Advances in Microbiology
Vol.06 No.04(2016), Article ID:65351,12 pages
10.4236/aim.2016.64025
Using a Collateral Damage Model to Explain Survival Data in West Nile Virus Infections
James K. Peterson1, Alison M. Kesson2,3, Nicholas J. C. King4
1Department of Mathematical and Biological Sciences, Clemson University, Clemson, SC, USA
2Department of Infectious Diseases and Microbiology, The Children’s Hospital, Sydney, Australia
3Discipline of Pediatrics and Child Health, The Marie Bashir Institute for Emerging Infectious Diseases and Biosecurity, University of Sydney, Sydney, Australia
4Discipline of Pathology, University of Sydney, Sydney, Australia

Copyright © 2016 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 28 January 2016; accepted 4 April 2016; published 7 April 2016
ABSTRACT
Simulation code for a model of the adaptive immune response seen in flavivirus infections is used to explain the immunopathological consequences seen in West Nile Virus virus (WNV) infections. We use a model that specifically handles the differences in how the virus infects resting cells, the G0 state, versus dividing cells, the G1 state, which includes vastly increased MHC-I upregulation for resting cells over dividing cells. The simulation suggests how the infection progresses in a one host model and the results shed insight into the unusual survival curve data obtained for this infection: there is an increase in health even though viral load has increased.
Keywords:
Auto Immune Responses, West Nile Virus, Host Survival Models, MHC-I Upregulation, IFN-g Upregulation, Free Antigen Levels

1. Introduction
The code for the general simulation framework has been discussed in [1] with results in [2] , and the reader is referred to those works for the background details. Further, in that work, we outline how a flavivirus such as West Nile Virus infects its host, and so we will only go over some of that information here.
The family Flaviviridae are single-stranded, plus sense RNA viruses transmitted by arthropods such as mosquitoes and ticks. Flaviviruses induce an increase in expression of major histocompatibility complex, MHC-I and II, as well as other immune recognition molecules, including intracellular adhesion molecule-1 (ICAM-1, CD54), vascular adhesion molecule-1 (VCAM-1, CD106) and E-selectin (CD62E) variously and on a wide variety of cells [3] . WNV-induced increases in cell surface expression of these molecules therefore result in increased efficiency of recognition and killing of infected cells by WNV-specific cytotoxic T lymphocytes, CTL [4] [5] . Increases in MHC-I concentration on infected cells enhance the avidity of interaction of virus-specific CTL with the infected target cell. The increased avidity enables the interaction of infected cells with CTL clones previously below the recognition threshold because of their low affinity for MHC-virus peptide ligand. Furthermore, a polyclonal anti-viral CTL population can include self-reactive low-affinity clones that recognize MHC-I-self peptide configurations as discussed in [5] - [7] . The previous simulation results in [2] offered support for this point of view. Due to the increased avidity of their interaction, these low-affinity, self-reactive clones can now lyse both infected and uninfected target cells that express high cell surface MHC-I concentrations [8] .
Because the avidity of interaction between T cells and their targets can be increased via MHC associated upregulation, any increases in MHC and/or ICAM-1 expression further enhance CTL recognition of target cells. This in turn increases the chances of low-affinity, self-reactive CTL recognizing these target cells. It is also known that the position of the cell cycle is important in flavivirus infection. Non-dividing cells when infected with WNV increase their MHC-I expression 6-10-fold, while infected cycling cells increase MHC-I expression by only 2-3-fold [9] . This results in some 10-fold more lysis of infected target cells than cycling cells by the same CTL [4] . Thus, there is enhanced avidity of interaction between CTL and WNV-infected cells in the nondividing state while infected cycling cells are less easily recognized. Further, WNV replicates significantly better in dividing cells than in non-dividing cells. Thus, virus may be eradicated with relatively poor efficiency in a population of infected cycling cells. In vivo, since most cells are not dividing, the decoy hypothesis asserts it is the small population of infected dividing cells that supports virus replication while maintaining a low immunological profile, thus increasing the probability of virus transmission [5] . High MHC-I expressing (uninfected) targets are therefore susceptible to lysis by low affinity self-reactive CTL clones. A review summarizing the case for an autoimmune component to the West Nile virus infections is in [10] .
In most virus infections, the probability of death of the host varies predictably with viral dose in a dose response curve. Thus, a lower dose results in viral clearance by an efficient immune response that runs its complete course in an infection with no mortality. In contrast, the highest doses produce 100% mortality, which interrupts the immune response before it has managed to clear the virus. In flavivirus infection, however, the mortality associated with intervening doses is unpredictable over several log10 dilutions of virus dose. This results in a ragged dose-response curve depicting population mortality [7] whose data we show in Figure 1. The work in this paper builds on the model of the host-pathogen interactions for flaviviruses detailed in [2] and is used to
Figure 1. Actual survival curve.
explain the ragged dose survival curves seen in these infections.
2. Simple Survival Model Calculations
We can do a simplified calculation to help us see that the presence of MHC-I upregulation should lead to local oscillations in the survival rate. We will do another proof of concept calculation in Section 3 once we have built a model of collateral damage which also suggests that there is a potential for auto immune interaction with the WNV infections. Let 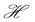 denote the population of uninfected dividing and non-dividing cells and
denote the population of uninfected dividing and non-dividing cells and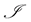 , the population of infected non-dividing cells (this is the population of interest). The size of these populations is dependent on the viral dose in pfu/cell (plaque-forming units),
, the population of infected non-dividing cells (this is the population of interest). The size of these populations is dependent on the viral dose in pfu/cell (plaque-forming units), 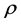 and the population of non dividing cells that are infected, respectively. Let r denote the overall growth rate of the uninfected dividing and non-dividing cell population
and the population of non dividing cells that are infected, respectively. Let r denote the overall growth rate of the uninfected dividing and non-dividing cell population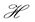 . We assume the rate of misrecognition (i.e., death of an uninfected cell) due to MHC-I upregulation
. We assume the rate of misrecognition (i.e., death of an uninfected cell) due to MHC-I upregulation 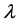 is given by
is given by 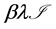 for some fraction
for some fraction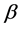 .
.
Then the net rate of change of 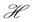 with respect to
with respect to 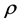 is
is 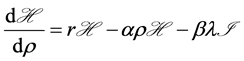 where we assume a
where we assume a
linear model for the dependence of the rate of infection on the viral load level , with
, with  being the fraction of viral load which is effectively translated into infection. Now if r is the growth rate of the uninfected dividing and non-dividing cell population, then
being the fraction of viral load which is effectively translated into infection. Now if r is the growth rate of the uninfected dividing and non-dividing cell population, then 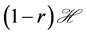 is the size of the pool of cells which can be infected. The rate of
is the size of the pool of cells which can be infected. The rate of
change of  with respect to
with respect to 

For our purposes here, the only parameter of interest is the viral dose
where
Let’s assume 
At such a point, approximate the system by the constant term





This has roots
Note the discriminant D is given by 













The survival model discriminant function can be negative leading to oscillations:

The figure shows us that the discriminant is negative in the range 
















3. Healthy Cell Approximations
When the immune system response causes healthy cells to be lysed, this is called collateral damage and in the case of West Nile virus infections, it has been discussed in [2] . We can use the damage model outlined there to shed insight in yet another way. We can rewrite the healthy cell update equation as

Figure 2. The survival model discriminant function can be negative leading to oscillations:

Figure 3. The survival model discriminant function has a smaller range leading to oscillations:

Figure 4. The survival model discriminant function does not allow oscillations:

where at time t, 








In Equation 2, N represents how we discretize our simulation universe of possible cells. We use about 108 cells in the simulation (coding details are in [1] and the general derivations in [2] ) and use a three dimensional box of size 217 on a side to give us 108 possible cells. We divide this universe of cells into 











We let the fraction 



small but for the moment think of 




Further, ignore the slight modification due to the intercept term b giving
Next, assume this is true for all coarse cubes, giving
We think it is reasonable to assume that if the number of healthy cells increases, the amount of collateral damage will increase also. We also believe that the amount of collateral damage will depend on the level of the initial viral dose which we will call I. Hence, let’s assume the parameter 

Next, assume this is true for all coarse cubes, giving the final model
We let 




Let this new proportionality constant be denoted by
Hence, we have 


We can solve this model numerically using the initial conditions 

























Figure 5. The Healthy Cell Approximations using a positive and negative reinforcement of the form
collateral damage will increase also. However, let’s now assume the amount of collateral damage will depend on both the level of the initial viral dose which we will call I and the upregulation level







This is not necessarily true, but we want to show how assuming the dependence is not linear allows for 

Hence, we have
Thus, an approximate dynamical model for the healthy cell level 
Now, the sign of the correction is determined by the function






So we can see that various assumptions about the dependencies of the new infection levels and collateral damage levels on 

4. Survival Models
In a survival experiment, a population of mice of size N is all infected with the same amount of virus, and the number of animals that die is determined. The amount of virus used is measured in plaque forming units (pfu). This determines the concentration of infectious virus by virtue of the number of areas of cell death in a cell monolayer in vitro. The experiment is then repeated, using N mice per group for a range of virus concentrations. If we did this for various pfu levels, we could then graph the number of mice that survive versus the virus concentration. Such a survival experiment is expensive to undertake, requiring many mice and hence, rarely done. With most viruses, a traditional survival experiment in which the virus is titrated in this way, gives a classical dose-response curve which progressively and smoothly decays down to a survival of 0 at high virus concentrations. WNV has a peculiar survival curve which was shown in Figure 1. Our simulations show similar results, although we use an artificial parameter in our simulations for the pfu level and in Figure 6. In this simulation, we modeled host infections in 18 groups using initial viral concentrations ranging from 100 to 
Figure 6. Simulated survival curve.
Table 1. Sample raw simulation results.
host fatality in a different way. For a given viral dose, we compute many plots and each plot is terminated at a time, 
Normalized Health; First we calculate 









Hence, we adjust the low value of the plot by extending it over the full 
terminates early. A measure of health is then
the plots for this viral dose to find an average normalized health value.
Survived: If the health value we have calculated, 

Survived 2: We can also calculate another measure of survival. If the lowest health value, 



If the normalized smallest health level, 
Survived 3: The third way we can use to estimate survival is to calculate the time interval that the value 



We show normalized health versus the logarithm of the infection level in Figure 6. In Figure 7, we plot all
Figure 7. The Percentage of hosts surviving.
three different survival measures versus the logarithm of the virus concentration but only for the range of viral dose where there is an increase in survival. We see the characteristic increase in survival with increasing viral load in all three measures as well as in the normalized health plot. We see in both simulation plots, survival sometimes increases with increasing virus concentration, in contrast to the data seen in most other viral infections.
5. Conclusion
We show the decoy hypothesis is a reasonable way to explain collateral damage and the existing survival data measured in WNV infections. In addition to the simulation results, we have also included simple arguments based on approximations that also show we can expect some sorts of oscillatory behavior in the level of healthy cells versus viral load. In Section 2, we let 




from which we could also reason that oscillations in the survival rate could be expected. We then presented simulation results that supported these suggested behaviors. A more general model, [11] , can also be developed where we assume a large population of cells 









Cite this paper
James K. Peterson,Alison M. Kesson,Nicholas J. C. King,1 1, (2016) Using a Collateral Damage Model to Explain Survival Data in West Nile Virus Infections. Advances in Microbiology,06,251-262. doi: 10.4236/aim.2016.64025
References
- 1. Douglas, D.W., Kesson, A.M. and King, N.J.C. (1994) CTL Recognition of West Nile Virus-Infected Fibroblasts Is Cell Cycle Dependent and Is Associated with Virus-Induced Increases in Class I MHC Antigen Expression. Immunology, 82, 561-570.
- 2. Kesson, A.M., Cheng, Y. and King, N.J.C. (2002) Regulation of Immune Recognition Molecules by Flavivirus, West Nile. Viral Immunology, 15, 273-283. http://dx.doi.org/10.1089/08828240260066224
- 3. King, N.J.C., Getts, D.R., Getts, M.T., Rana, S., Shrestha, B. and Kesson, A.M. (2007) Immunopathology of Flavivirus Infections. Immunology & Cell Biology, 85, 33-42.
http://dx.doi.org/10.1038/sj.icb.7100012 - 4. King, N.J.C. and Kesson, A.M. (2003) Interaction of Flaviviruses with Cells of the Vertebrate Host and Decoy of the Immune Response. Immunology & Cell Biology, 81, 207-216.
http://dx.doi.org/10.1046/j.1440-1711.2003.01167.x - 5. King, N.J.C. and Kesson, A.M. (1988) Interferon-Independent Increases in Class I Major Histocompatibility Complex Antigen Expression Follow Flavivirus Infection. The Journal of General Virology, 69, 2535-2543.
http://dx.doi.org/10.1099/0022-1317-69-10-2535 - 6. King, N.J.C., Müllbacher, A., Tian, L., Rodger, J.C., Lidbury, B. and Hla, R.T. (1993) West Nile Virus Infection Induces Susceptibility of in Vitro Outgrown Murine Blastocysts to Specific Lysis by Paternally Directed Allo-Immune and Virus-Immune Cytotoxic T Cells. Journal of Reproductive Immunology, 23, 131-144.
http://dx.doi.org/10.1016/0165-0378(93)90003-Z - 7. King, N.J.K., Davison, A., Getts, D., Lu, D.P., Getts, M., Yeung, A., Peterson, J. and Kesson, A.M. (2008) Enhanced Antigen Processing or Immune Evasion? West Nile Virus and the Induction of Immune Recognition Molecules. In: Diamond, M.S., Ed., West Nile Encephalitis Virus Infection: Viral Pathogenesis and the Host Immune Response, Springer-Verlag, New York, 309-339.
- 8. Peterson, J.K., King, N.J.C. and Kesson, A.M. (2014) Modeling West Nile Virus One Host Infections. Technical Report, Clemson University, Clemson.
- 9. Peterson, J., Kesson, A.M. and King, N.J.C. (2016) A Model of Auto Immune Response. BMC Immunology, 1-40. (Draft Submitted)
- 10. Peterson, J., Kesson, A.M. and King, N.J.C. (2015) A Simulation for Flavivirus Infection Decoy Responses. Advances in Microbiology, 5, 123-142. http://dx.doi.org/10.4236/aim.2015.52013
- 11. Peterson, J., Kesson, A.M. and King, N.J.C. (2016) A Theoretical Model of the West Nile Virus Survival Data. BMC Immunology, 1-34. (Draft Submitted)
- 12. Peterson, J., Kesson, A.M. and King, N.J.C. (2016) Viral Infections and the Central Nervous System Infection Models. 1-40. (Draft)
- 13. Shen, J., T-To, S.S., Schrieber, L. and King, N.J.C. (1997) Early E-Selectin and VCAM-1 and ICAM-1 and Late Major Histocompatibility Complex Antigen Induction on Human Endothelial Cells by Flavivirus and Comodulation of Adhesion Molecule Expression by Immune Cytokines. Journal of Virology, 71, 9323-9332.




























