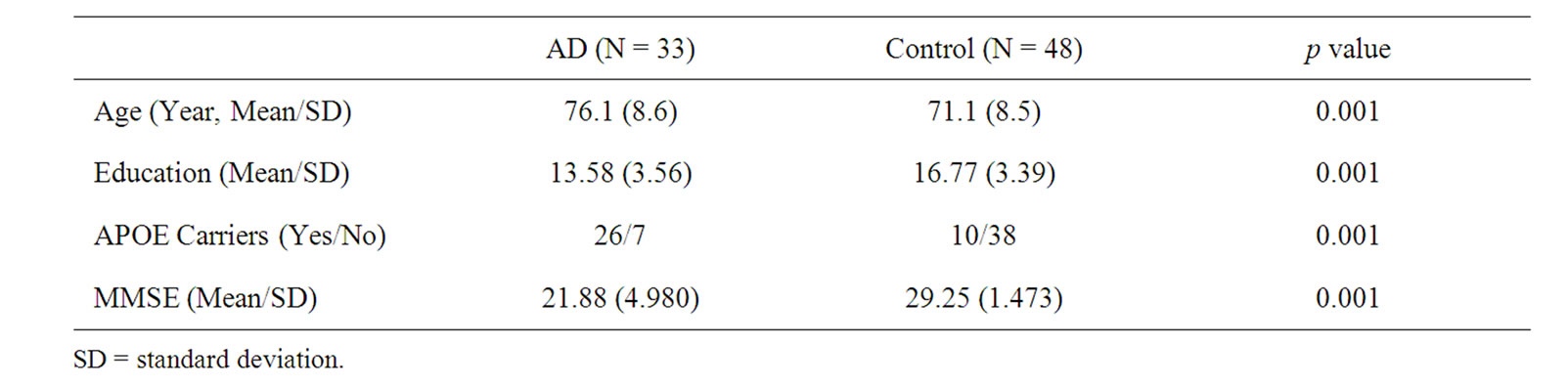
Table 2. Demographics of the study group.
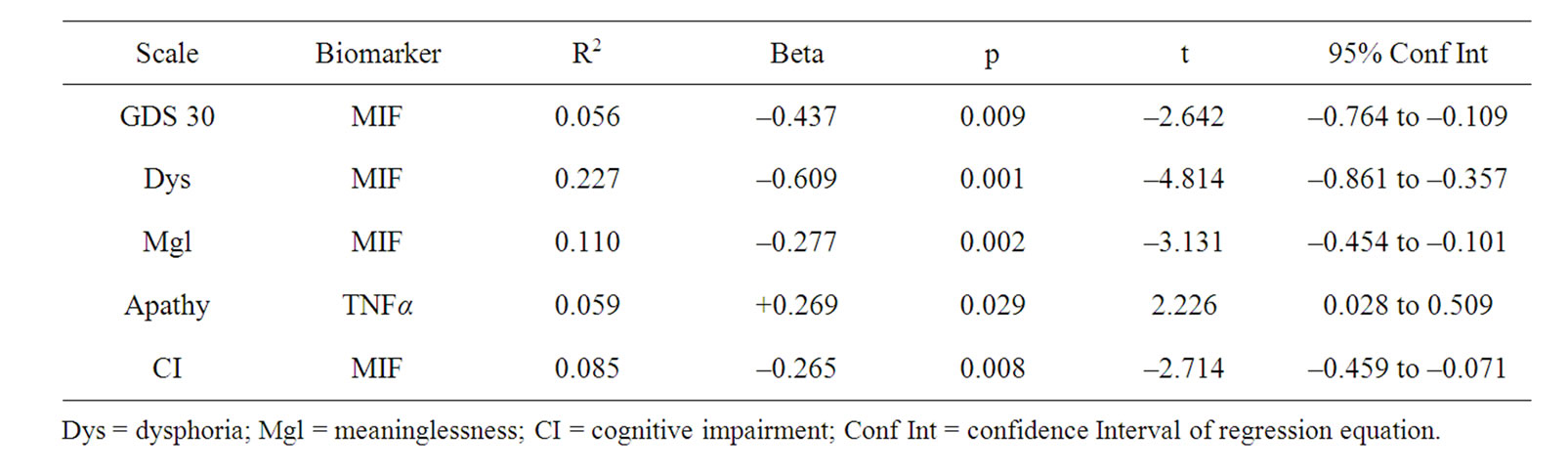
Table 3. All males N = 81.
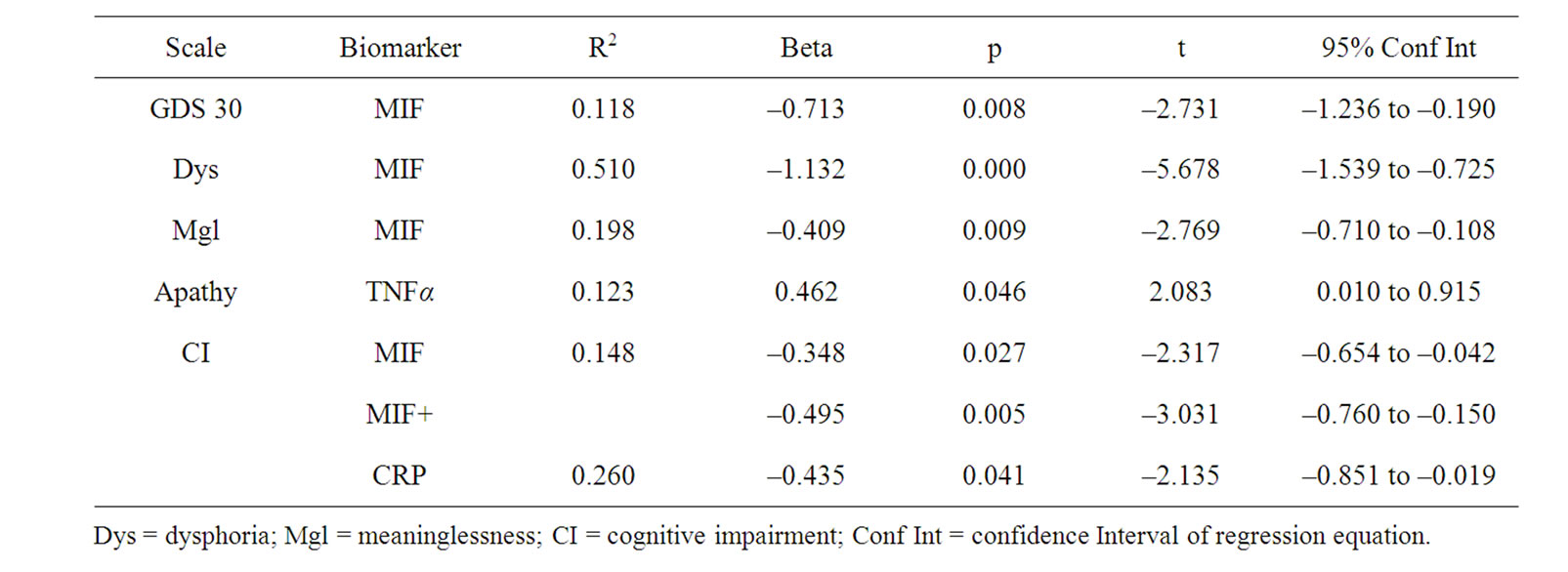
Table 4. AD N = 33.
MIF and measures of cognitive functioning revealed no significant relationships on any of the cognitive measures in the TARCC battery for AD males suggesting that MIF levels are related to mood but not level of cognitive impairment in AD males. The negative relationship between CRP and Cognitive Impairment in the second significant regression model is puzzling in that higher CRP has been related to increased depression in other studies. Since the CI subscale assesses perceived cognitive decline, it may be that level of CRP is in some way related to level of awareness of decline. In a mixedsample study, TNF-α was positively associated with depressive symptoms in healthy elderly men and women [6], which is consistent with current findings. Accordingly, our data suggest that TNF-α does have a positive relationship with Apathy in AD male patients.
Differences in current and prior findings are attributable, at least partly, to effects of mixed gender analysis, which may have obscured significant findings. In addition, methodological differences are also attributable to differences in prior findings. The present study is not only one of the first to evaluate AD and control males only, but also is the first to examine this significant question using subclassifications of depression rather than only global symptoms. These differences are significant because symptoms of depression have been known to manifest differently in women and men. For instance, women are more likely to report dysphoria, appetite disturbances, and fatigue although both men and women experiences similar levels of subjective, social, and occupational impairment [32].
The current study is also unique in that it evaluated both cognitively intact and AD samples. The present research advocates for the importance of continuing to explore inflammatory processes in older patients with and without cognitive impairment across gender. Current data also suggest the value of using subscales rather than global depression scores as outcome measures.
5. Study Limitations
Limitations to the current study include small and uneven sample sizes. Efforts to replicate the current findings are important for the current understanding of biomarkers in late-life depression among cognitively impaired and unimpaired elders. The present study suffers from its cross-sectional nature and relatively small sample of individuals with a higher level of depressive symptoms. The overall level of depressive symptoms was low and the small number of patients with at least mild depression reflects a sampling bias in the TARCC cohort where individuals with high initial levels of depression were excluded from the study. The current study did not use diagnostic criteria to determine the presence of depressive symptoms but rather relied on scores as a measure of depression. It may well be that individuals with higher levels of symptoms or a Major Depressive Disorder may present with a distinct biomarker profile. Another limitation is the absence of IL-6 in the current analysis. While some studies have suggested the significance of IL-6, findings have not been consistent. Among the characteristics of the sample, the AD group, on average, was older and less well educated than the cognitively intact group. Despite it limitations, the present study is the first to specifically examine the relationship between males, cognitive status, clusters of depressive symptoms and biomarkers. Additional efforts are warranted in this research area to utilize biomarkers to more accurately predict latelife depression among males and females with varying cognitive capacities and varying levels of depression.
6. Acknowledgements
This study was made possible by the Texas Alzheimer’s Research & Care Consortium (TARCC) funded by the state of Texas through the Texas Council on Alzheimer’s Disease and Related Disorders. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Investigators from the Texas Alzheimer’s Research Consortium: Baylor College of Medicine: Eveleen Darby, Aline Hittle; Texas Tech University Health Science Center: Paula Grammas, Benjamin Williams, Andrew Dentino, Gregory Schrimsher, Parastoo Momeni, Larry Hill; University of North Texas Health Science Center: Janice Knebl, Lisa Alvarez, Douglas Mains; University of Texas Southwestern Medical Center: Roger Rosenberg, Ryan Huebinger, Joan Reisch, Janet Smith, Mechelle Murray, Tomequa Sears; University of Texas Health Sciences Center—San Antonio: Don Royal, Ray Palmer.
REFERENCES
- P. Gudmundsson, I. Skoog, M. Waern, K. Blennow, S. Palsson, et al., “The Relationship between Cerebrospinal Fluid Biomarkers and Depression in Elderly Women,” American Journal of Geriatric Psychiatry, Vol. 15, No. 10, 2007, pp. 832-838. doi:10.1097/JGP.0b013e3180547091
- J. Wiltfang, P. Lewczuk, P. Riederer, et al., “Consensus Paper of the WFSBP Task Force on Biological Markers of Dementia: The Role of CSF and Blood Analysis in the Early and Differentia Diagnosis of Dementia,” World Journal of Biological Psychiatry, Vol. 6, No. 2, 2006, pp. 69-84. doi:10.1080/15622970510029786
- S. Palsson, L. Larsson, E. Tengelin, et al., “The Prevalence of Depression in Relation to Cerebral Atrophy and Cognitive Performance in 70- and 74-Year-Old Women in Gothenburg,” The Women’s Health Study. Psychological Medicine, Vol. 31, 2001, pp. 39-49.
- S. E. Starkstein, P. Gustavo, E. Chemerinski, et al., “Syndromic Validity of Apathy in Alzheimer’s Disease,” American Journal Psychiatry, Vol. 158, 2001, pp. 872- 877. doi:10.1176/appi.ajp.158.987.872
- G. S. Alexopoulos and S. S. Morimoto, “The Inflammation Hypothesis in Geriatric Depression,” International Journal of Geriatric Psychiatry, Vol. 26, No. 11, 2011, pp. 1109-1118.
- B. W. Penninx, S. B. Kritchevsky, K. Yaffe, et al., “Inflammatory Markers and Depressed Mood in Older Persons: Results from the Healthy, Aging and Body Composition Study,” Biological Psychiatry, Vol. 54, No. 5, 2003, pp. 566-572. doi:10.1016/S0006-3223(02)01811-5
- Y. Dowlati, N. Herrmann, W. Swardfager, et al., “A Meta-Analysis of Cytokines in Major Depression,” Biological Psychiatry, Vol. 67, No. 5, 2010, pp. 446-457. doi:10.1016/j.biopsych.2009.09.033
- M. A. Bremmer, A. T. F. Beekman and D. J. H. Deeg, “Inflammatory Markers in Late-Life Depression: Results from a Population-Based Study,” Journal of Affective Disorders, Vol. 106, 2008, pp. 248-255. doi:10.1016/j.jad.2007.07.002
- M. Berk, A. A. Wadee, R. H. Kuschke, et al., “Acute Phase Proteins in Major Depression,” Journal of Psychosomatic Research, Vol. 43, No. 5, 1997, pp. 529-534. doi:10.1016/S0022-3999(97)00139-6
- A. Sluzewska, J. Rybakowski, E. Bosmans, et al., “Indicators of Immune Activation in Major Depression,” Psychiatry Research, Vol. 64, No. 3, 1996, pp. 161-167. doi:10.1016/S0165-1781(96)02783-7
- A. Reichenberg, R. Yirmiya, A. Schuld, et al., “CytokineAssociated Emotional and Cognitive Disturbances in Humans,” Archives of General Psychiatry, Vol. 58, 2001, pp. 445-452. doi:10.1001/archpsyc.58.5.445
- H. Moshage, “Cytokines and the Hepatic Acute Phase Response,” Journal of Pathology, Vol. 181, No. 3, 1997, pp. 257-266. doi:10.1002/(SICI)1096-9896(199703)181:3<257::AID-PATH756>3.0.CO;2-U
- E. M. Sternberg, G. P. Chrousos, R. L. Wilder, et al., “The Stress Response and the Regulation of Inflammatory Disease,” Annals of Internal Medicine, Vol. 117, No. 10, 1992, pp. 854-866.
- K. S. Lee, J. H. Chung, K. H. Lee, et al., “Simultaneous Measurement of 23 Plasma Cytokines in Late-Life Depression,” Neurological Science, Vol. 30, No. 5, 2009, pp. 435-438. doi:10.1007/s10072-009-0091-1
- C. L. Varea, J. E. Castro, H. Sakouhi-Ouertatani, et al., “Macrophage Migration Inhibitory Factor Is Critically Involved in Basal and Flouxetine-Stimulated Adult Hypocampal Cell Proliferation and in Anxiety, Depression, and Memory-Related Behaviors,” Molecular Psychiatry, 2010, Epub ahead of Print: http://www.nature.com/mp/journal/vaop/ncurrent/full/mp201015a.html
- L. Pezawas, A. Meyer-Lindenberg, A. L. Goldman, et al., “Evidence of Biologic Epistasis between BDNF and SLC6A4 and Implications for Depression,” Molecular Psychiatry, Vol. 13, No. 7, 2008, pp. 709-716. doi:10.1038/mp.2008.32
- J. R. Hall, S. E. O’Bryant, L. Johnson, et al., “Neuropsychological Performance and Symptoms of Depression,” Scientific Meeting of the International Conference on Alzheimer’s Disease, Honolulu, Hawaii, July 2010.
- A. J. Thomas, I. N. Ferrier, R. N. Kalaria, et al., “Evaluation in Late-Life Depression in the Dorsolateral Prefrontal Cortex,” American Journal of Psychiatry, Vol. 157, No. 10, 2000, pp. 1682-1684. doi:10.1176/appi.ajp.157.10.1682
- A. J. Thomas, I. N. Ferrier, R. N. Kalaria, S. Davis and J. T. O’Brien, “Cell Adhesion Molecule Expression in the Dorsolateral Prefrontal Cortex and Anterior Cingulate Cortex in Major Depression in the Elderly,” British Journal of Psychiatry, Vol. 181, No. 2, 2002, pp. 129-134.
- T. Casoli, G. D. Stefano, M. Balietti, et al., “Peripheral Inflammatory Biomarkers of Alzheimer’s Disease: The Role of Platelets,” Biogerontology, Vol. 11, No. 5, 2010, pp. 627-633. doi:10.1007/s10522-010-9281-8
- A. M. Kulminski, S. V. Ukraintseva, I. V. Kulminskaya, et al., “Cumulative Deficits Better Characterize Susceptibility to Death in Elderly People than Phenotypic Frailty: Lessons from the Cardiovascular Health Study,” Journal of the American Geriatrics Society, Vol. 56, 2008, pp. 898-903. doi:10.1111/j.1532-5415.2008.01656.x
- M. Edwards, C. Mauer, J. R. Hall, R. C. Barber and S. E. O’Bryant, “APOE Moderates Link between Affective Status and Cognition: A Project Frontier Study,” The 119th Annual Meeting of the American Psychological Association, Washington D.C., August 2011.
- P. Gudmundsson, I. Skoog, M. Waern, et al., “Is There a CSF Biomarker Profile Related to Depression in Elderly Women?” Psychiatry Research, Vol. 176, No. 2-3, 2010, pp. 174-178. doi:10.1016/j.psychres.2008.11.012
- N. Andreasen and K. Blennow, “CSF Biomarkers for Mild Cognitive Impairment and Early Alzheimer’s Disease,” Clinical Neurology and Neurosurgery, Vol. 107, No. 3, 2005, pp. 165-173. doi:10.1016/j.clineuro.2004.10.011
- M. B. Howren, D. M. Lamkin and J. Suls, “Associations of Depression with C-Reactive Protein, IL-1, and IL-6: A Meta-Analysis,” Psychosomatic Medicine, Vol. 71, No. 2, 2009, pp. 171-186. doi:10.1097/PSY.0b013e3181907c1b
- V. Vaccarino, B. D. Johnson, D. S. Sheps, et al., “Depression, Inflammation, and Incident Cardiovascular Disease in Women with Suspected Coronary Ischemia: The National Heart, Lung, and Blood Institute-Sponsored WISE Study,” Journal of the American College of Cardiology, Vol. 50, No. 21, 2007, pp. 2044-2050. doi:10.1016/j.jacc.2007.07.069
- S. O’Bryant, J. Hall, K. Cukrowicz, et al., “The Differential Impact of Depressive Symptom Clusters on Cognition in a Rural Multi-Ethnic Cohort: A Project Frontier Study,” International Journal of Geriatric Psychiatry, Vol. 26, No. 2, 2011, pp. 199-205. doi:10.1002/gps.2514
- J. A. Yesavage, T. L. Brink, L. R. Terrence, et al., “Development and Validation of a Geriatric Depression Screening Scale: A Preliminary Report,” Journal of Psychiatry Research, Vol. 17, No. 1, 1983, pp. 37-49. doi:10.1016/0022-3956(82)90033-4
- S. Waring, S. E. O’Bryant, J. S. Reisch, R. Diaz-Arrastia, J. Knebl and R. Doody, “For the Texas Alzheimer’s Research Consortium. The Texas Alzheimer’s Research Consortium Longitudinal Research Cohort: Study Design and Baseline Characteristics,” Texas Public Health Journal, Vol. 60, No. 3, 2008, pp. 9-13.
- J. Hall and T. E. Davis, “Factor Structure of the Geriatric Depression Scale in Cognitive Impaired Older Adults,” Clinical Gerontology, Vol. 33, 2010, pp. 39-48. doi:10.1080/07317110903362127
- M. Bacher, O. Deuster, B. Aljabari, et al., “The Role of Macrophage Migration Inhibitory Factor in Azheimer’s Disease,” Molecular Medicine, Vol. 16, No. 3-4, 2010, pp. 116-121. doi:10.2119/molmed.2009.00123
- M. Piccinelli and G. Wilkinson, “Gender Differences in Depression,” British Journal of Psychiatry, Vol. 177, 2000, pp. 486-492. doi:10.1192/bjp.177.6.486

