Open Journal of Veterinary Medicine
Vol.3 No.5(2013), Article ID:36001,7 pages DOI:10.4236/ojvm.2013.35040
Experimental Haematobiochemical Alterations in Broiler Chickens Fed with T-2 Toxin and Co-Infected with IBV
1College of Veterinary Science, Mekelle University, Mekelle, Ethiopia
2Division of Pathology, Indian Veterinary Research Institute, Bareilly, India
Email: *john.asfaw@yahoo.com
Copyright © 2013 T. Yohannes et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received November 29, 2012; revised January 5, 2013; accepted February 5, 2013
Keywords: Anaemia; Hypoproteinenemia; IBV; Lymphocytopenia; T-2 Toxin; Thrombocytopenia
ABSTRACT
The purpose of this experimental study was to evaluate and record the effects of T-2 toxicity alone and in association with IBV infection on haematobiochemical parameters. A total of 128 one-week-old chicks were divided into four groups of 32 birds each and were treated respectively with T-2 toxin alone, IBV alone, T-2 toxin and co-infected with IBV, and no treatment (control) for a period of 6 weeks. Haematologically, the birds treated with T-2 toxin developed anaemia as indicated by significant decrease in haemoglobin levels, total erythrocyte counts and packed cell volume values; leucopenia, lymphocytopenia heterophilia and thrombocytopenia. The IBV infected birds exhibited lymphocytophilia and heteropoenia; the degrees of severity of leucopenia, lymphocytopenia heterophilia and thrombocytopenia were more pronounced in T-2+IBV groups. The serum biochemistry revealed hypoproteinemia and hypoalbuminemia in all the treated groups consistently. Besides, hypoglobulinemia and increased levels of alanine aminotransferase in T-2+IBV, and increased levels of alkaline phosphatase in toxin group alone were recorded. The changes in biochemical parameters were more in magnitude in the combination treatment group and their severity increased with duration of treatment. It was concluded that T-2 toxin made the birds more susceptible to IBV infection.
1. Introduction
Mycotoxins produced by the moulds, are the common contaminants of foods, feeds and agricultural products, which cause serious health problems (mycotoxicoses) in both animals and human beings [1-3]. T-2 toxin is a member of a large group of fungal metabolites with same basic chemical structure, called Trichothecene mycotoxins, produced by various species of Fusarium [4]. T-2 toxin has been commonly found in grains and poultry feed [5] and is well known for its genotoxic, cytotoxic and immunomodulatory effects. Actively dividing cells (cells of the gastrointestinal tract, bone marrow, lymph nodes, spleen, and liver) are found to be highly sensitive to T-2 toxin activity. Cytotoxic radiomimetic effects of T-2 toxin were considered as a major cause of impaired protein synthesis. In vivo T-2 toxin induced DNA damage in chicken peripheral lymphocytes [5] and apoptosis in vivo in haematopoietic tissues, spleen, liver and intestinal crypts of mice [6-8]. T-2 toxin was found to be haematotoxic having its effects on bone marrow, circulating blood cells and haemostasis [9]. Infectious bronchitis (IB), an acute, highly contagious and primarily respiratory infection, is characterised by causing respiratory and urogenital systems pathology; occurring at all ages and presenting in most poultry producing areas [10]. Infectious bronchitis virus (IBV) causes severe economic losses to the layers and broiler chickens industry [11]. T-2 mycotoxin induced alterations in immune function may contribute to susceptibility of the birds to certain infectious diseases [12] including IB. As such there appears no report in the perused available literatures on the effects of T-2 toxin in association with IBV infection. Hence, the present study was undertaken to find out T-2 toxicity alone or in combination with IBV infection on haematobiochemical alterations in broiler chickens.
2. Materials and Methods
Production and analysis of T-2 mycotoxin: T-2 mycotoxin was produced on sterile maize and wheat as described by [13]. The cultures of Fusarium sporotrichioioides var. Sporotrichioides NRRL 3299, supplied by National Centre for Agricultural Utilization Research (NCAUR), Peoria, Illinois, USA, and MTCC1894, procured from Institute of Microbial Technology (IMTC), Chandigarh, India, were used to produce T-2 mycotoxin on partially ground maize and intact wheat grains. The T-2 mycotoxin was estimated by TLC at Animal Feed Analytical and Quality Control Laboratory (AFAQCL), Veterinary College and Research Institute, Namakkal, Tamil Nadu (India).
IBV propagation and EID50 determination: Infectious bronchitis virus (IBV) isolate (India/LKW/56/IVRI/ 08) of chickens used in the study was obtained from the Avian Disease Section (Division of Pathology), IVRI, India. The isolate was propagated in embryonated chicken eggs by serial passages with its 10 fold serial dilutions. Five eggs were inoculated via allantoic membrane, with each dilution (as such, 102, 103, 104, 105, 106 and 107) and the presence of virus in each embryo was checked by observing the lesions (curling and dwarfing of embryos) from five to seven days after inoculation and embryo deaths were recorded. The virus titre in terms of 50 per cent egg infective dose (EID50) per 0.2 ml was calculated following the procedure of [14].
Experimental chicks: The experiment was conducted using a total of 128, one-week-old broiler chicks, procured from the Hatchery Unit of Central Avian Research Institute (CARI), Bareilly, India. All the experimental procedures were conducted as per the guidelines of the Institute Animal Ethics Committee (IAEC) and Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA). The chicks were kept under standard managemental conditions in the poultry shed of Avian Diseases Section (Division of Pathology), IVRI. The birds were checked for the presence of antibodies against IBV by agar gel precipitation test (AGPT) to ensure their seronegative status prior to experimental IBV infection. They were provided with standard basal feed free of toxin and fresh water ad libitum during the entire experimental period.
Experimental feed: The substrate (maize and wheat) containing the known amount of T-2 toxin (purified and detected using chromatography) was mixed to the basal feed (tested negative for mycotoxin contamination by ELISA) to make the desired concentration of T-2 toxin in the diet i.e. 2 ppm (2 mg/kg feed) [2]. Aliquots were taken from the mixed feed and the toxin was quantified by ELISA (Romer lab, Singapore) to ensure proper mixing.
Experimental design: One hundred twenty eight 1- week-old broiler chicks were weighed and randomly distributed to four groups (T-2, IBV, T-2+IBV and control groups) of 32 birds each. The experimental diets were given to each group of chicken for a period of 6 weeks as per following: T-2 group (toxin feed from 0 - 6 weeks), IBV group (toxin free control feed from 0 - 6 weeks and infected with IBV at 3rd week), T-2+IBV group (toxin feed from 0 - 6 weeks and co-infected with IBV at 3rd week), and control group (toxin free control feed from 0 - 6 weeks).
Parameters studied: Blood samples (2 ml) were collected at 3 (3), 4 (10), 5 (17) and 6 (21) WTF (DPI) from jugular vein or at times directly from heart. Half of it was taken in dry sterilized vial containing anticoagulant, EDTA (Ethylene diamine tetra acetic acid, at 1 mg/ml) for haematology and remaining half in dry test tubes with no anticoagulant for harvesting serum to be used for biochemical parameters. The haematological parameters included haemoglobin concentration (Hb), packed cell volume (PCV), total erythrocyte count (TEC), total leucocyte count (TLC) and differential leucocyte count (DLC) which were carried out as per standard procedures [15]. The values of mean corpuscular volume (MCV), mean corpuscular haemoglobin (MCH) and mean corpuscular haemoglobin concentration (MCHC) were derived from the values of Hb, PCV and TEC [15], using the following formulae.
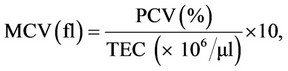
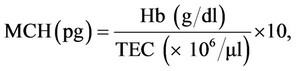
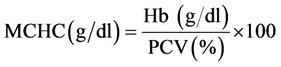
The biochemical parameters included total serum protein (TSP), albumin, globulin, uric acid, creatinine, alkaline phosphatise (AP), AST(SGOT) and ALT(SGPT) by using commercial kits (Span Diagnostics, India) and following the procedure of manufacturer of the kits.
Data management: The data were analysed using GraphPad Prism statistical package. Descriptive (%) statistics and Chi-square were used to compare results across treatment groups, and P-value ≤ 0.05 was taken as level of significance.
3. Results
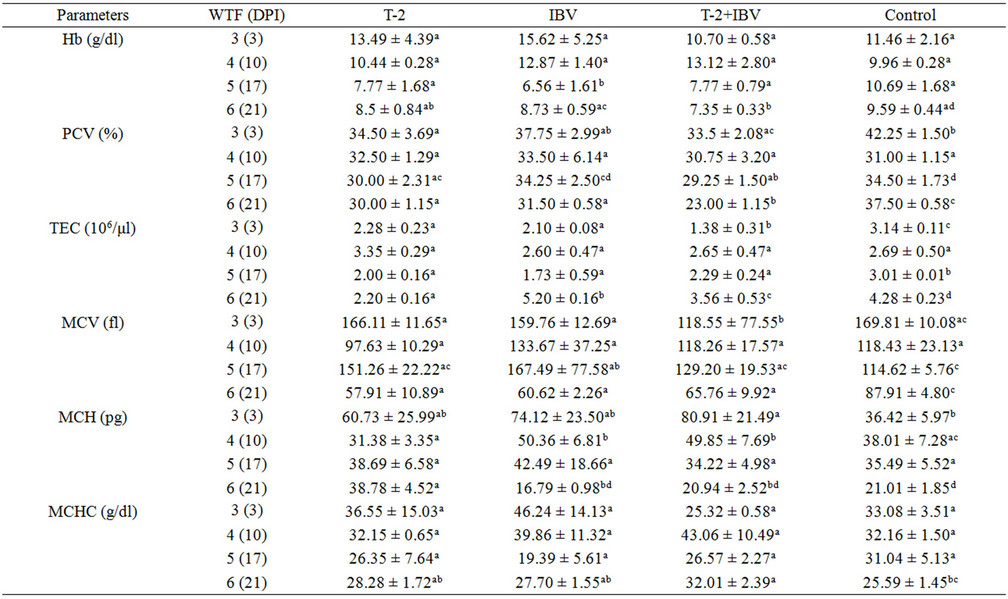
The values of haematological parameters at various intervals in different treatment groups have been presented in Tables 1(a) and (b). Haemoglobin (Hb) values were significantly reduced in T-2+IBV group (7.35 ± 0.33) as compared to IBV group (8.73 ± 0.59), T-2 group (8.5 ± 0.84) and control group (9.59 ± 0.44) at 6 (21) WTF (DPI). Packed cell volume (PCV) at 5 (17) WTF (DPI) of T-2+IBV group (29.25 ± 1.50) was significantly lower than IBV group (34.25 ± 2.50) and control group (34.50 ± 1.73). The PCV value of T-2 group (30.00 ± 2.31) was
 (a)
(a)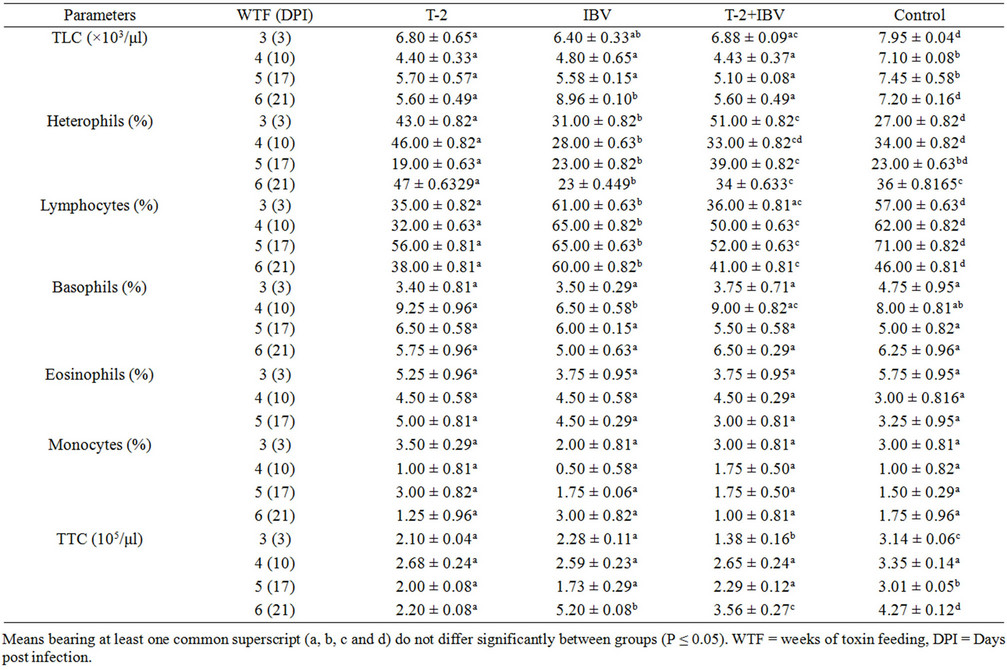 (b)
(b)
Table 1. Effects of T-2 toxin and IBV infection, on various haematological parameters (Means ± S.E) at different intervals.
also significantly (P < 0.05) reduced as compared to that of control. Moreover, at 6 (21) WTF (DPI), mean PCV values of all treatment groups were significantly (P < 0.05) lower when compared with the control. Total erythrocyte count (TEC) values in all treatment groups at 3 (3), 5 (17) and 6 (21) WTF (DPI) were significantly (P < 0.05) lower as compared to that of the control group. The TEC counts were lowest in T-2+IBV group at 3 (3) and 6 (21) WTF (DPI). The values of erythrocyte indices did not exhibit any specific trend with duration of treatment and were fluctuating. However, T-2+IBV group birds showed lower values at most of the times (Table 1(a)).
Total leucocyte counts (TLC) decreased significantly (P < 0.05) in all the treatment groups as compared with those in control group at all intervals. However, TLC counts in IBV group were significantly higher than the control as well as other treatment groups, at 21 DPI (Table 1). Toxin fed birds showed significant reduction in per cent lymphocyte counts as compared to control. The per cent lymphocyte count was, however, higher in IBV group (Table 1(b)). Total thrombocyte counts (TTC) revealed that T-2 fed chicks had significantly (P < 0.05) lesser number of thrombocytes at all intervals, except 4 (10) WFT (DPI), than those in control chicks. The TTC in T-2+IBV group chicks was significantly lower as compared to the number in IBV group chicks (at 3 (3) and 6 (21) WFT (DPI) intervals (Table 1(b)). The percent lymphocytes in T-2+IBV groups were lower (lymphocytopenia) from 3 (3) WTF (DPI) till the end and the value in T-2 groups was significantly lower than T-2+ IBV groups. However, the value in IBV was higher than control. Significant differences in percent basophils, eosoniphils and monocytes were not observed in any of the treatment groups.
Blood biochemical analyses revealed significant reduction in serum total protein (STP) at 5 (17) and 6 (21) WFT (DPI) in combined toxin and virus groups (Table 2). Toxicated birds belonging to T-2+IBV groups showed
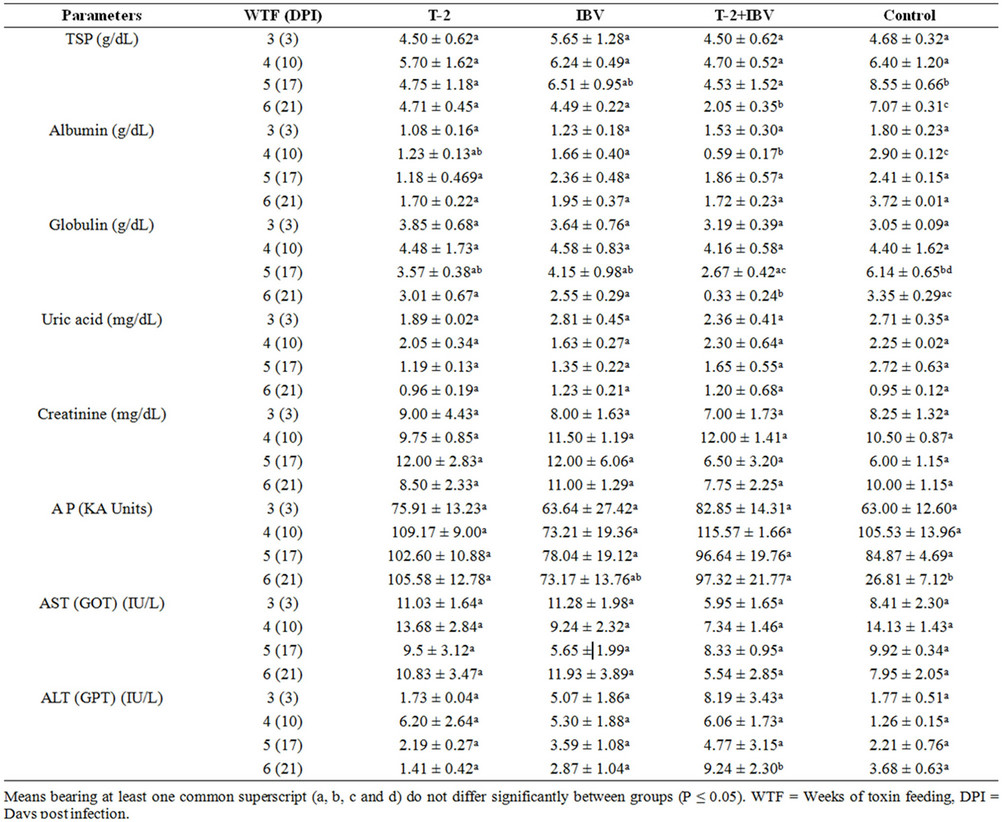
Table 2. Effects of T-2 toxin and IBV infection on various biochemical parameters (Means ± S.E) at different intervals.
significant increment in levels of alkaline phosphatase and ALT at 6 (21) WTF (DPI), respectively (Table 2). Whereas, no significant variation was seen in uric acid, creatinine and aspartate aminotransferase (AST) values at all intervals and amongst all groups (Table 2). Biochemical changes were not conspicuously observed in chicks belonging to IBV groups.
4. Discussion
The toxin treated and combination treatment group birds showed anaemia with significant reduction in haemoglobin (Hb) concentration, PCV values and TEC counts. The present findings are in accordance with report of [16] where reduced Hb values were observed in chicks fed T-2 toxin for 42 days at 1, 2 and 4 ppm levels. However, the authors did not observe any significant difference in TEC. Significant decrease in PCV and Hb values in 48 day-old broiler chicks fed with 0.5 ppm T-2 toxin mixed diets from 0 to 4 weeks of age was also observed by [17]. The intensity of anaemia was increased in the broiler chickens received T-2 toxin and co-infected with IBV and with time of toxin feeding in both the toxin groups. The anaemia might be attributed by inhibition of protein synthesis and haematopoietic depletion in the bone marrow [18] and lowered feed conversion efficiency [19] leading to iron deficiency due to inhibition of iron absorption in T-2 toxin fed birds [20].
The observation of significant leucocytopenia, lymphocytopenia and thrombocytopenia in T-2 toxin fed groups was in agreement with [21-23], however, previous workers [16] did not observe significant reduction in TLC in broiler chicks fed with 1, 2 and 4 ppm of T-2 toxin for 42 days. Both T-2+IBV and T-2 groups had lymphocytopenia which might be due to the cytotoxic effects of T-2 toxin on lymphoid organs that led in failure of mounting of cell mediated immunity against IBV infection and. Among the WBC differential count, percent heterophils were higher in T-2+IBV groups than in T-2 as well as IBV groups, which might be due to the relative reduction of lymphocytes (lymphocytopenia). Similar findings were also observed by [22] and [23]. T-2 fed Chicks had significantly less number of thrombocytes (thrombocytopenia) at almost all intervals and this is supported by work of [24] wherein birds given 0.5 - 1 mg/kg of T-2 toxin for 3 weeks exhibited significantly decreased thrombocyte counts with concomitant increase in blood clotting and bleeding times. The TTC in T-2+IBV group chicks were lower as compared to those in IBV group chicks at 3 (3) and 6 (21) WFT (DPI).
The decrease in TLC (leukocytopenia) is due to the reduced number of circulating lymphocytes (lymphocytopenia). Reduction in number of lymphocytes attributed to the negative effects of T-2 toxin on their blastogenesis and induced DNA damage in chicken peripheral lymphocytes [5]. This signified that the chicks were suffering from lymphocytopenia and severe IBV infection. Depletion of lymphocytes and lymphocytolysis in lymphoid organs were also observed histopathologically in birds fed with T-2 toxin at 2 ppm levels for 6 weeks and indicated that the birds were immunosupressed and thus lost resistance to infectious agent like IBV in present study [19]. More importantly, thrombocytopenia appeared to be the main haematological finding in toxin fed birds which might have resulted in loss of blood haemostasis resulting in haemorrhages as had been observed histopathologically in thymus, pectoral muscles, kidneys and lungs [19].
The occurrence of hypoproteinemia matched with previous works by [16] who reported dietary treatment of T-2 toxin at levels of 1, 2 and 4 ppm in broiler chicks, resulted significant reduction in STP. It was also observed decrease in STP and albumin [25]. Other workers like [17] and [26] also observed that T-2 toxin caused reduction in STP; however, [27], in contrast, reported no change in STP in breeder hens fed with a mixture of T-2 and other Fusarium toxins. The hypoproteinemia might be due to inhibition of protein synthesis [28-30] and is indirect indication of reduced immunoglobulin levels and other immune response factors like cytokines which are protein in nature and thus leading to some degree of immunosuppression in toxicated birds. Toxicated birds belonging to T-2+IBV groups showed significant increment in levels of alkaline phosphatase and ALT at end of the experiment. This findings agree with [25] and [17] who reported increased level of ALT whiling contradict with the previous observations made by [31] and [26] who reported reduced AST and ALT.
Biochemical changes were not conspicuously observed in chicks belonging to IBV groups. However, the reduction as well as increment of the levels of the various blood chemistry parameters in this experimental study might be attributed to T-2 toxicity which damaged liver and kidneys [19]. The present findings of increased serum ALT finds support from the histopathological changes in liver in toxin fed groups [19]. Little or no changes in serum biochemistry of birds of IBV groups indicated that the isolated of IBV used was not much pathogenic and produced changes of milder intensity.
5. Conclusion
From the present study, it was concluded that T-2 toxin caused anaemia and immunosuppression in broiler birds and potentiated the effect of IBV strain which was otherwise less pathogenic.
REFERENCES
- H. S. Hussein and J. M. Brasel, “Toxicity, Metabolism, and Impact of Mycotoxins on Humans and Animals,” Toxicology, Vol. 167, No. 2, 2001, pp. 101-134. doi:10.1016/S0300-483X(01)00471-1
- E. M. Binder, L. M. Tan, L. J. Chin, J. Handl and J. Richard, “Worldwide Occurrence of Mycotoxins in Commodities Feeds and Feed Ingredients,” Animal Feed Science Technology, Vol. 137, No. 3-4, 2007, pp. 265-282. doi:10.1016/j.anifeedsci.2007.06.005
- A. E. Glenn, “Fusarium and Their Toxins: Mycology, Occurrence, Toxicity, Control and Economic Impact by Morgavi,” Animal Feed Science Technology, Vol. 137, No. 3, 2007, pp. 199-200.
- A. E. Desjardins, “Fusarium Mycotoxins: Chemistry, Genetics, and Biology,” American Phytopathological Society, 2006, p. 260.
- M. Sokolovic, V. Garaj-Vrhovac, S. Ramic and B. Simpraga, “Chicken Nucleated Blood Cells as a Cellular Model for Genotoxicity Testing Using the Comet Assay,” Food Chemical Toxicology, Vol. 45, No. 7, 2007, pp. 2165-2170. doi:10.1016/j.fct.2007.05.013
- J. Shinozuka, G. Li, K. Uetsuka, H. Nakayama and K. Doi, “Process of the Development of T-2 Toxin-Induced Apoptosis in the Lymphoid Organs of Mice,” Experimental Animal, Vol. 46, 1997, pp. 117-126.
- A. M. Murshedul, M. Nagase, T. Yoshizawa and N. Sakato, “Thymocyte Apoptosis by T-2 Toxin in Vivo in Mice Is Independent of Fas/Fas Ligand System,” Bioscience and Biotechnology and Biochemistry, Vol. 64, No. 1, 2000, pp. 210-213. doi:10.1271/bbb.64.210
- A. Poapolathep, R. Ohtsuka, W. Kiatipattanasakul, N. Ishigami, H. Nakayama and K. Doi, “Nivalenol-Induced Apoptosis in Thymus, Spleen and Peyer’s Patches of Mice,” Experimental Toxicological Pathology, Vol. 53, No. 6, 2002, pp. 441-446. doi:10.1078/0940-2993-00211
- G. M. Meissonnier, J. Laffitte, I. Raymond, E. Benoit, A. M. Cossalter, P. Pinton, G. Bertin, I. P. Oswald and P. Galtier, “Subclinical Doses of T-2 Toxin Impair Acquired Immune Response and Liver Cytochrome P450 in Pigs,” Toxicology, Vol. 247, No. 1, 2008, pp. 46-54. doi:10.1016/j.tox.2008.02.003
- P. Villegas, “Viral Diagnosis of Respiratory System,” Poultry Science, Vol. 77, 1998, pp. 1143-1175.
- D. Cavanagh and S. A. Naqi, “Infectious Bronchitis,” In: H. J. Barnes, J. R. Glisson, A. M. Fadly, L. R. McDougald, D. E. Swayne and Y. M. Saif, Eds., Diseases of Poultry, 11th Edition, Iowa State University Press, Ames, 2003, pp. 101-119.
- I. P. Oswald, D. E. Martin, S. Bouhet, P. Pinton, I. Taranu and F. Accensi, “Immunological Risk of Mycotoxins for Domestic Animals,” Food Additives and Contaminants, Vol. 22, No. 4, 2005, pp. 354-360. doi:10.1080/02652030500058320
- AOAC, “Official Method of Analysis,” 16th Edition, Association of Official Analytical Chemists, Washington DC, 1995.
- L. J. Reed and H. Muench, “A Simple Method of Estimating 50% End-Point,” American Journal of Hygiene, Vol. 27, 1983, p. 493.
- N. C. Jain, “Schalm’s Veterinary Haematology,” 4th Edition, Lea Febiger, Philadelphia, 1986.
- V. V. Pande, N. V. Kurkure and A. Bhandarkar, “Effects of T-2 Toxin on Growth, Performance and Haematobiochemical Alterations in Briores,” Indian Journal of Experimental Biology, Vol. 44, 2006, pp. 86-88.
- P. Krishnamoorthy, S. Vairamuthu, C. Balachandra and B. Muralimanohar, “Chlorpyriphos and T-2 Toxin Induced Haemato-Biochemical Alterations in Broiler Chicken,” International Journal of Poultry Science, Vol. 5, No. 2, 2006, pp. 173-177. doi:10.3923/ijps.2006.173.177
- F. J. Hoerr, W. W. Carlton, B. Yagen and A. Z. Joffe, “Mycotoxicosis Caused by Either T-2 Toxin or Diacetoxyscirpenol in the Diet of Broiler Chickens,” Fundamental Applied Toxicology, Vol. 2, 1982, pp. 121-124. doi:10.1016/S0272-0590(82)80092-4
- T. A. Yohannes, “Clinicopathological, Pathological and Immunological Studies on Experimental T-2 Mycotoxicosis and IBV Infection in Broiler Chicken,” M.V.Sc. Thesis, Indian Veterinary Research Institute, India, 2010.
- W. E. Huff, J. A. Doerr and P. B. Hamilton, “Decreased Glycogen Mobilization during Ochratoxicosis in Broiler Chickens,” Applied and Evironmental Microbiology, Vol. 37, No. 1, 1979, pp. 122-126.
- F. J. Hoerr, “Mycotoxicoses: Diseases of Poultry,” 11th Edition, Iowa State University Press, Ames, Iowa, 2003, pp. 1103-1132.
- A. M. Shareef, “Some Immunosuppressive Effect of T-2 Toxin in Broiler Chicks,” Iraqi Journal of Veterinary Sciences, Vol. 19, No. 2, 2005, pp. 99-108.
- S. R. Chowdhury, T. K. Smithm, H. J. Boermans and B. Woodward, “Effects of Feed-Borne Fusarium Mycotoxins on Hematology and Immunology of Laying Hens,” Poultry Science, Vol. 84, 2005, pp. 1841-1850.
- U. K. Mishra, P. K. Dwarakanath, S. P. Agrawal and M. I. Hossain, “Effect of T-2 Toxin on Haemostatic Profile in Growing Chickens,” Indian Veterinary Journal, Vol. 73, No. 11, 1996, pp. 1133-1137.
- T. H. Nataraja, H. D. N. Swamy, S. K. Vijayasarathi, G. C. Prakash and B. S. Kumar, “Biochemical Alterations in Combined Mycotoxicosis of Broiler Chicks,” Indian Veterinary Journal, Vol. 81, No. 3, 2004, pp. 264-266.
- R. Madheswaran, C. Balachandran and B. M. Manohar, “Pathological Effects of Feeding Aflatoxin and T-2 Toxin in Japanese Quail,” Indian Journal of Veterinary Pathology, Vol. 29, No. 1, 2005, pp. 23-26.
- M. Yegani, T. K. Smith, S. Leeson and H. J. Boermanst, “Effect of Feeding Grains Naturally Contaminated with Fusarium Mycotoxins on Performance and Metabolism of Broiler Breeders,” Poultry Science, Vol. 85, 2006, pp. 1541-1549.
- E. Cundliffe, M. Cannon and J. Davies, “Mechanism of Inhibition of Eukaryotic Protein Synthesis by Trichothecene Fungal Toxins,” Proceedings of National Academy of Sciences, Vol. 71, 1974, pp. 30-40. doi:10.1073/pnas.71.1.30
- D. E. Corrier, “Mycotoxicosis: Mechanisms of Immunosuppression,” Veerinary Immunology and Immunopathology, Vol. 30, No. 1, 1991, pp. 73-87.
- S. Leeson, G. Diaz and J. D. Summers, “Poultry Metabolic Disorders and Mycotoxins,” University Books, Ontario, 1995.
- K. L. Arvind, V. S. Patil, G. Devegowda, B. Umakantha and S. P. Ganpule, “Efficacy of Esterified Glucomannan to Counteract Mycotoxicosis in Naturally Contaminated Feed on Performance and Serum Biochemical and Hematological Parameters in Broilers,” Poultry Science, Vol. 82, No. 4, 2003, pp. 571-576.
NOTES
*Corresponding author.

