Surgical Science
Vol. 3 No. 3 (2012) , Article ID: 18036 , 5 pages DOI:10.4236/ss.2012.33022
The Frequency of K-ras Mutation in Colorectal Adenocarcinomas with Absence of Distant Metastasis at Diagnosis
1Department of Pathology, School of Medicine, Baskent University, Istanbul, Turkey
2Medical Genetics Laboratory, Goztepe Park Medical Hospital, Istanbul, Turkey
3Department of General Surgery, School of Medicine, Baskent University, Istanbul, Turkey
Email: *ebrudemiralay@yahoo.co.uk
Received December 1, 2011; revised January 16, 2012, accepted January 25, 2012
Keywords: Colon Adenocarcinoma; K-ras; HER-2/Neu
ABSTRACT
Purpose: K-ras mutations were reported to be in 40% - 60% of patients with sporadic colorectal cancers (CRCs). HER-2/neu oncogene that is important in breast carcinoma was reported in CRCs in several studies. The aims of our study are to determine; the frequency of K-ras mutation in CRC and positivity of HER-2/neu, the relation between K-ras mutation and HER-2/neu positivity, and also the relation between clinicopathological findings with K-ras mutation and HER-2/neu expression. Methods: Total of 35 colon resection specimen from patients without distant metastasis who were operated due to colorectal adenocarcinoma were included in the study. The sections were examined with light microscopy. Vascular and perineural invasions and pericolonic tumor deposits (PCTD) were investigated. HER- 2/neu was applied immunohistochemically. DNA sample obtained from tumor paraffin block was screened for presence of 20 mutations in K-ras gene-codon 12, 13, 61 by AutoGenomics and Infinity Analyzer. Results: K-ras gene mutation was detected in 14 of 35 patients (40%). HER-2/neu positivity was detected in 7 cases (28.57%). There was not any significant correlation between HER-2/neu positivity, K-ras gene mutation and clinicopathological findings. There was direct correlation between PCTD and vascular invasion. Conclusions: There was not correlation between K-ras mutation and clinicopathological findings similar to several other studies. The relation between HER-2/neu expression and clinicopathological findings was not found.
1. Introduction
The colorectal adenocarcinoma (CRC) are leading in all malignancies worldwide. Its incidence varies according to geography [1]. CRC development is a result of acquisition of multiple genetic changes such as mutations of tumor suppressor genes and various prooncogenes. In recent studies, K-ras mutations were detected in rate of 30% - 50% in colorectal adenocarcinomas [2-12]. K-ras gene is located on chromosome 12 and encodes G protein showing GTPase activity [2]. The presence of K-ras mutations in prognosis of established colorectal cancer is still unclear [13].
The HER2/neu gene is located on chromosome 17 and encodes the cell membrane p185HER2. HER-2/neu oncogene that is important in breast carcinoma was reported in CRCs in several studies [14].
The aims of our study are to determine; the frequency of K-ras mutation in CRC and positivity of HER-2/neu, the relation between K-ras mutation and HER-2/neu positivity, and also the relation between clinicopathological findings of age, gender, tumor localization, vascular and perineural invasion, tumor differentiation, tumor stage with K-ras mutation and HER-2/neu expression.
2. Materials and Methods
Total of 35 colon resection specimen from patients operated due to colorectal adenocarcinoma in Baskent University Istanbul Hospital between 2007 and 2011 were included in the study. 24 (68.5%) of the patients were female and 11 (31.5%) were male, the mean age of patients was 72 ± 12 (mean ± SD).
There was not any distant metastasis in clinical findings, laboratory and radiological (abdomen and thorax Computed Tomography) and PET (Positron Emission Tomography) scan screening before operation. Tumor localizations were as following; rectum in 5 patients, left colon in 18 patients, right colon in 12 patients.
The sections taken from paraffin-embedded blocks from tissues were examined with light microscopy according to colon and rectum tumor pTNM classification (7.ed). Vascular and perineural invasions and pericolonic tumor deposits (PCTD) were investigated.
The sections prepared from tumor paraffin blocks were applied immunohistochemically with HER-2/neu (Monoclonal Antibody to c-erbB-2 Protein/HER-2/neu, BioGenex). The results were classified as 1+, 2+, 3+ and negative (dense membranous staining in more than 10% of tumor cells was 3+, intermediate membranous staining in more than 10% of tumor cells was 2+, indistinguishable membranous staining in more than 10% of tumor cells was 1+, negative staining was 0).
DNA sample obtained from tumor paraffin block was screened for presence of 20 mutations in K-ras genecodon 12, 13, 61.
Auto Genomics and Infinity Analyzer Method
Genotyping: Genomic DNA was extracted from whole blood using a MiniAmp extraction kit (Qiagen, Venlo, the Netherlands). Multiplex amplification of each sample was performed in an individual well of a 24-well plate using an Eppendorf Mastercycler (Hamburg, Germany). Template and Platinum Taq Polymerase (Life Technologies, Carlsbad, California) were added to an analytespecific amplification mix (Auto Genomics Inc., Carlsbad, California). After amplification, the plate was placed in the INFINITI analyzer (Auto Genomics Inc.) where detection primer extension occurred, followed by hybridization of detection primers to individual oligonucleotides arrayed on the Bio-Film Chip. After hybridization, the Biofilm Chips were washed and scanned in the INFINITI optics module.
The Auto genomics KRAS assays were used to detect the presence of 20 mutations in codon 12, codon 13, and codon 61, respectively (Table 1).
The Infiniti® KRAS assay is designed to detect the most prevalent K-ras amino acid changes in positions known to affect the function of the proteins. Detecting their codon variants or nucleotide changes identifies these mutations.
The assay protocol includes the following five major processes:
1) Multiplex PCR amplification of DNA.
2) Fluorescent label incorporation using analyte specific primer extension (ASPE).
3) Hybridization of the ASPE primers to a microarray followed by washing.
4) Scanning of the microarray.
5) Signal detection and analysis.
(Steps 2) through 5) are performed using the INFINITI® Analyzer).
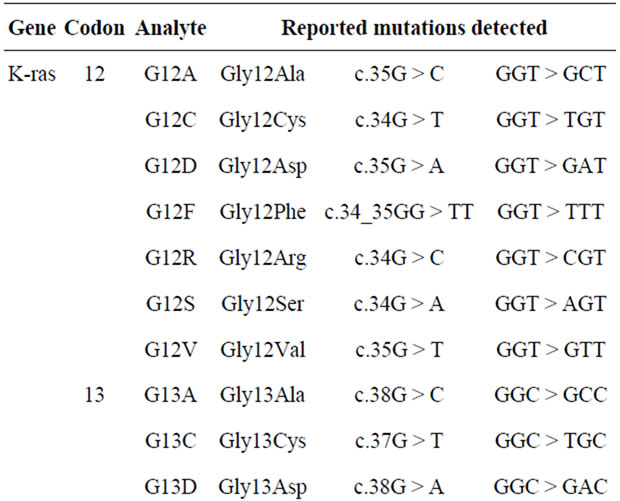
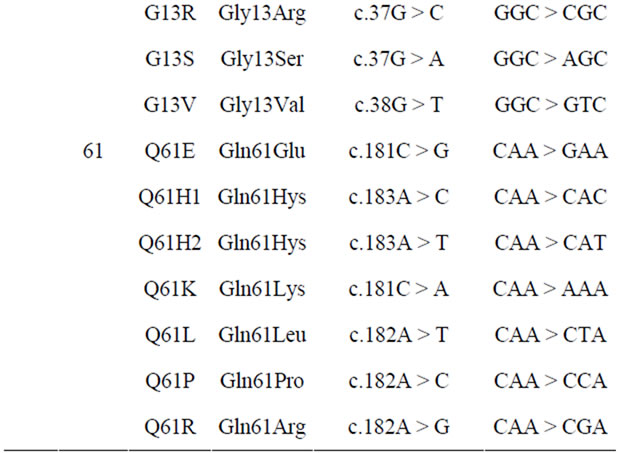
Table 1. Mutations of codons in K-ras gene.
3. Statistical Analysis
The statistical analysis was performed using Graph Pad Prism (USA). Results were considered statistically significant if p value was less than 0.05. Variables were checked for normality. Data are shown as “mean ± standard deviation (SD)”. Comparisons between two groups were assessed by means of Mann-Whitney U test and between more than two groups were by Kruskal-Wallis followed by Dunn’s Multiple Comparison Test for nonnormally distributed continuous variables. We used Spearman’s correlation analysis for correlation analysis of nonparametric variables.
4. Results
K-ras gene mutation was detected in 14 of 35 patients (40%). 9/24 of females and 5/11 (45.5%) of male patients have been shown to have K-ras gene mutation. There was not any significant correlation between K-ras gene mutation and age, gender, tumor differentiation, stage, vascular and perineural invasion, tumor localization, PCTD, lymph node positivity and HER-2/neu expression.
In 7 of the cases, HER-2/neu was 2+, in 3 of them 3+, in others it was negative (Figure 1). Total of 28.57% HER-2/neu positivity was detected. No correlation was found between HER-2/neu positivity and K-ras mutation, age, gender, tumor differentiation, stage, vascular and perineural invasion, tumor localization, PCTD and lymph node positivity. The ANOVA analysis of tumor localization with HER-2/neu presence did not show any signifycant significance (p = 0.330). HER-2/neu positivity was found in 3 of 11 male patients and 7 of 24 female patients. There was any significance with t-test analysis (p = 0.946).
PCTD was found in 9 cases (Figure 2). Perineural invasion was in 14 and vascular invasion was in 19 cases. There was direct correlation between PCTD and vascular invasion (r = 0.539, p = 0.008; Spearman correlation analysis).
In lymph node classification was; 20 of the cases were N0, 2 were N1a, 4 were N1b, 8 were N1c, 1 was N2a. 7 of the cases passed over serosa, 31 were passed over muscularis propria. Histologically, 5 were well differenttiated (Grade 1), 26 were moderate (Grade 2) and 4 were poorly differentiated (Grade 3; Table 2).
5. Discussion
The frequency of K-ras mutation was reported to be 22.64% in the study of Sameer et al. [1], 39% in study of Plesec et al. [15], 32.7% in Yunxia et al. study [12] and it was 37% in work of Brink et al. [6]. The ratio of 40% in our study is consistent with the other studies.
The role of K-ras mutation in prognosis of CRC is not clear. In several small-sized studies, the relation of K-ras mutation with survey was not found [13,14]. In the study of Ahnen et al. two thirds of K-ras mutations were found in codon 12, and K-ras mutated tumors were more fre-
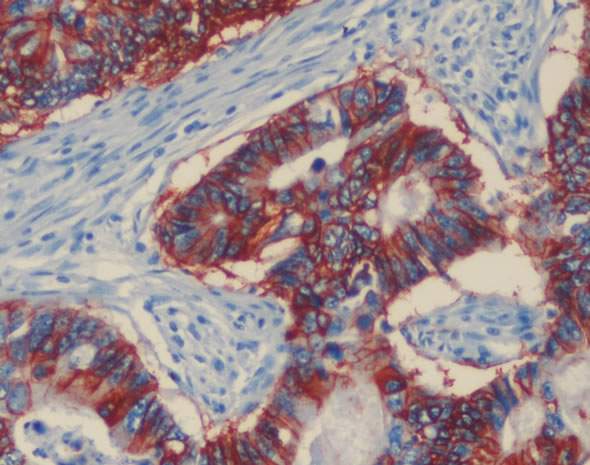
Figure 1. HER2/neu immunostain demonstrates intense membrane staining of all tumor cells (3+), ×200.
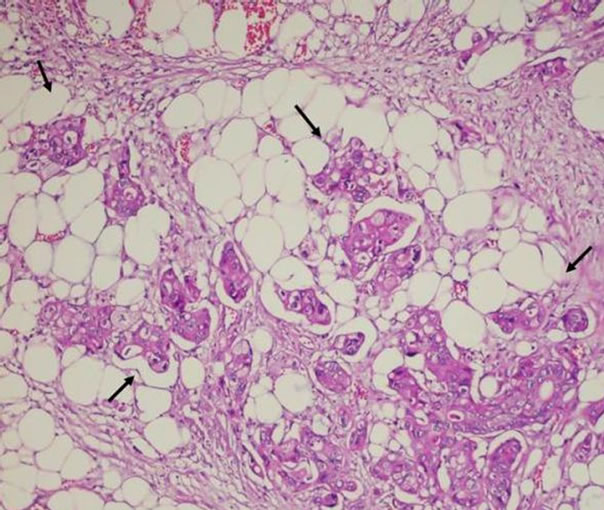
Figure 2. Pericolonic tumor deposits: Tumor islands in pericolonic adipose tissue (arrows), H&E, ×100
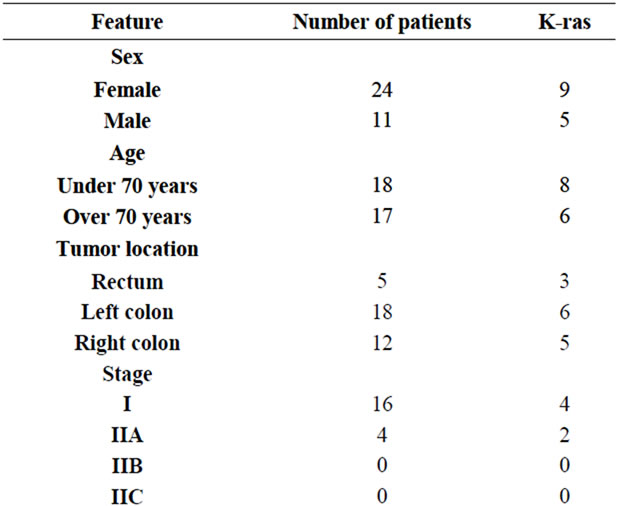
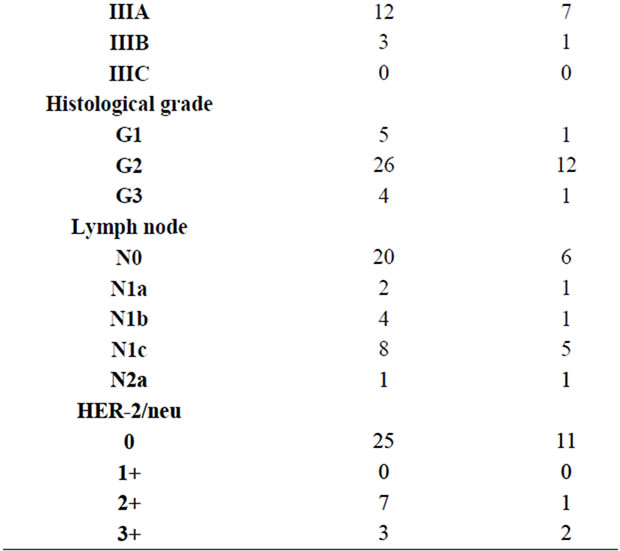
Table 2. K-ras gene mutation and clinicopathologic findings.
quently observed in patients with more than 3 metastatic regional lymph nodes [16].
In some studies, connection between K-ras mutation and resistance to EGFR inhibitors in metastatic CRCs were shown [13]. There are studies demonstrating that presence of codon 12 and 13 mutation in K-ras oncogene indicates poor response of patients to EGFR-targeted therapy [11].
The K-ras mutation was detected in high ratio in poor differentiated tumors in study of Yunxia et al. [12]. In our study, we did not find any relationship between histological grade and K-ras mutation.
The most frequently detected mutations in tumors were in rectum according to localization in study of Brink et al. and any correlation between tumor differentiation and K-ras mutation were not detected [6]. Samowitz et al. showed K-ras gene codon 12 mutations to be widespread in proximal tumors and advanced stages [17]. There was not any correlation between tumor localization and K-ras mutation in our study.
The correlation between K-ras mutation and tumor stage is not clear. Significant correlation was found between K-ras mutation, Duke’s stage and lymph node in the study of Sameer et al. [1].
In the current study, there was not any correlation between tumor stage and K-ras mutation. In another study, K-ras mutation was found to be correlated with vascular invasion and hematogenous metastasis [18].
In some reports, PCTDs were proposed to be vascular metastasis and that they are areas of vascular invasions [11,19,20]. In our cases, correlation between vascular invasion and PCTD presence was investigated and direct correlation was detected between PCTD and vascular invasion (r = 0.539, p = 0.0008). There was not signifycant correlation between K-ras mutation and PCTDs.
HER-2/neu is an important oncogene in breast cancer. While HER-2/neu positivity is high in some studies, it was found to be lower in most of them. The HER2/neu mutation was 16 % in one study whereas it was 59.4% in 69 tumors in another study [21]. In the study of Schuell et al., there was not any correlation between clinicopathological characteristics and HER-2 overexpression [22].
Ghaffarzadegan et al. found correlation between HER- 2/neu expression and age, gender, tumor localization and tumor type [21]. In our cases, HER-2/neu expression was found as 28.5% positive immunohistochemically. No correlation between the presence of K-ras mutation and HER-2/neu positivity was found. Osaka et al. presented immunohistochemical analysis of 146 colorectal tumors, and showed that overexpression of HER-2/neu protein occurred to be an independent indicator of poor prognosis [23].
In conclusion, rate of K-ras mutation in our cases was similar to other studies. Also, there was not correlation between K-ras mutation and clinicopathological findings. All of the cases were investigated for distant metastasis at the time of diagnosis and found as negative. These findings also support that there is no significant correlation between tumor stage and K-ras mutation. The serial in our study is in short-term and the relation between therapy response and K-ras mutation has not been well clarified.
The relation between HER-2/neu expression and clinicopathological findings was not found and only 3 cases noted to have 3+ staining. As HER-2/neu expression is quiet low, larger serials with in situ hybridization technique can yield more significant results.
REFERENCES
- A. S. Sameer, N. A. Chowdhri, S. Abdullah, Z. A. Shah and M. A. Siddiqi, “Mutation Pattern of K-ras Gene in Colorectal Cancer of Kashmir: A Report,” Indian Journal of Cancer, Vol. 46, No. 3, 2009, pp. 219-225. doi:10.4103/0019-509X.52956
- K. S. Al-Kuraya, “KRAS and TP53 Mutations in Colorectal Carcinoma,” Saudi Journal of Gastroenterology, Vol. 15, No. 4, 2009, pp. 217-219. doi:10.4103/1319-3767.56087
- H. J. Andreyev, A. R. Norman, D. Cunningham, et al., “Kirsten Ras Mutations in Patients with Colorectal Cancer: The ‘RASCAL II’ Study,” British Journal of Cancer, Vol. 85, 2001, pp. 692-696. doi:10.1054/bjoc.2001.1964
- R. T. Belly, J. D. Rosenblatt, M. Steinmann, J. Toner, J. Sun, J. Shehadi, J. L. Peacock, R. F. Raubertas, N. Jani and C. K. Ryan, “Detection of Mutated K12-ras in Histologically Negative Lymph Nodes as an Indicator of Poor Prognosis in Stage II Colorectal Cancer,” Clinical Colorectal Cancer, Vol. 1, No. 2, 2001, pp. 110-116. doi:10.3816/CCC.2001.n.011
- J. Brevik, G. I. Meling, A. Spurkland, T. O. Rognum and G. Gaudernack, “K-ras Mutation in Colorectal Cancer: Relations to Patient Age, Sex and Tumour Location,” British Journal of Cancer, Vol. 69, 1994, pp. 367-371. doi:10.1038/bjc.1994.67
- M. Brink, A. F. P. M. de Goeij, P. M. Weijenberg, G. M. J. M. Roemen, M. H. F. M. Lentjes, M. M. M. Pachen, R. A. Goldbohm and P. A. van den Brandt, “K-ras Oncogene mutAtions in Sporadic Colorectal Cancer in the Netherlands Cohort Study,” Carcinogenesis, Vol. 24, No. 4, 2003, pp. 703-710. doi:10.1093/carcin/bgg009
- U. Kressner, J. Bjorheim, S. Westring, S. S. Wahlberg, L. Pahlman, et al., “Ki-ras Mutations and Prognosis in Colorectal Cancer,” European Journal of Cancer, Vol. 34, No. 4, 1998, pp. 518-521. doi:10.1016/S0959-8049(97)10111-3
- S. G. Martinez-Garza, A. Nunez-Salazar, A. L. CalderonGarciduenas, F. J. Bosques-Padilla, A. Niderhauser-Garcia and H. A. Barrera-Saldana, “Frequency and Clinicopathology Associations of K-ras Mutations in Colorectal Cancer in a Northeast Mexican Population,” Digestive Diseases, Vol. 17, No. 4, 1999, pp. 225-229. doi:10.1159/000016940
- E. Saraga, D. Bautista, G. Dorta, P. Chaubert, P. Martin, et al., “Genetic Heterogeneity in Sporadic Colorectal Adenomas,” The Journal of Pathology, Vol. 181, No. 3, 1997, pp. 281-286. doi:10.1002/(SICI)1096-9896(199703)181:3<281::AID-PATH777>3.0.CO;2-M
- N. Urosevic, K. Krtolica, A. Skaro-Milic, S. KnezevicUsaj and A. Dujic, “Prevalence of G-to-T Transversions among K-ras Oncogene Mutations in Human Colorectal Tumors in Yugoslavia,” International Journal of Cancer, Vol. 54, No. 2, 1993, pp. 249-254. doi:10.1002/ijc.2910540215
- J. H. J. M. Van Krieken, A. Jung, T. Kirchner, F. Carneiro, R. Seruca, et al., “KRAS Mutation Testing for Predicting Response to Anti-EGFR Therapy for Colorectal Carcinoma: Proposal for an European Quality Assurance Program,” Virchows Archiv, Vol. 453, No. 5, 2008, pp. 417-431. doi:10.1007/s00428-008-0665-y
- Y. X. Zuo, J. Cao, G. S. Zhu, Y. C. Lu, X. K. Zhou and J. Li, “Mutations in Epidermal Growth Factor Receptor and K-ras in Chinese Patients with Colorectal Cancer,” BMC Medical Genetics, Vol. 11, 2010, p. 34. doi:10.1186/1471-2350-11-34
- N. Sharma, M. Saifo, I. R. Tamaskar, et al., “KRAS Status and Clinical Outcome in Metastatic Colorectal Cancer Patients Treated with First-Line Folfox Chemotheraphy,” Journal of Gastrointestinal Oncology, Vol. 1, No. 2, 2010, pp. 90-96.
- W. Kruszewski, R. Kowara, R. Rzepko, et al., “K-RAS Mutation, and Amplification of C-MYC and C-ERBB2 in Colon Adenocarcinoma,” Folia Histochemica et Cytobiologica, Vol. 42, No. 3, 2004, pp. 173-178.
- T. P. Plesec and J. L. Hunt, “KRAS Mutation Testing in Colorectal Cancer,” Advances in Anatomic Pathology, Vol. 16, No. 4, 2009, pp. 196-203. doi:10.1097/PAP.0b013e3181a9d4ed
- D. J. Ahnen, P. Feigl, F. G. Quan, C. Feneglio-Presier, L. C. Lovato, et al., “Ki-ras Mutation and p53 Overekspression Predict the Clinical behavior of Colorectal Cancer: A Southwest Oncology Group Study,” Cancer, Vol. 58, 1998, pp. 1149-1158.
- W. S. Samowitz, K. Curtin, D. Schaffer, M. Robertson, M. Leppert and M. L. Slattery, “Relationship of Ki-ras Mutations in Colon Cancers to Tumor Location, Stage and Survival: A Population-Based Study,” Cancer Epidemiology, Biomarkers & Prevention, Vol. 9, 2000, pp. 1193-1197.
- M. Tanaka, K. Omura, Y. Watanabe, Y. Oda and I. Nakanishi, “Prognostic Factors of Colorectal Cancer: K-ras Mutation, Overexpression of the p53 Protein, and Cell Proferative Activity,” Journal of Surgical Oncology, Vol. 57, No. 1, 1994, pp. 57-64. doi:10.1002/jso.2930570115
- N. S. Goldstein and J. R. Turner, “Pericolonic Tumor Deposits in Patients with T3N+M0 Colon Adenocarcinomas,” Cancer, Vol. 88, No. 10, 2000, pp. 2228-2238. doi:10.1002/(SICI)1097-0142(20000515)88:10<2228::AID-CNCR5>3.0.CO;2-1
- G. Puppa, P. Maisonneuve, A. Sonzogni, M. Masullo, P. Capelli, et al., “Pathological Assessment of Pericolonic Tumor Deposits in Advanced Colonic Carcinoma: Relevance to Prognosis and Tumor Staging,” Modern Pathology, Vol. 20, 2007, pp. 843-855. doi:10.1038/modpathol.3800791
- K. Ghaffarzadegan, N. Sharifi, H. Vosooghynia, et al., “HER2/neu Expression in Colon Adenocarcinoma and Its Correlation with Clinicopathologic Variables,” IJBMS, Vol. 9, No. 1, 2006, pp. 64-69.
- B. Schuell, T. Gruenberger, W. Scheithauer, C. Zielinski and F. Wrba, “HER2/neu Protein Expression in Colorectal Cancer,” BMC Cancer, Vol. 6, 2006, p. 123. doi:10.1186/1471-2407-6-123
- T. Osaka, M. Miyahara, S. Uchino, M. İnomata, S. Kitano and M. Kabayashi, “Immunohistochemical Study of cerbB-2 Protein in Colorectal Cancer and the Correlation with Patient Survival,” Oncology, Vol. 55, No. 6, 1998, pp. 548-555. doi:10.1159/000011911
NOTES
*Corresponding author.

