Surgical Science
Vol. 4 No. 7 (2013) , Article ID: 33789 , 4 pages DOI:10.4236/ss.2013.47061
Leiomyosarcoma of the Duodeno-Jejunal Angle: Two Case Reports and Literature Review
1Surgical Unit “B”, Charles Nicolle Hospital, Faculty of Medicine of Tunis, Tunis, Tunisia
2Surgical Unit, Habib Bourguiba Hospital, Faculty of Medicine of Sfax, Sfax, Tunisia
Email: *jerrayahichem@yahoo.fr
Copyright © 2013 Hichem Jerraya et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received April 13th, 2013; revised May 11th, 2013; accepted May 20th, 2013
Keywords: Leiomyosarcoma; Duodenal Tumors; Surgery
ABSTRACT
The duodenal leiomyosarcoma is a rare tumor. Its location in the duodenum makes diagnosis difficult and often delayed. Surgical treatment is not consensual because it must take into account anatomical imperatives which vary depending on the duodenal portion and oncological requirements that are not currently well known especially with regard to the utility of lymphadenectomy. We report 2 cases of leiomyosarcomas located at the angle of Treitz. This location is exceptional and only few cases were reported in the literature. From these 2 new cases and a review of the literature, we analyze the clinical features, diagnosis and treatment of duodenal leiomyosarcoma.
1. Introduction
Leiomyosarcomas are rare malignant tumors which constitute with stromal tumors (GISTs) the majority of gastrointestinal mesenchymal tumors. These two tumors correspond to 1% of all gastrointestinal tumors [1]. Leiomyosarcomas may develop from all parts of the gastrointestinal tract, but they are found mainly in the stomach [1]. The duodenal localization is rare and to date, only a hundred cases have been reported in the literature. The location at the angle of Treitz has never been reported, hence the interest of our 2 case reports.
2. Case No. 1
A 73 year-old male was admitted for exploration of hypochromic anemia with hemoglobin at 6 g/100ml. Physical examination was normal. Upper gastrointestinal endoscopy and gastrointestinal series were normal. Colonoscopy showed a left colic diverticulosis without bleeding and two millimetric polyps which were resected. Pathological examination concluded to adenoma in moderate dysplasia. The patient was given a martial treatment. Six months later, evolution was marked by persistent anemia at 4.5 g/100ml. A 2nd upper gastrointestinal endoscopy showed atrophic gastritis. A 2nd colonoscopy showed a non-complicated colic diverticulosis. Explorations were then supplemented by an abdominal ultrasound which showed a 6 cm hypoechoic and heterogeneous mass, in front of the left renal pedicle. The computed tomography (CT) demonstrated a 6 × 5 × 3 cm intra peritoneal and tissular mass with an area of central necrosis. This mass expanded to small intestine close to the 4th duodenum which seemed to depend on. Gastrointestinal series showed a filling defect on the 4th duodenum (Figure 1). So a jejunoscopy was performed which visualized a non-ulcerated tumor at the angle of Treitz.
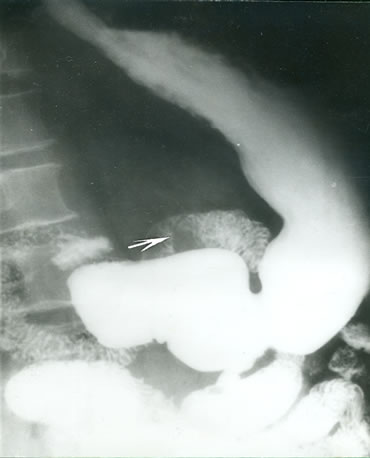
Figure 1. Gastrointestinal contrast x-ray studies demonstrating a filling defect on the 4th duodenum (arrow).
Surgical exploration disclosed a tumor arising from the duodeno-jejunal angle (Figure 2). The tumor was not invading the adjacent organs and there were no lymph nodes or distant metastases. Resection of the tumor was performed, taking the fourth duodenum, the first jejunal loop with its mesentery and then end-to-end anastomosis anterior to the mesenteric vessels was done. The postoperative course was uneventful. Histological examination coupled with immunohistochemistry confirmed leiomyosarcoma.
3. Case No. 2
A 52 year-old man was hospitalized for exploration of epigastric pain associated with gradual weakness and failing strength evolving since two months. Physical examination was normal. The Upper gastrointestinal endoscopy was normal. Different morphological examinations, including abdominal CT, revealed a tumor developed at the root of mesentery, under the body of the pancreas, with a probably invasion of the aorta and left renal vein (Figure 3). The surgical exploration found a large tumor which was developed behind the duodeno-jejunal angle and the fourth duodenum. This tumor was based on the anterior surface of the aorta and left renal vein which was invaded. Palliative resection was performed, taking the
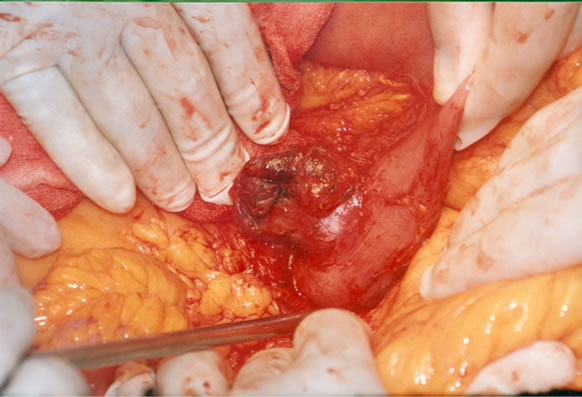
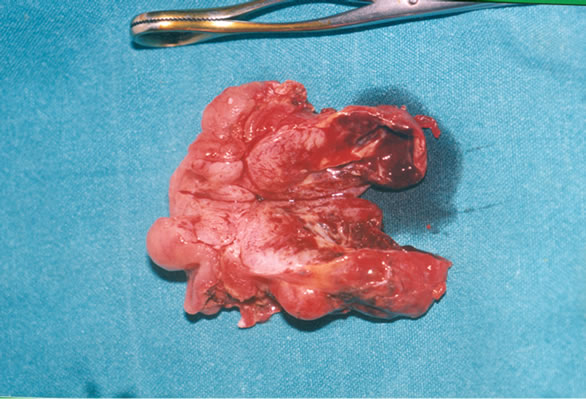
Figure 2. (A) Operative picture of the tumor of the duodeno-jejunal angle (arrow). (B) Gross appearance of the tumor which is formed by a cerebral-like tissue with central necrosis.
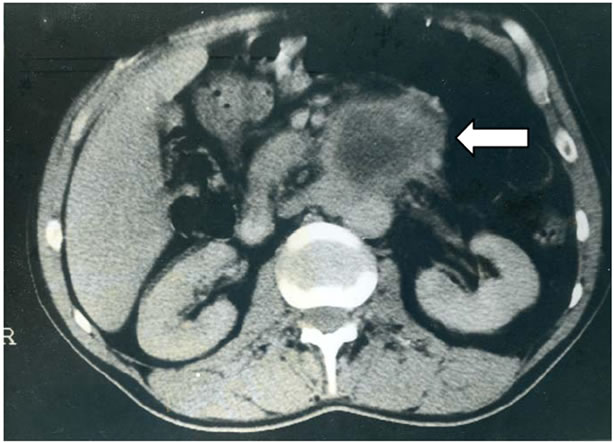
Figure 3. CT scan showing a tumor of the duodeno-jejunal angle (arrow) with invasion of the aorta and left renal vein.
angle of Treitz and the fourth duodenum with leaving a residual tumor in contact with the aorta and renal vessels, and then end-to-end anastomosis anterior to the mesenteric vessels was done. The postoperative course was uneventful. Histological examination found a leimyosarcoma. The patient died after 3 years of follow-up in an array of cachexia related to his neoplastic disease.
4. Discussion
The first case of duodenal leiomyosarcoma was reported by Von Salis in 1920 [2]. Since that date, we have achieved a search in the Pubmed database using “duodenal leiomyosarcoma” as key words and we have found 92 cases of duodenal leiomyosarcoma.
From these various observations, it was found that duodenal leiomyosarcoma occurred mainly in the age range of 50 - 70 years, but it could also affect younger [3]. The tumor occurred in the two sexes with a slight female predominance. Of the 72 cases in which sex was mentioned, 40 were women and 32 were men.
As noted by Starzl in his review of the literature [2], 53% of duodenal leiomyosarcoma occurred in the 2nd duodenum. The location at the fourth duodenum was observed in 8 cases (11%). The location at the duodenojejunal angle has never been reported hence the originality of our cases.
The symptoms were not characteristic and gastrointestinal symptoms were often poor and delayed because of slow and often exoluminal development of the tumor [4]. It was characterized also by the development of a rich vascular supply from adjacent organs which contrasted with central necrosis. This could lead to ulcers which were the source of gastrointestinal bleeding [5] or perforations in the peritoneal cavity or in the retroperitoneum [6].
The diagnosis of duodenal leiomyosarcoma remains difficult despite improvement of means of endoscopic and morphological investigations. As for our two patients, in no case the diagnosis was made in before surgery. This can be explained by two reasons: firstly, there are no specific radiological signs of the tumor and secondly, the distal location in the duodenum makes tumor inaccessible to endoscopy. In addition, even if endoscopic visualization of the tumor is possible, its extra mucosal development makes biopsies often negative [4,7].
The tumor spreads most frequently to adjacent organs and through blood, while lymphatic spread is rare. Distant metastases were observed in 20% of published cases. The Liver was involved in 75% of cases of distant metastases. The extension to adjacent organs was observed in 23% of cases.
The organs most affected were the pancreas, the mesocolon and the transverse colon. The extension can also be done backwards with invasion of great vessels as was the case for our second observation. Furthermore, our 2 patients showed no nodal involvement. This invasion has been reported in the literature only in three cases [2].
In the absence of proven efficacy of adjuvant treatments, the treatment of duodenal leiomyosarcoma is surgery. The surgical treatment is not consensual. Indeed, on one hand the anatomical complexity of the duodenum imposes different procedures adapted to the location of the tumor, and on the other hand, the rarity of lymph node metastases arouses debate for the need for node dissection. Among the published cases, only 52 cases had a curative surgery [2-4,7-22]. The review of the various procedures carried out (Table 1) found that the choice of surgical procedure depended much more on the location and the size of the tumor that on the need for node dissection. Indeed, most tumors of the 1st and 2nd duodenum were treated by pancreaticoduodenectomy. Only the tumors of small sizes were treated by segmental resection or simple excision. For leiomyosarcoma of the third and fourth duodenum, they were treated either by resection or by segmental excision. For our part, the choice was focused on the achievement of tumor resection with lymphadenectomy. Therefore, the location of the tumor at the Treitz angle required a resection of the first loop with its mesentery, a section of the duodenum at the junction of D2-D3 followed by end-to-end duodenojejunal anastomosis which was anterior to the mesenteric vessels.
Analysis of the results of surgical treatment of leiomyosarcoma (Table 2) showed that tumor recurrence occurred among both the patients who underwent a segmental resection and those who underwent pancreaticoduodenectomy while no recurrence was noted among patients who underwent simple excision of the tumor. Although these procedures have been made for different tumor stage, we can deduce that the most important factor for prolonged survival with no recurrence does not seem to be the lymphadenectomy but the healthy resection margins. Therefore, surgery of leiomyosarcoma should be aggressive to get healthy margins of resection. To achieve this, the resection should remove when possible all the adjacent invaded organs [6,8]. As for our second patient, the survival becomes compromised if the resection margins are invaded [9].
In case of locally advanced tumor with doubtful resectability, we believe that resection should be attempted even if it leaves in place some residual tumor tissue. In-

Table 1. Procedures depending on the tumor location in published cases.
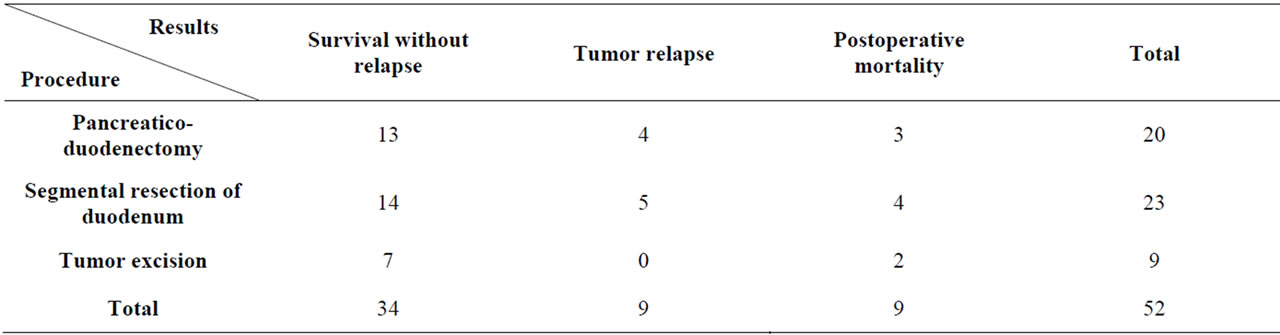
Table 2. Results of surgical treatment in published cases.
deed, the diversion procedures keeping the tumor in place do not prevent the complications of the tumor such perforation and hemorrhage, especially as leiomyosarcomas have a slow progression.
The presence of liver metastases does not appear to be against the surgical treatment as evidenced by the case published by Cage into which no recurrence had occurred after 26 months of follow-up after pancreaticoduodenectomy and left lobectomy [10]. On the other hand, iterative surgery seems to be justified in cases of relapse as evidenced by the two cases described by Pedrazzoli and Colovic with a disease-free survival of 3 and 2 years respectively [11,12].
5. Conclusion
Duodenal leiomyosarcoma is a rare tumor. Its clinical latency makes that the diagnosis is often done at an advanced stage. Surgery should be aggressive in carrying the tumor and its contiguous extensions. This is the only guarantee for prolonged survival. The need for a lymphadenectomy remains to be demonstrated.
REFERENCES
- T. Diamond, T. G. Parks and M. H. D. Danton, “Smooth Muscle Tumours of the Alimentary Tract,” Annals of the Royal College of Surgeons of England, Vol. 72, No. 5, 1990, pp. 316-320.
- T. E. Starzl, V. M. Bernhard and G. C. Henegar, “Leiomyosarcoma of the Duodenum,” International Abstracts of Surgery, Vol. 110, 1960, pp. 313-322.
- D. G. Marshall and F. Kim, “Leiomysorcoma of the Duodenum,” Journal of Pediatric Surgery, Vol. 122, No. 11, 1987, pp. 1007-1008. doi:10.1016/S0022-3468(87)80498-0
- M. Kanematsu, T. Imaeda, G. Iinuma, S. Mori, et al., “Leiomyosarcoma of the Duodenum,” Gastrointestinal Radiology, Vol. 16, No. 2, 1991, pp. 109-112. doi:10.1007/BF01887321
- M. Elabsi, M. Amraoui, M. Elouannani, M. Echarrab, et al., “Duodenal Leiomyosarcoma Revealed by Gastrointestinal Hemorrhage,” La Presse Médicale, Vol. 36, No. 11, 2007, pp. 1585-1588. doi:10.1016/j.lpm.2007.03.039
- M. Pitcher, A. Colfor, E. Moskovic and J. M. Thomas, “Duodenal Leiomyosarcoma as a Cause of Retroperitoneal Sepsis,” European Journal of Surgical Oncology, Vol. 21, No. 1, 1995, pp. 85-86. doi:10.1016/S0748-7983(05)80075-7
- S. Yassinger, T. J. Imperato, R. Midgley, et al., “Leiomyosarcoma of the Duodenum,” Gastrointestinal Endoscopy, Vol. 24, No. 1, 1977, pp. 38-40. doi:10.1016/S0016-5107(77)73440-6
- A. Watanabe, D. Korenaga, H. Baba, et al., “Long Survival of a Woman with a Huge Leiomyosarcoma of the Duodenum after Pancreaticoduodenectomy and Transverse Colectomy: A Case Report,” Surgery, Vol. 108, No. 1, 1990, pp. 110-113.
- G. A. Petralia, P. D. Hansen, R. C. Bowyer and R. C. Williamson, “Duodenal Leiomyosarcoma,” Digestive Surgery, Vol. 16, No. 1, 1999, pp. 22-25. doi:10.1159/000018689
- M. Cagetti, A. Nicolosi and A. Mereu, “Duodenal Leiomyosarcoma with Left Hepatic Metastasis Treated by Total Exeresis,” Annales De Chirurgie, Vol. 35, No. 10, 1981, pp. 854-856.
- S. Pedrazzoli, C. Sperti, C. Militello, A. Chiappetta, et al., “Duodenal Leiomyosarcoma and Its Multiple Recurrences: Good Surgical Result after Three Years of FollowUp,” The Italian Journal of Surgical Sciences, Vol. 14, No. 4, 1984, pp. 321-326.
- R. Colović, M. Micev and S. Zogović, “Leiomyosarcoma of the Duodenum. A Successful Reoperation after Recurrency,” Acta Chirurgica Iugoslavica, Vol. 48, No. 2, 2001, pp. 45-48.
- J. J. Dodds and O. H. Beahrs, “Leiomyosarcoma of the Duodenum,” American Journal of Surgery, Vol. 105, No. 2, 1963, pp. 245-249. doi:10.1016/0002-9610(63)90298-8
- C. M. Peterson, C. U. Franklin and W. R. Peterson, “Leiomyosarcoma of the Duodenum: A Case Report,” Journal of the National Medical Association, Vol. 56, No. 4, 1964, pp. 331-332.
- M. A. Meyers, “Leiomyosarcoma of the Duodenum,” Clinical Radiology, Vol. 22, No. 2, 1971, pp. 257-260. doi:10.1016/S0009-9260(71)80069-7
- C. Sperti, C. Pasquali, F. Di Prima, R. Baffa and S. Pedrazzoli, “Duodenum Leiomyosarcoma Mimicking a Pancreatic Pseudocyst,” HPB Surgery, Vol. 8, No. 1, 1994, pp. 49-52. doi:10.1155/1994/71873
- M. Fleet, A. F. Mellon, J. A. Lee, et al., “Duodenal Leiomyosarcoma Presenting with Iron Deficiency Anemia,” Journal of Pediatric Surgery, Vol. 29, No. 12, 1994, pp. 1601-1603. doi:10.1016/0022-3468(94)90233-X
- L. Sebkova, N. Saccà, S. Rodinòet, et al., “Duodenal Leiomyosarcoma Revealed by a Gastrointestinal Hemorrhage,” Digestive and Liver Disease, Vol. 40S, 2008, pp. S1-S195.
- S. H. uzuki and A. Yasui, “Pancreas-Sparing Duodenectomy for a Huge Leiomyosarcoma in the Third Portion of the Duodenum,” Journal of Hepato-Biliary Pancreatic Surgery, Vol. 6, No. 4, 1999, pp. 414-417. doi:10.1007/s005340050142
- N. Kawano, M. Ryu, T. Kinoshita, M. Konishi, et al., “Segmental Resection of the Duodenum for Treating Leiomyosarcoma Associated with von Recklinghausen’s Disease: A Case Report,” Japanese Journal of Clinical Oncology, Vol. 25, No. 3, 1995, pp. 109-112.
- Y. N. Ho, Y. P. Leong, A. S. Sakijan, M. Swaminathan and K. Sarvananthan, “Duodenal Leiomyosarcoma: A Report of a Rare and Aggressive Tumour,” Singapore Medical Journal, Vol. 33, No. 3, 1992, pp. 297-298.
- R. Orda, J. Sayfan and I. Wasserman, “Surgical Treatment of Leiomyosarcoma of the Distal Duodenum,” Digestive Surgery, Vol. 17, No. 4, 2000, pp. 410-412. doi:10.1159/000018890
NOTES
*Corresponding author.

