Open Journal of Veterinary Medicine
Vol.06 No.02(2016), Article ID:63859,12 pages
10.4236/ojvm.2016.62005
Evidence-Based Use of Antibiotics in Veal Calves with Diarrhea
Michael Hässig, Susanne Kretschmar
Section for Ambulatory Field Clinic and Herd Health, Department of Farm Animals, University of Zurich, Zurich, Switzerland

Copyright © 2016 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 6 January 2016; accepted 21 February 2016; published 26 February 2016
ABSTRACT
Diarrhea is the leading cause of mortality in beef and dairy calves during the first week of life and results in substantial financial loss [1] . Diarrhea is a multifactorial disease and can be infectious or non-infectious. However, in the majority of calves, infectious organisms, especially Cryptosporidium parvum, rotavirus, coronavirus, and E. coli, are the primary cause [2] . The aim of this study was to generate a decision tree, based on prevalence, diagnostic testing and treatment and to estimate associated costs or risk. For each of the four main pathogens, two principal approaches are outlined and compared. The first approach relies on a detailed diagnostic workup and allows for specific etiological treatment. The second approach relies on the trial-and-error method, which involves the use of a first-choice antibiotic, followed by a second- and third-choice antibiotic if the previous ones failed to resolve the disease. In Switzerland, the prevalence of diarrheic calves infected with E. coli is approximately 1% suggesting that the use of antimicrobials for the treatment of scouring calves, in the absence of a diagnostic workup, is not always justified. However, for all four major pathogens, the trial-and-error method affords cheaper treatment compared with treat- ment based on an etiological diagnosis. This creates a quandary in view of the current worldwide efforts to reduce the use of antibiotics in animal agriculture.
Keywords:
Bovine, Calf, Antibiotic, Antimicrobial Susceptibility Testing, Decision Tree Analysis, Diarrhea

1. Introduction
Diarrhea is the leading cause of death in beef and dairy calves in the first week of life [1] and has a major economic impact on animal production. Calf losses, reduced weight gains, increased workload, and the cost of treatment and preventive measures result in substantial financial expenditures [3] .
Diarrhea is a multifactorial disease and may be caused by viruses, bacteria or parasites. Infectious diarrhea is often a sequel to failure of passive transfer because of inadequate colostrum quality or quantity. Crowding, immune status, and environmental and management factors also play important roles in the pathogenesis of diarrhea [5] . The four most prevalent infectious microorganisms found in calves with diarrhea are Cryptosporidium parvum, rotavirus, coronavirus, and enterotoxic E. coli. These agents are responsible for 75% to 95% of all cases of diarrhea worldwide in newborn calves [2] . Mixed infections and consecutive infections by different infectious agents are common [2] [4] .
The etiological diagnosis in a calf with diarrhea usually requires laboratory testing and typically cannot be made based on the clinical signs and the type of diarrhea alone. The establishment of an etiological diagnosis is crucial, especially in herd problems, to initiate specific treatment and metaphylactic and prophylactic measures [5] . Selection of an effective treatment for scouring calves can be difficult without an etiological diagnosis.
The use of antibiotics for the treatment of diarrhea in calves is controversial. A recent review article on antimicrobial decision making [1] referred to studies that favored the use of antibiotics for the treatment of calves with diarrhea [6] as well as studies that discouraged the use of antibiotics because of contraindication or lack of efficacy [7] . Several studies have shown that antibiotics can reduce the mortality rate and shorten the duration of diarrhea [8] . The two main indications for the use of antibiotics for the treatment of scouring calves are the prevention of secondary bacteremia and the reduction in the number of coliform bacteria in the small intestine [8] . This review investigated the overall cost of veterinary services, diagnostic tests, treatment, reduced weight gain, and mortality in calves with diarrhea. The goal was to create a decision tree to show how calf diarrhea can be addressed diagnostically and therapeutically. Special emphasis was given to comparison of the trial-and-error method and treatment based on an etiological diagnosis after the identification of the infectious agent and evaluation of potential resistance to therapeutic drugs. The different risks that were compared using this decision tree were defined as cost multiplied by probability. The prevalence of the four major diarrheal pathogens in calves in Switzerland was multiplied by veterinary costs. A further goal was to examine whether the cost of antimicrobial susceptibility testing was justified; the trial-and-error method minimized the cost of diagnostic testing but also minimized the likelihood of successful treatments, whereas antimicrobial susceptibility testing increased the cost of diagnostic testing but minimized the number of futile antibiotic treatments.
2. Animals, Materials and Methods
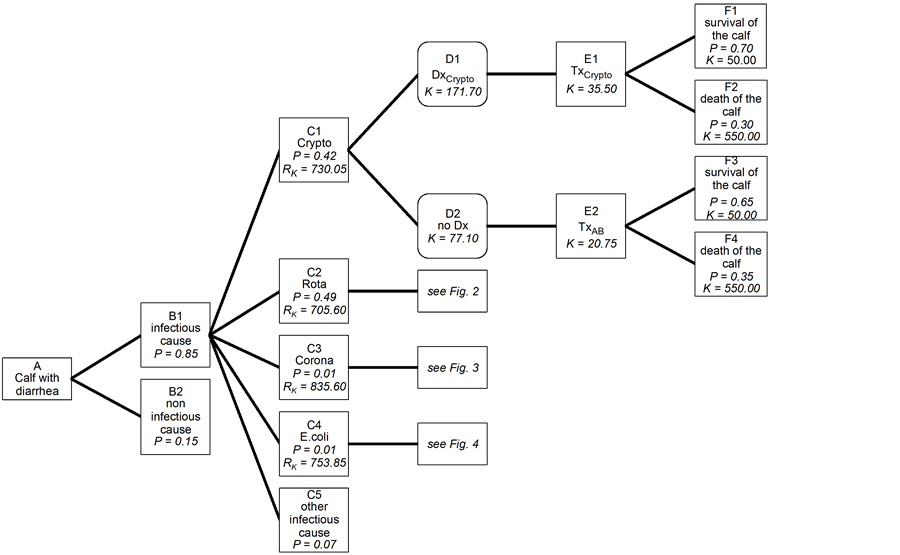
The operational approach needed no clearance by an animal protection ethics committee, since all animals were treated when clinically ill. All raw data were extracted from a Filemaker®-database (FileMaker, Inc. 5201 Patrick Henry Drive, Santa Clara, CA) where all herds transferred to the Section for Ambulatory Field Clinic and Herd Health, Department of Farm Animals, University of Zurich were registered from 1990 until 2013. The data were transferred to Stata® for further statistical evaluation such as means and counts for specific selections (Stata statistical software: release 12.0. Statcorp LP, College Station, TX, USA). The decision tree was analyzed according to Altman [9] . The decision tree was divided into four parts with one overview for convenience (Figures 1-5). Four trees have been drawn that show only one branch in detail. The division was made according to the four main diarrhea pathogens. Each field was marked with a combination of a letter and a number. The boxes with the rounded corners represent a free choice. In addition, they contain costs (K). The boxes with the pointed corners show the possible consequences of a given decision. They include the probability (P) linked to a particular decision and the costs thereby incurred (K). The information relating to probability and cost is given in Tables 1-6. The risks expressed as probability multiplied by cost (RK) are listed. The boxes in Figures 1-5, labeled “B2 non-infectious cause” and those containing information about other infectious agents are shown for the sake of comprehensiveness and are not dealt with further because they do not relate to the comparison of targeted diagnostic procedures and and the trial-and-error method.
The sources for the decision tree were visited between January 2014 and March 2015 in the following three databases: PubMed (http://www.ncbi.nlm.nih.gov/pubmed), ScienceDirect (http://www.sciencedirect.com/) and cabdirect (http://www.cabdirect.org/). The following search terms were used in different combinations: calf/ calves diarrhea/scours, cryptosporidiosis, rotavirus, coronavirus, E. coli, treatment, fluids, antimicrobials, prevalence Switzerland. The weighted average of the listed prevalence was calculated for the calculation of the prevalences in the decision tree. The drugs were selected according to the treatment guidelines used at our clinic (Cryptosporidium parvum: Halofuginone (Halocur®, MSD Animal Health GmbH, 2 ml/10 kg BW on 7 days,
Figure 1. Decision tree for treatment outcome in diarrheic calves infected with Cryptosporidium parvum. Crypto: Cryptosporidium parvum; Rota: rotavirus; Corona: coronavirus; Dx: diagnosis; Tx: therapy; AB: antibiotics; P: probability; K: costs; RK: risks as probability multiplied by cost.
Figure 2. Decision tree for treatment outcome in diarrheic calves infected with rotavirus. Crypto: Cryptosporidium parvum; Rota: rotavirus; Corona: coronavirus; Dx: diagnosis; Tx: therapy; AB: antibiotics; P: probability; K: costs; RK: risks as probability multiplied by cost.
Figure 3. Decision tree for treatment outcome in diarrheic calves infected with coronavirus. Crypto: Cryptosporidium parvum; Rota: rotavirus; Corona: coronavirus; Dx: diagnosis; Tx: therapy; AB: antibiotics; P: probability; K: costs; RK: risks as probability multiplied by cost.
Figure 4. Decision tree for treatment outcome in diarrheic calves infected with E. coli. Crypto: Cryptosporidium parvum; Rota: rotavirus; Corona: coronavirus; Dx: diagnosis; Tx: therapy; AB: antibiotics; ABN: N = choice, i.e. first, second or third choice according to materials and methods; AB special: Choice of AB according to bacteriological examination and resistance test. P: probability; K: costs; RK: risks as probability multiplied by cost.
Figure 5. Decision tree for outcome of diarrhea in calves. Crypto: cryptosporidiosis; Rota: rotavirus; Corona: coronavirus. P: probability; RK: risks as probability multiplied by the cost.
100 µg/kg BW on 7 days), rotavirus and coronaviruses: symptomatic therapy with rehydration, E. coli: antibiotics). The order of antibiotics was chosen according to the treatment guidelines used at our clinic [4] : 1st choice: Ampicillin (Albipen® LA, MSD Animal Health GmbH, Switzerland, Ampicillinum anhydricum 100 mg, E312 0.0875 mg; Aluminii monostearas, Oleum cocos ad susp. pro 1 ml), 2nd choice: trimethoprim-sulfonamide (Borgal® 24%, MSD Animal Health GmbH, Sulfadoxinum 200 mg, Trimethoprimum 40 mg, Natrii hydroxidum, Glyceroli formalum, Aqua ad iniectabilia pro 1 ml), 3rd choice: enrofloxacin (Baytril® 10%, Provet AG, Switzerland, Enrofloxacinum 100 mg, Kalii hydroxidum, Alcohol butylicus, Aqua ad iniectabilia qs ad 1 ml). Treatment success was defined as resolution of diarrhea in a sick calf or survival of a treated calf. Treatment failure was defined as continuation of diarrhea despite treatment, death of the calf, or the need to euthanize the calf on humane grounds. Information regarding the spontaneous cure rate in calves with diarrhea was gained from studies that used placebo-treated control groups of experimentally or naturally infected calves (references are given in Tables 3-5). Spontaneous cure was defined as resolution of diarrhea in the absence of treatment or survival of a diarrheic calf with resolution of diarrhea. Prevalence of different infectious agents is given in Table 1. Treatment success and spontaneous cure rates are shown in Table 2 for diarrheic calves with cryptosporidiosis, in Table 3 for calves infected with rotavirus, in Table 4 for calves infected with coronavirus, and in Table 5 for calves with E. coli diarrhea. The dosages and prices used for calculation in Tables 6-8 are based on the fee schedule of our clinic as of June 2015. For calculations, the amount of 0.00 CHF (Swiss Francs) was used for a surviving calf, 500.00 CHF was used for a dead calf, and 5.00 CHF was used for the cost of treatment, diagnostic testing, and to account for the loss in weight gain per day [23] .
3. Results
The prevalence of important pathogens causing diarrhea in neonatal calves in Switzerland is shown in Table 1 and the prevalence of treatment success for different pathogens is shown in Tables 2-5. Prevalence is used to express probabilities. The cost of treatment is given in Tables 6-8 and the steps in the decision tree explain how the cost was calculated. Individual risks are additive and generate the total risk contained in a main branch of the decision tree [23] . Table 9 shows the formulae for the risk calculations (RK) for the fields C1 to C4 in Figures 1-5. For clarity, the branches for cryptosporidiosis (Figure 1), rotavirus (Figure 2), coronavirus (Figure 3) and E. coli (Figure 4) are shown separately. Figure 5 provides a synopsis of Figures 1-4 and details the costs associated with the different branches. An infectious pathogen was isolated in 85% of scouring calves in a Swiss
Table 1. Prevalence of major pathogens causing diarrhea in calves in Switzerland.
Table 2. Therapeutic success and spontaneous cure rate in diarrheic calves infected with Cryptosporidium parvum.
Tx: treatment.
Table 3. Therapeutic success and spontaneous cure rate in diarrheic calves infected with rotavirus.
Tx: treatment; OES: oral electrolyte solution.
Table 4. Therapeutic success and spontaneous cure rate in diarrheic calves infected with coronaviruses.
Tx: treatment; OES: oral electrolyte solution.
Table 5. Therapeutic success and spontaneous cure rate in diarrheic calves infected with E. coli. The therapeutic success is based on results of susceptibility testing of isolated fecal E. coli strains to various antibiotics.
Tx: Treatment; NA: not available.
Table 6. Treatment costs for diarrheic calves infected Cryptosporidia based on the fee schedule at our clinic as of June 2015 and [23] .
Dx: diagnosis; Tx: treatment; PU: parasitological examination; VU: viral examination; BU: bacteriological examination; CHF: Swiss francs; BW: body weight; i/v: intravenous; i/m: intramuscular; s/c: subcutaneous. §: decision according to decision tree in Figures 1-4.
Table 7. Treatment costs for diarrheic calves infected with rotavirus and coronavirus based on the fee schedule at our clinic as of June 2015 and [23] .
Table 8. Treatment costs for diarrheic calves infected with E. coli based on the fee schedule at our clinic as of June 2015 and [23] .
AB: antibiotic; Dx: diagnosis; Tx: treatment; PU: parasitological examination; VU: viral examination; BU: bacteriological examination; CHF: Swiss francs; BW: body weight; i/v: intravenous; i/m: intramuscular; s/c: subcutaneous; §: decision according to decision tree in Figures 1-4.
Table 9. Calculation of the risk (RK) for each of the 4 main infectious agents according to Figures 1-4. For probabilities (P) see Figures 1-4, for costs CN to FNN see Tables 6-8.
study [3] , [5] ; rotavirus and Cryptosporidium parvum each accounted for 40% to 50% of all cases and coronavirus and E. coli each accounted for approximately 1% of cases. As can be seen in the decision trees (Figures 1-4), the costs are higher for all four infectious agents when diagnostic testing was done compared with the trial-and-error method. The risk, defined by probability of occurrence multiplied by cost, is equivalent to CHF 700.00 to 840.00 for all four infectious agents; it is highest for coronavirus (CHF 835.60) and lowest for rotavirus (CHF 705.60, Figure 5). The branches B2 (non-infectious causes) and C5 (other infectious causes) are also shown in Figures 1-5 but detailed calculations were not done.
4. Discussion
Calf diarrhea is a complex disease caused by a variety of infectious microorganisms and non-infectious factors. Infectious agents include viruses, parasites and bacteria [2] . The decision trees shown in this study are limited to a few variations of cost and risk analysis in calves with diarrhea. By virtue of the public health axiom ’intention to treat’ [24] , only those branches of the decision tree are shown that involve treatment. The premise was that calves with diarrhea presented for veterinary examination were either treated or euthanized and that non-treat- ment was not an option for humane reasons.
Some pathogens including Cryptosporidium parvum, rotavirus, and coronavirus have intermittent shedding patterns [5] and therefore, negative results must be interpreted accordingly regardless of the sensitivity and specificity of the tests used. Furthermore, diarrhea pathogens also are commonly isolated from clinically healthy calves [5] .
Two studies of the prevalence of diarrhea pathogens in calves in Switzerland found an infectious cause in 85% of affected calves; 93% of the infectious agents belonged to the four main pathogens Cryptosporidium parvum, rotavirus, coronavirus, and E. coli [3] [5] . This is consistent with the observation that 75% - 95% of diarrheic calves worldwide are affected by these pathogens [2] . Other potential diarrhea pathogens mentioned in the literature include vero-cytotoxic and necrotoxic E. coli, bovine torovirus, calicivirus, norovirus, Giardia spp., Salmonella spp., Clostridium perfringens type B and C, Eimeria spp., and Campylobacter jejuni [2] but these were not considered for calculation in this study.
The prevalences presented in this study confirm that viral and parasitic pathogens are much more commonly involved in calf diarrhea than bacterial pathogens suggesting that in the majority of cases the use of antibiotics is not justified. A Swedish study reported that 30% of calves with diarrhea in the first three months of life were treated with antibiotics [7] , and a similar substantial over-treatment of diarrheic calves with antimicrobial drugs was reported in another study [25] . The use of antibiotics for the treatment of calves with diarrhea is controversial [1] and several authors have been critical of this practice [7] [25] [26] . Calves treated prophylactically for calf diarrhea during the first two weeks of life with neomycin or tetracycline in the milk had lower weight gain, lower feed intake, and more days with diarrhea than calves not receiving antibiotics in the milk. However, the use of antimicrobials other than in-milk antimicrobials used in calves with diarrhea, fever, anorexia and depression was beneficial [27] . The use of antibiotics is recommended in diarrheic calves with systemic signs of disease including anorexia, dehydration, lethargy, fever, or when the diarrheic stool contains blood or sloughed mucous membranes. This is to prevent bacteremia following bacterial overgrowth in the intestines and to reduce the duration of the disease and the mortality rate [28] .
There are three main management tools aimed at the reduction of antimicrobial use in young calves [1] . Maternal vaccination against enterotoxic E. coli, rotavirus and coronavirus in the last trimester increases specific colostral immunity against these pathogens. A sufficient amount of high-quality colostrum fed to newborn calves within a few hours after birth prevents failure of passive transfer. Finally, attention to good hygiene and other appropriate management factors reduces the pathogen load in the environment.
Regardless of the etiology of the diarrhea, fluid therapy and replacement of electrolyte deficits are a crucial part of treatment of diarrheic calves [26] . The inclusion of an alkalinizing agent in the electrolyte mixture seems to be beneficial; between 83% and 92% of diarrheic calves infected with rotavirus or coronavirus recovered after treatment with electrolyte solutions that contained bicarbonate or acetate compared with 33% of calves that did not receive an alkalinizing agent [16] .
The decision trees show that empirical treatment of diarrheic calves infected with Cryptosporidium parvum, rotavirus or coronavirus in the absence of a diagnostic workup is significantly cheaper than specific treatment according to a laboratory diagnosis. This means that from a strictly economic point of view, the indiscriminate use of antibiotics compares favorably with targeted treatment based on an etiological diagnosis. This difference is caused by the high cost of diagnostic testing. Calves infected with rotavirus or coronavirus receiving fluid therapy had a significantly higher survival rate than the same calves treated with antibiotics alone, whereas this difference was less pronounced for calves with cryptosporidiosis treated with fluids, antibiotics, or halofuginone alone.
With respect to the branch of the decision tree for E. coli, the costs are highest when bacteriological culture and antimicrobial susceptibility testing are carried out. The trial-and-error methods provided cheaper treatment even in cases in which three different antibiotics were used consecutively.
In contrast to calves with pneumonia, in which diagnostic laboratory testing is advantageous from an economic standpoint [18] [23] , the same does not seem to be true for calves with diarrhea. Nevertheless, the producer should always be given the option for additional diagnostic testing, particularly when a herd problem exists or when antimicrobial misuse is suspected. Once an etiological diagnosis has been made, specific prophylactic measures can be instituted to reduce further cases of diarrhea and associated costs. This will pay off in the long term and offset the cost of the initial testing; however, this aspect was not taken into account in these calculations.
The risk analysis calculations generated costs between CHF 700.00 and 840.00 for diarrheic calves infected by any of the four pathogens. The costs are highest for calves infected with coronavirus, which accounts for only about 1% of diarrheic calves. The high cost associated with coronavirus diarrhea is attributable mainly to to the low spontaneous cure rate and loss of the calf [29] .
5. Conclusion
There is a need for standard operating procedures for the management of diarrhea in young calves when the goal is to prevent unnecessary use of antibiotics in diarrheic calves. It is expected that the implementation of standard operating procedures for evidenced-based treatment of calves with diarrhea will increase therapeutic costs. This is also likely to increase the prices in the entire chain of animal food products. However, if the use of antimicrobial drugs must be reduced, consumers must tolerate paying higher food prices.
Cite this paper
MichaelHässig,SusanneKretschmar, (2016) Evidence-Based Use of Antibiotics in Veal Calves with Diarrhea. Open Journal of Veterinary Medicine,06,28-39. doi: 10.4236/ojvm.2016.62005
References
- 1. Smith, G. (2015) Antimicrobial Decision Making for Enteric Diseases of Cattle. Veterinary Clinics of North America: Food Animal Practice, 31, 47-60.
http://dx.doi.org/10.1016/j.cvfa.2014.11.004 - 2. Radostits, O.M., Gay, C. C., Hinchcliff, K.W. and Constable, P.D. (2007) Veterinary Medicine. 10 Issues. Saunders, Elsevier.
- 3. Luginbühl, A., Reitt, K., Metzler, A., Kollbrunner, M., Corboz, L. and Deplazes, P. (2005) Field Study about Prevalence and Diagnostics of Diarrhea Causing Agents in the New-Born Calf in a Swiss Veterinary Practice Area. Schweizer Archiv für Tierheilkunde, 147, 245-252.
http://dx.doi.org/10.1024/0036-7281.147.6.245 - 4. Braun, U. (2013) Durchfall beim Kalb in den ersten Lebenswochen. Script Innere Medizin Rind, Departement für Nutztiere, Universität Zürich, Zürich.
- 5. Uhde, F.L., Kaufmann, T., Sager, H., Albini, S., Zanoni, R., Schelling, E. and Meylan, M. (2008) Prevalence of Four Enteropathogens in the Faeces of Young Diarrhoeic Dairy Calves in Switzerland. Veterinary Record, 163, 362-366.
http://dx.doi.org/10.1136/vr.163.12.362 - 6. Lofstedt, J., Miller, L., Duizer, G. and Daley, J. (1996) Comparison of Efficacy of Sulbactam: Ampicillin to Ampicillin and Saline for Treatment of Experimentally Induced Escherichia coli Diarrhea in Neonatal Calves. Canadian Journal of Veterinary Research, 60, 210-215.
- 7. Ortman, K. and Svensson, C. (2004) Use of Antimicrobial Drugs in Swedish Dairy Calves and Replacement Heifers. Veterinary Record, 154, 136-140.
http://dx.doi.org/10.1136/vr.154.5.136 - 8. Valente, C., Fruganti, G., Tesei, B., Ciorba, A., Cardaras, P., Floris, A. and Bordoni, E. (1988) Vaccination of Pregnant Cows with K99 Antigen of Enterotoxigenic Escherichia coli and Protection by Colostrum in Newborn Calves. Comparative Immunology, Microbiology & Infectious Diseases, 11, 189-198.
http://dx.doi.org/10.1016/0147-9571(88)90037-9 - 9. Hässig, M., Eugster, S. and Lewis, F.I. (2015) Evidence-Based Use of Antibiotics in Meat Calves. Open Journal of Veterinary Medicine, 5, 68-72.
http://dx.doi.org/10.4236/ojvm.2015.53009 - 10. Bonita, R., Beaglehole, R. and Kjellström, T. (2013) Einführung in die Epidemiologie. WHO Press, Colorado.
- 11. Björkman, C., Svensson, C., Christensson, B. and de Verdier, K. (2003) Cryptosporidium parvum and Giardia intestinalis in Calf Diarrhoea in Sweden. Acta Veterinaria Scandinavica, 44, 145-152.
http://dx.doi.org/10.1186/1751-0147-44-145 - 12. Grove-White, D. (2007) Practical Intravenous Fluid Therapy in the Diarrhoeic Calf. In Practice, 29, 404-408.
http://dx.doi.org/10.1136/inpract.29.7.404 - 13. Berge, A.C., Moore, D.A., Besser, T.E. and Sischo, W.M. (2009) Targeting Therapy to Minimize Antimicrobial Use in Pre-Weaned Calves: Effects on Health, Growth, and Treatment Costs. Journal of Dairy Science, 92, 4707-4714.
http://dx.doi.org/10.3168/jds.2009-2199 - 14. Constable, P.D. (2009) Treatment of Calf Diarrhea: Antimicrobial and Ancillary Treatments. Veterinary Clinics of North America: Food Animal Practice, 25, 101-120.
http://dx.doi.org/10.1016/j.cvfa.2008.10.012 - 15. Heiniger, D., van den Borne, B.H.P., Lechner, I., Tschopp, A., Strabel, D., Steiner, A. and Meier, H. (2014) Kosten-Nutzen-Analyse einer Intervention zur Verbesserung der Eutergesundheit in Schweizer Milchviehbetrieben. Schweizer Archiv für Tierheilkunde, 156, 473-481.
http://dx.doi.org/10.1024/0036-7281/a000634 - 16. Pereira, R.V.V., Santos, T.M.A., Bicalho, M.L., Caixeta, L.S., Machado, V.S. and Bicalho, R.C. (2011) Antimicrobial Resistance and Prevalence of Virulence Factor Genes in Fecal Escherichia coli of Holstein Calves Fed Milk with and without Antimicrobials. Journal of Dairy Science, 94, 4556-4565.
http://dx.doi.org/10.3168/jds.2011-4337 - 17. De Verdier, K., Nyman, A., Greko, C. and Bengtsson, B. (2012) Antimicrobial Resistance and Virulence Factors in Escherichia coli from Swedish Dairy Calves. Acta Veterinaria Scandinavica, 54, 2.
http://dx.doi.org/10.1186/1751-0147-54-2 - 18. ARCH-Vet (2013) Bericht über den Vertrieb von Antibiotika in der Veterinärmedizin und das Antibiotikaresistenzmonitoring bei Nutztieren in der Schweiz. Eidgenössisches Departement des Innern, Bundesamt für Lebensmittelsicherheit und Veterinärwesen.
http://www.anresis.ch/index.php/resistenzdaten-veterinaermedizin.html - 19. Eugster, S. (2013) Antibiotikaresistenz in Kälbermastbetrieben. Master’s Thesis, Vetsuisse Faculty, University of Zurich, Zürich.
- 20. Castrucci, G., Ferrari, M., Frigeri, F., Traldi, V. and Angelillo, V. (1994) A Study on Neonatal Calf Diarrhea Induced by Rotavirus. Comparative Immunology, Microbiology & Infectious Diseases, 17, 321-331.
http://dx.doi.org/10.1016/0147-9571(94)90051-5 - 21. Naylor, J.M., Petrie, L., Rodriguez, M.I. and Skilnick, P. (1990) A Comparison of Three Oral Electrolyte Solutions in the Treatment of Diarrheic Calves. Canadian Veterinary Journal, 31, 753-760.
- 22. Bouda, J., Doubek, J., Medina-Cruz, M., Paasch, M.L., Candanosa, A.E., Dvorák, R. and Soska, V. (1997) Pathophysiology of Severe Diarrhoea and Suggested Intravenous Fluid Therapy in Calves of Different Ages under Field Conditions. Acta Veterinaria Brno, 66, 87-94.
http://dx.doi.org/10.2754/avb199766020087 - 23. Glover, A.D., Puschner, B., Rossow, H.A., Lehenbauer, T.W., Champagne, J.D., Blanchard, P.C. and Aly, S.S. (2013) A Double-Blind Block Randomized Clinical Trial on the Effect of Zinc as a Treatment for Diarrhea in Neonatal Holstein Calves under Natural Challenge Conditions. Preventive Veterinary Medicine, 112, 338-347.
http://dx.doi.org/10.1016/j.prevetmed.2013.09.001 - 24. Keidel, J. and Daugschies, A. (2013) Integration of Halofuginone Lactate Treatment and Disinfection with P-Chloro-M-Cresol to Control Natural Cryptosporidiosis in Calves. Veterinary Parasitology, 196, 321-326.
http://dx.doi.org/10.1016/j.vetpar.2013.03.003 - 25. Naciri, M., Mancassola, R., Yvore, P. and Peeters, J.E. (1993) The Effect of Halofuginone Lactate on Experimental Cryptosporidium parvum Infections in Calves. Veterinary Parasitology, 45, 199-207.
http://dx.doi.org/10.1016/0304-4017(93)90075-X - 26. Joachim, A., Krull, T., Schwarzkopf, J. and Daugschies, A. (2003) Prevalence and Control of Bovine Cryptosporidiosis in German Dairy Herds. Veterinary Parasitology, 112, 277-288.
http://dx.doi.org/10.1016/S0304-4017(03)00006-2 - 27. Lallemond, M., Villeneuve, A., Belda, J. and Dubreuil, P. (2006) Field Study of the Efficacy of Halofuginone and Decoquinate in the Treatment of Cryptosporidiosis in Veal Calves. Veterinary Record, 159, 672-676.
http://dx.doi.org/10.1136/vr.159.20.672 - 28. Altman, D.G. (1994) Practical Statistics für Medical Research. Chapman & Hall, London.
- 29. Jacks, T.M., Schleim, K.D., Miller, B.M., Shungu, D.L., Weinberg, E. and Gadebusch, H.H. (1983) Quaternary Heterocyclylamino Beta-Lactams. V. L-640,876 Treatment of Induced Enterotoxigenic Colibacillosis (Scours) in Calves and Piglets. The Journal of Antibiotics, 36, 70-75.
http://dx.doi.org/10.7164/antibiotics.36.70