Surgical Science
Vol.3 No.11(2012), Article ID:24413,4 pages DOI:10.4236/ss.2012.311102
Regeneration of Mastoid Air Cells in Vivo Using Autologous Cortical Bone
1Department of Otolaryngology-Head and Neck Surgery, Nagasaki University Graduate School of Biomedical Sciences, Nagasaki, Japan
2Department of Otolaryngology, Foundation for Biomedical Research and Innovation, Kobe, Japan
3Department of Otolaryngology-Head and Neck Surgery, Tazuke Kofukai Medical Research Institute, Kitano Hospital, Osaka, Japan
4Department of Radiology, Fukui Red Cross Hospital, Fukui, Japan
Email: *kkaneko@nagasaki-u.ac.jp
Received September 1, 2012; revised September 30, 2012; accepted October 9, 2012
Keywords: Regeneration; Mastoid Air Cells; Mastoid Cortex Plasty; Tissue Engineering; Cholesteatoma
ABSTRACT
Purpose: This was a preliminary study to assess surgical construction and regeneration of mastoid air cells in the treatment of cholesteatoma. Methods: Two-stage tympanoplasty with mastoidectomy was performed in four cases of unilateral cholesteatoma with sclerotic mastoid. During the first-stage operation, small fragments of autologous cortical bone were inserted into the cavity after mastoidectomy to form a honeycomb-like structure. Reconstruction of the lateral wall of the mastoid cavity was performed using the mastoid cortical bony plate. Preand postoperative mastoid volume was evaluated by three-dimensional reconstruction based on high-resolution computed tomography (HR-CT) images. Results: HR-CT images after the first-stage operation showed that mastoid volume had increased in all subjects. Macroscopic inspection during the second-stage operation revealed that the honeycomb-like structure made of bony fragments and covered by thin mucosa in the mastoid cavity was stable, with no evidence of effusion or granulation tissue. No retraction of the eardrum, middle ear effusion or recurrence of cholesteatoma was observed, and the hearing level on a pure-tone audiogram was improved in any subject 60 - 94 months after the second-stage operation. Conclusion: Surgical construction and regeneration of mastoid air cells using autologous cortical bone can be useful in treatment of cholesteatoma with arrested mastoid pneumatization.
1. Introduction
Middle ear gases are exchanged through the Eustachian tube and the middle ear mucosa. Well-developed mastoid air cells aid in ventilation of the middle ear cavity and may prevent occurrence of middle ear atelectasis. However, in many cases of chronic otitis media and cholesteatoma, mastoid cells are undeveloped and the ability to exchange middle ear gases is consequently poor. Disease recurrence is a danger in such cases even after tympanoplasty if middle ear gas exchange is not restored.
Kanemaru et al. [1,2] reported a new tissue engineering method to construct and regenerate mastoid air cells. This technique, mastoid air cell plasty, uses three-dimensional hydroxyl apatite (3D-HA) in patients with severe cholesteatoma, adhesive otitis media, and purulent chronic otitis media. This method could improve transmucosal gas exchange and may prevent recurrent intractable middle ear diseases.
In the present preliminary study, this method was modified and applied in four subjects with cholesteatoma using autologous cortical bone, which is easily accessible to most surgeons. In addition, modified mastoid cortex plasty [3] was performed, including reconstruction of the lateral wall of the mastoid cavity after mastoidectomy using the mastoid cortical bony plate. Mastoid volume was evaluated preand postoperatively by three-dimensional reconstruction based on high-resolution computed tomography (HR-CT) images.
2. Subjects and Methods
Subjects included three adults and a child with unilateral cholesteatoma (Table 1). The contralateral ear was free of disease in all subjects. Two-stage tympanoplasty with mastoidectomy was performed in all cases. The procedures for mastoid air cell plasty with mastoid cortex plasty using the autologous cortical bone were as follows: in the first tympanoplastic procedure, the mastoid corti-
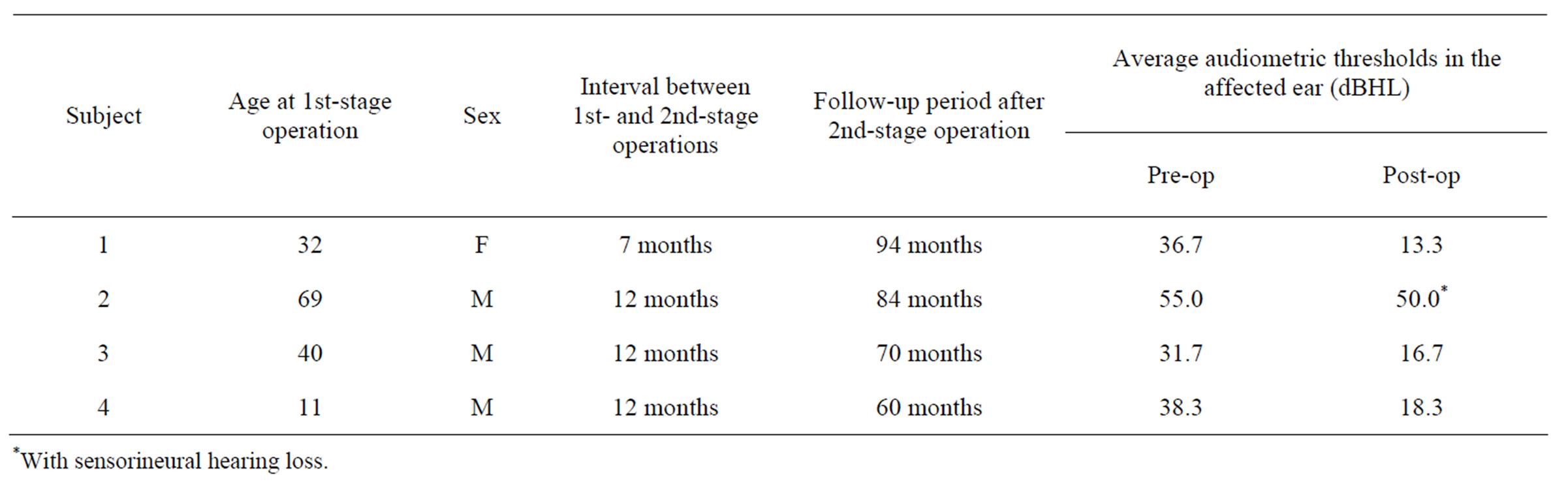
Table 1. Subjects’ characteristics and outcome of hearing level.
cal bony plate was collected from the surface of the temporal bone posterior to the external auditory canal prior to mastoidectomy (Figure 1(a)) and used to close the mastoid cortex defect at the end of the operation. Canal-wall-up mastoidectomy was performed. For cases in which a portion of the canal wall was removed, the defect was reconstructed with bone or cartilage. After removal of the cholesteatoma matrix and granulation tissue, small fragments of autologous cortical bone (1 - 6 mm in diameter) harvested from the temporal bone were inserted into the opened mastoid cavity to form a honeycomb-like structure. They were then fixed with fibrin glue (Figure 1(b)). A silastic sheet was placed in the tympanic cavity and antrum, and an elastic drainage tube was positioned in the mastoid cavity. Finally, the mastoid cortical bony plate was used to close the mastoid cavity and fixed with bone pate and fibrin glue. The drainage tube was removed 1 - 2 weeks postoperatively.
The second-stage operation was performed 7 - 12 months after the first to provide a second look and the opportunity for ossiculoplasty type III in all cases. In this procedure, the silastic sheet was removed from the tympanic cavity and antrum. The mastoid cavity was examined for air cells construction by lifting the mastoid cortex bony plate slightly. After examination of the mastoid cavity, the bony plate was repositioned and fixed with bone pate and fibrin glue as in the first-stage operation.
Volume of the mastoid (aditus ad antrum, mastoid antrum, and mastoid air cells) was evaluated using multislice HR-CT images. As observed on CT images, total volume (air part plus soft tissue part) and aerated volume (air part only) of the mastoid before and after the firststage operation were constructed using image reconstruction software (AZE Virtual Place, Aze Ltd., Tokyo, Japan). Average conductive hearing levels at three frequencies (500, 1000, and 2000 Hz) on a puretone audiogram were compared prior to the first-stage operation and after the second-stage operation.
This study was approved by the ethics committee of Fukui Red Cross Hospital. Prior to the study, its proce-
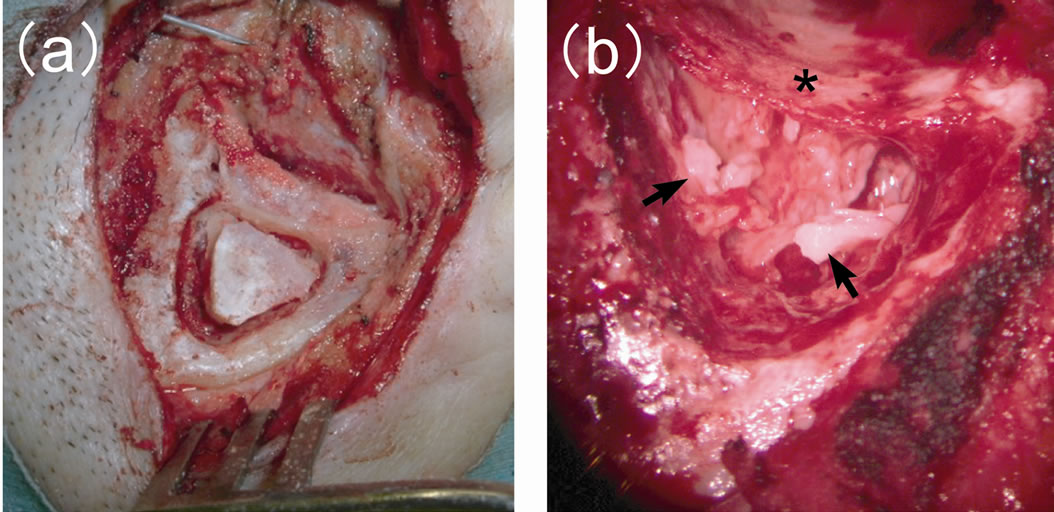
Figure 1. Intraoperative views (a) Mastoid cortical bony plate collected from the surface of the temporal bone posterior to the external auditory canal before mastoidectomy; (b) Small fragments of autologous cortical bone (arrows) inserted into the opened mastoid cavity to form a honeycomb-like structure. Asterisk: posterior wall of the external auditory canal.
dures and objectives were explained to all subjects and an informed consent was obtained.
3. Results
Macroscopic inspection during the second-stage operation revealed that the honeycomb-like structure made of bony fragments and covered by thin mucosa in the mastoid cavity (Figure 2) was stable in all subjects, with no evidence of effusion or granulation tissue. Histopathological examination of the implanted autologous cortical bone taken as a sample from the reopened mastoid cavity in subject 3 showed monolayer cuboidal epithelium covering the surface of the bone (Figure 3).
On CT images, sclerotic mastoid was evident in all subjects. Total mastoid volume (0.4 - 0.7 mL) and aerated mastoid volume (0.1 - 0.4 mL) had decreased in the affected ear compared with that in the healthy ear (1.5 - 8.8 mL in both total and aerated mastoid). After the first-stage operation, the total mastoid volume increased and aerated mastoid volume had recovered (1.0 - 1.7 mL in both total and aerated mastoid) in all subjects (Figures 4 and 5). Here, total and aerated mastoid volumes were the same in the healthy ear and the affected ear after the
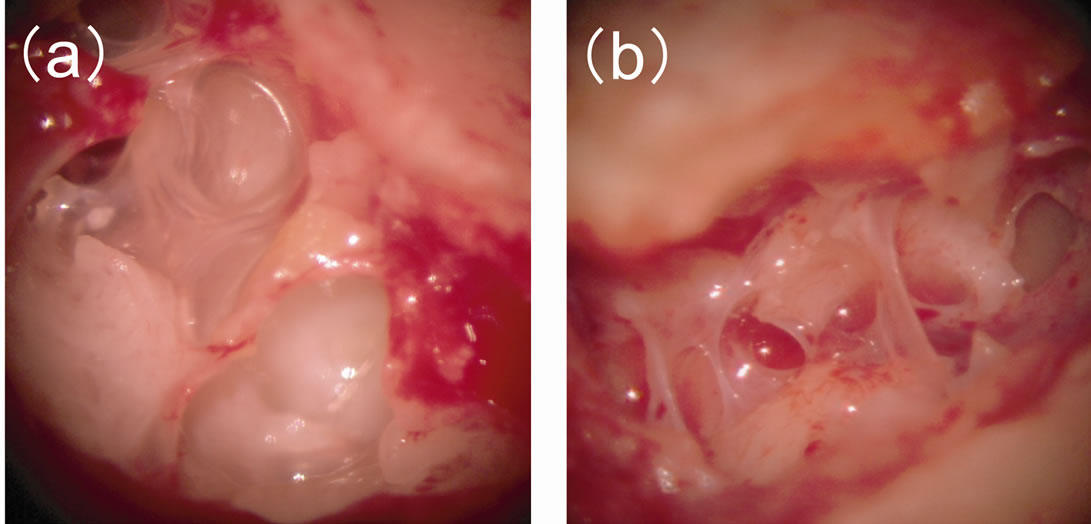
Figure 2. Macroscopic inspection during the second-stage operation in subject 1 (a) and subject 2 (b). The honeycomb-like structure made of bony fragments and covered by thin mucosa in the mastoid cavity was stable.
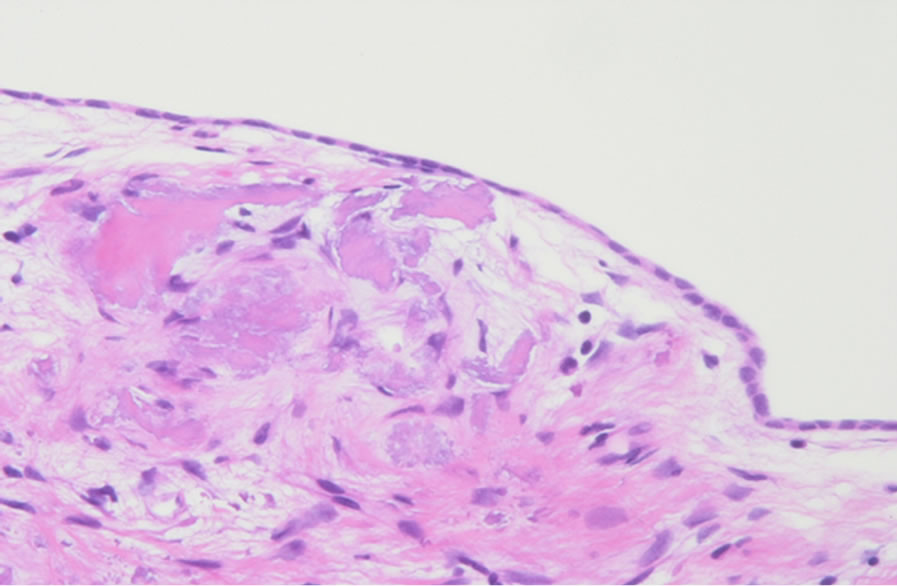
Figure 3. A Hematoxylin-eosin stained specimen of implanted autologous cortical bone taken from the reopened mastoid cavity during the second-stage operation in subject 3. Normal monolayer mucosa with blood capillaries covering the surface of the bone is observed.
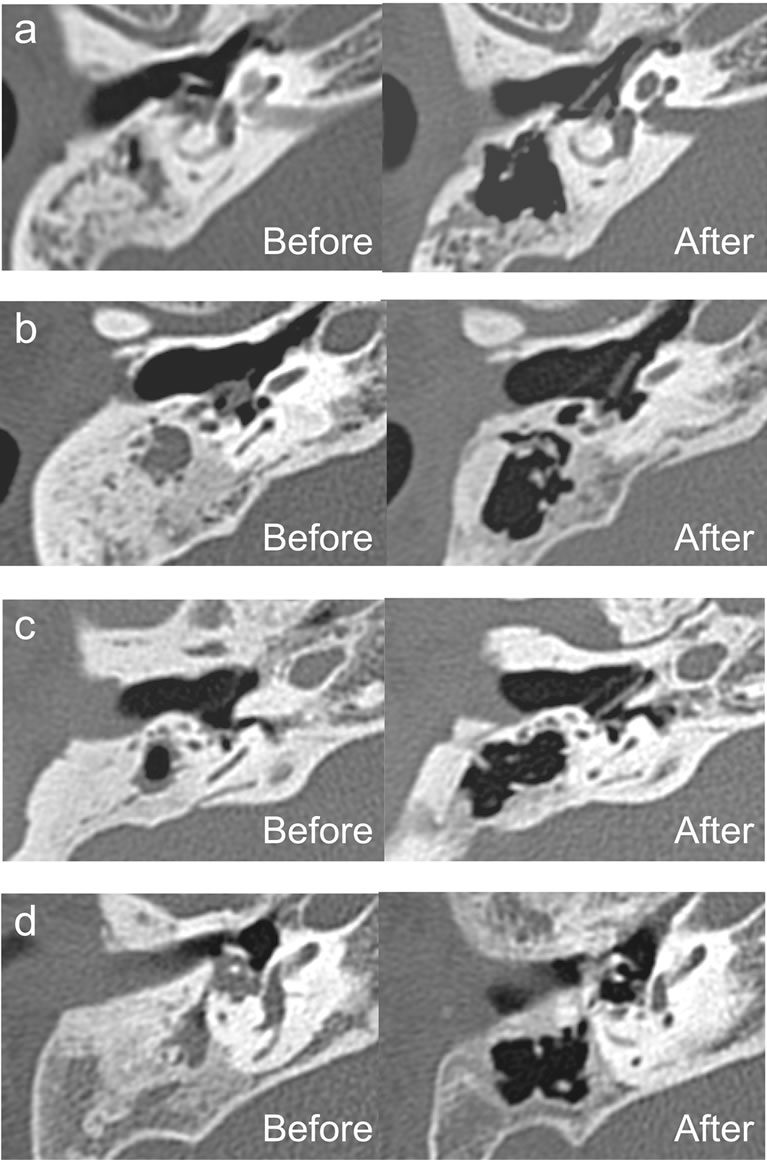
Figure 4. CT images of the mastoid before and after the first-stage operation. (a)-(d) represent subjects 1 - 4, respectively. Preoperatively, sclerotic mastoid was evident in all subjects. Both total and aerated mastoid volume increased postoperatively. Constructed air cells were observed in the mastoid.
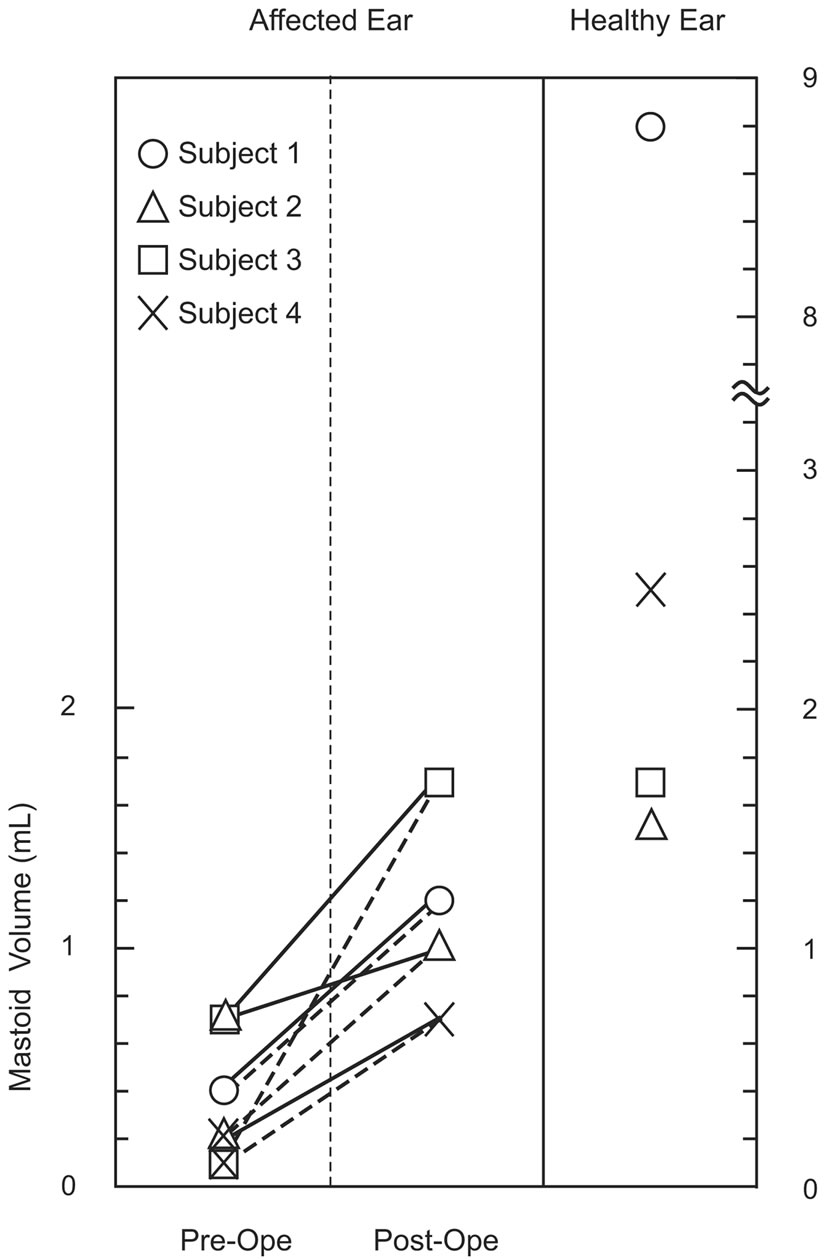
Figure 5. Comparison of total and aerated mastoid volume preand postoperatively estimated by three-dimensional reconstructions on CT images. Solid and broken lines show changes in the total and aerated mastoid volume of the affected ear, respectively.
first-stage operation because there was almost no soft tissue in the mastoid.
No retraction of the eardrum, middle ear effusion or recurrence of cholesteatoma was observed in any subject 60 - 94 months after the second-stage operation. Postoperative hearing level had improved in all subjects (Table 1). Average audiometric thresholds reached normal levels in subjects 1, 3, and 4, and air-bone gaps decreased to 5 - 15 dB in subject 2 in whom sensorineural hearing loss remained.
4. Discussion
Kanemaru et al. [1,2] reported a new surgical procedure in tympanoplasty with mastoidectomy using a 3D-HA tissue-engineering approach to treat intractable chronic otitis media. It was an epoch-making method involving morphological construction of functional mastoid air cells. This study describes a modified version of this surgical procedure. Firstly, small fragments of autologous cortical bone were used instead of 3D-HA as scaffolding for mastoid cells. Although 3D-HA is a good material for this purpose [1,2], most surgeons are not yet familiar with it. Fragments of cortical bone are easily accessible during mastoidectomy. These autologous fragments are functionally equivalent to 3D-HA in the construction of mastoid air cells.
Secondly, reconstruction of the lateral wall of the mastoid cavity was added by returning of the mastoid cortical bony plate harvested before mastoidectomy. Yanagihara et al. originally reported reconstruction of the mastoid cortical defect using bone pate in mastoid cortex plasty [3]. Reconstruction of the mastoid cortex has also been performed using a titanium mesh [4,5] and hyaluronatebased bioreabsorbable membrane [6]. These were effective in preventing connective tissue from invading the mastoid cavity while preserving the mastoid space and preventing postoperative retroauricular dimpling. In the procedure reported here, mastoid cortical bony plate was useful because of its firm structure. No special material is needed in this procedure, and foreign body reaction is not an issue. Some practice is needed to harvest the bony plate correctly. Mastoid cortex plasty using the mastoid cortical bony plate is a very important procedure for successfully constructing mastoid air cells.
In this study, CT images revealed increased mastoid volume in all subjects postoperatively. During the second-stage operation, we confirmed that the honeycomblike structure made of bony fragments and covered by thin mucosa in the mastoid cavity was stable. Thus, mastoid air cells were successfully constructed using the method reported here. Postoperatively, a larger functional mucosa-lined area and lower middle ear impedance were thought to be acquired than those before surgery, facilitating better mastoid gas exchange. Though the function of the constructed mastoid air cells could not be proven directly, the long-term good results, including release from retraction pockets and improved hearing level, indirectly demonstrate the favorable condition of the newly constructed middle ear.
Hasebe et al. [7] conducted a study to determine if cholesteatoma is controlled by conservative treatment with mastoid ventilation. Their clinical observations suggest that progression of cholesteatoma could be related to the ventilatory conditions in the mastoid rather than the Eustachian tube function. They concluded that conservative treatment may be effective when ears with cholesteatoma have aeration in the mastoid. In conjunction with conservative treatment, construction and regeneration of mastoid air cells should be effective against recurrence of cholesteatoma.
We believe that surgical construction and regeneration of mastoid air cells using autologous cortical bone can be useful in treatment of intractable chronic otitis media with arrested mastoid pneumatization.
REFERENCES
- S. Kanemaru, T. Nakamura, K. Omori, A. Magrufov, M. Yamashita, Y. Shimizu, H. Takahashi and J. Ito, “Regeneration of Mastoid Air Cells: Clinical Applications,” Acta Otolaryngol Supplement, Vol. 124, No. 551, 2004, pp. 80-84. doi:10.1080/03655230310016690
- S. Kanemaru, T. Nakamura, K. Omori, A. Magrufov, M. Yamashita and J. Ito, “Regeneration of Mastoid Air Cells in Clinical Applications by in Situ Tissue Engineering,” Laryngoscope, Vol. 115, No. 2, 2005, pp. 253-258. doi:10.1097/01.mlg.0000154728.23657.3a
- N. Yanagihara, Y. Hinohira and H. Sato, “Mastoid Cortex Plasty Using Bone Pate,” Ontology & Neurotology, Vol. 23, No. 4, 2002, pp. 422-424. doi:10.1097/00129492-200207000-00004
- T. T. Jung and S. K. Park, “Reconstruction of Mastoidectomy Defect with Titanium Mesh,” Acta Oto-Laryngologica, Vol. 124, No. 4, 2004, pp. 440-442. doi:10.1080/00016480410016450
- H. H. Kim and D. F. Wilson, “Titanium Mesh for Functional Reconstruction of the Mastoid Cortex after Mastoidectomy,” Ontology & Neurotology, Vol. 27, No. 1, 2006, pp. 33-36. doi:10.1097/00129492-200601000-00007
- R. Caylan and D. Bektas, “Preservation of the Mastoid Aeration and Prevention of Mastoid Dimpling in Chronic Otitis Media with Cholesteatoma Surgery Using Hyaluronate-Based Bioresorbable Membrane (Seprafilm),” European Archives of Oto-Rhino-Laryngology, Vol. 264, No. 4, 2007, pp. 377- 380. doi:10.1007/s00405-006-0193-9
- S. Hasebe, H. Takahashi, I. Honjo, M. Miura and M. Tanabe, “Mastoid Condition and Clinical Course of Cholesteatoma,” Journal for Oto-Rhino-Laryngology and Its Related Specialties, Vol. 63, No. 3, 2001, pp. 160-164. doi:10.1159/000055733
NOTES
*Corresponding author.

