Journal of Sustainable Bioenergy Systems
Vol.2 No.2(2012), Article ID:19755,8 pages DOI:10.4236/jsbs.2012.22003
Enhanced Succinic Acid Production from Sake Lees Hydrolysate by Dilute Sulfuric Acid Pretreatment and Biotin Supplementation
1Faculty of Bioresource Sciences, Akita Prefectural University, Akita, Japan
2College of Food Science and Light Industry, Nanjing University of Technology, Nanjing, China
3State Key Laboratory of Materials-Oriented Chemical Engineering, College of Biotechnology and Pharmaceutical Engineering, Nanjing University of Technology, Nanjing, China
Email: *zhangh@akita-pu.ac.jp
Received March 9, 2012; revised April 23, 2012; accepted May 7, 2012
Keywords: Succinic Acid; A. succinogenes; Sake Lees Hydrolysate; Pretreatment; Biotin
ABSTRACT
Succinic acid is valued as a potential starting point for the production of chemicals of the C4 family or in the preparation of biodegradable polymers. For sustainable development in this era of petroleum shortage, production of succinic acid by microbial fermentation of renewable feedstock has attracted great interest. In this study, pretreatment with sulfuric acid and biotin supplementation were used to enhance succinic acid production by Actinobacillus succinogenes 130Z from sake lees, a byproduct of Japanese rice wine. Pretreatment with sulfuric acid resulted in little change of glucose, total nitrogen and succinic acid content in the sake lees hydrolysate but had a positive effect on succinic acid fermentation, which caused a 25.0% increase in succinic acid yield in batch fermentation. Biotin supplementation was used to further enhance the fermentability of sake lees hydrolysate. As a result, a 30 h batch fermentation of 0.5% sulfuric acid pretreated sake lees hydrolysate with 0.2 mg/L biotin gave a succinic acid yield of 0.59 g/g from 61.6 g/L of glucose, with a productivity of 1.21 g/(L·h). A 22.9% increase in succinic acid yield and a 101.7% increase in succinic acid productivity were obtained compared with untreated sake lees hydrolysate.
1. Introduction
Succinic acid, an intermediate metabolite of the tricarboxylic acid (TCA) cycle and one of the fermentation end-products of anaerobic metabolism, has been used widely in the agricultural, food and pharmaceutical industries [1]. Recently, succinic acid has been valued because it can be used for the production of biodegradable polymers, such as polybutylene succinate and polyamides, and as an intermediate for synthesis of C4 chemicals, including 1,4-butanediol, tetrahydrofuran, N-methyl pyrrolidinone, 2-pyrrolidinone and gamma-butyrolactone [2-4]. Today, succinic acid is mainly produced by a chemical process from butane through maleic anhydride, a petroleum-based raw material. For sustainable development in this era of petroleum shortages, biological production of succinic acid from renewable feedstock and carbon dioxide has attracted great interest.
A wide variety of bacteria have been investigated to produce succinic acid, such as Mannheimia succiniciproducens [5,6], Actinobacillus succinogenes [7,8], Anaerobiospirillum succiniciproducens [9,10], recombinant Escherichia coli [11,12] and Corynebacterium glutamicum [13]. Among the succinic acid-producing strains, A. succinogenes has been considered to be one of the most promising microorganisms for industrial succinic acid production because it can produce a large amount of succinic acid, tolerate high concentrations of organic acids and use a wide range of carbon sources [14]. The fermentation cost of bio-based succinic acid is, however, a key factor when competing with petroleum-based succinic acid production. Therefore, the utilization of cheap carbon sources rather than glucose is important for the economical production of succinic acid. Recently, many bioresources have been used for succinic acid production by A. succinogenes, including whey [15], cane molasses [16], straw [17], crop stalk wastes [18], and corn cob [19]. However, A. succinogenes is a fastidious organism [20] and high concentrations of complex growth supplements, mainly yeast extract, are required to utilize these renewable resources. The cost of such supplements is not feasible for economical fermentation of succinic acid. Therefore, minimizing their use is also important for industrial succinic acid fermentation.
Sake lees, a byproduct of Japanese rice wine (sake), can serve as an attractive, low-cost feedstock for producing bio-based chemicals because they contain significant quantities of carbohydrate, protein, vitamins and other nutrients [21]. A previous study showed sake lees hydrolysate could be used not only as a carbon source for efficient production of succinic acid but also to partially replace nitrogen source supplementation [22]. However, the components in sake lees could influence the metabolism of A. succinogenes 130Z, causing a rather low succinic acid yield, while a low yeast extract supplementation also led to a rather low succinic acid productivity. In this study, dilute sulfuric acid pretreatment, combined with biotin supplementation, was used to enhance succinic acid production from sake lees hydrolysate by A. succinogenes 130Z.
2. Materials and Methods
2.1. Chemicals
Yeast extract was obtained from Oxoid Ltd (Cambridge, UK). Other chemicals were of reagent grade and obtained from Wako (Tokyo, Japan). CO2 gas was obtained from Akisan Co. (Akita, Japan).
2.2. Preparation of Sake Lees Hydrolysate
Sake lees were obtained from Akita Shurui Seizoh Co., Ltd. (Akita, Japan). The crude sake lees contained 39.2% (w/w) water, 12.0% (w/w) protein, 5.0% (w/w) succinic acid, 14.8% (w/w) glucose, and the residual composition was starch, fat, ethanol, etc. The conditions used for preparation of sake lees hydrolysate were the same as previously reported [22] and proceeded as follows: the crude sake lees were diluted with distilled water to obtain a concentration of 25% (w/w, on wet basis). For liquefaction treatment, sake lees slurry was adjusted to pH 6.0 with 3% (w/v) NaOH, 20 U amylase (Wako, Japan) was added per gram wet sake lees and the slurry kept at 70˚C for 4 h with a water bath. At the end of liquefaction, the slurry was adjusted to pH 4.5 with 5% (w/v) HCl and the temperature was decreased to 58˚C. 200 U glucoamylase (Amano, Japan) was added per gram wet sake lees for saccharification over 5 h. After centrifugation at 6000 rpm for 15 min, the supernatant was designated as sake lees hydrolysate (SLH).
2.3. Pretreatment of SLH
Pretreatment of SLH was carried out under alkaline, oxidative and acidic conditions. The alkaline condition was obtained with 0.5% (w/v) Ca(OH)2, the oxidative condition with 0.5% (w/v) H2O2, and the acidic conditions with 1.0% (w/v) HCl, and 0.5% and 1.0% (w/v) H2SO4. All pretreatments were carried out at 60˚C for 90 min in a water bath. After pretreatment, SLH was centrifuged at 20˚C and 6000 rpm for 15 min. The supernatants from pretreatments with Ca(OH)2 and H2O2 were adjusted to pH 5.0 with 98% (w/v) H2SO4, while the supernatants from pretreatments with acids were adjusted to pH 5.0 with Mg(OH)2. After pH adjustment, SLHs from the different pretreatments were used as substrates. SLH from the pretreatment with 0.5% H2SO4 was designated as PSLH.
2.4. Microorganism and Growth Conditions
A. succinogenes 130Z (ATCC55618) was obtained from the American Type Culture Collection. Cells were grown in 100 mL sealed anaerobic bottles containing 50 mL medium. The medium for inoculum cultures was composed of (per liter): 20 g glucose, 5.0 g yeast extract, 2.5 g corn steep liquid (dry power), 2.0 g NaH2PO4·H2O, 3.0 g K2HPO4 and 20 g MgCO3. The medium was heat sterilized at 121˚C for 15 min. The anaerobic bottles were inoculated with 1 mL of a –80˚C glycerol stock culture and incubated at 37˚C.
For anaerobic bottle fermentation, exponentially growing cells were inoculated into 100 mL sealed anaerobic bottles containing 30 mL of the RM medium, which contained (per liter): 2.5 g yeast extract and a salt mixture including 3.0 g K2HPO4, 2.0 g NaH2PO4·H2O, 0.2 g MgCl2·6H2O and 1.0 g NaCl. The pH of the medium was maintained by addition of 60 g/L of MgCO3 at 6.0. Separately autoclaved SLH was added aseptically to the medium to make up the desired glucose concentration and was designated as the SLH-based medium. The medium was sparged with aseptic carbon dioxide in the anaerobic bottle for 1 min after inoculation to create anaerobic conditions. Anaerobic bottle fermentation was carried out in a rotary shaker at 37˚C and 120 rpm.
Batch fermentation was carried out in a 3 L fermenter (Mitsubishi, Japan) with an initial broth volume of 1.5 L. All fermentations were performed at 37˚C at an agitation speed of 200 rpm and a CO2 flow rate of 0.5 L/min. The pH was set at 6.8 and maintained by addition of 2 mol/L Na2CO3. All batch fermentation results were calibrated for alkali neutralization.
2.5. Analytical Methods
The total nitrogen (TN) content in samples was measured by the Kjeldahl method (AOAC, 1999) [23], using a protein analyzer (2300 Kjeltec Analyzer Unit, ACTAC, Japan). Glucose and organic acids were analyzed by highperformance liquid chromatography (Class-VP server monitor, LC-10AD pump, Shimadzu, Japan). For determining glucose, a refractive index detector, RID-10A, and an ion exchange chromatographic column (Shimpack SPR-Na 7.8 mm × 250 mm, Shimadzu, Japan) were used. The mobile phase was water at a flow rate of 0.6 mL/min at 80˚C. To determine the organic acids, a UV detector, SPD-M 10A, and an ion exchange chromatographic column (Shim-pack SPR-H 7.8 mm × 250 mm, Shimadzu, Japan) were used. The mobile phase was 5 mmol/L perchloric acid at a flow rate of 0.6 mL/min at 50˚C.
The cell growth was represented by dry cell weight (DCW) and was computed from a curve relating optical density at 660 nm (OD660) to dry weight. An OD660 of 1.0 represented 542 mg dry weight per liter when using the SLH-based medium and 645 mg dry weight per liter when using the glucose-based medium.
The yield of products, including succinic acid and acetic acid, was defined as the amount of the final organic acid minus the initial organic acid from 1 g glucose consumption, expressed as g/g. Succinic acid productivity was calculated as the final succinic acid minus the initial succinic acid in g/L of broth divided by the fermentation time (the time at which fermentation was stopped). Glucose consumption rate was defined as the glucose consumed in g/L of broth divided by the fermentation time.
3. Results and Discussion
3.1. Effect of Pretreatment on SLH
The effects of different pretreatment methods on the main composition of SLH are shown in Figure 1. It can be seen that after liquefaction and saccharification treatments of sake lees, SLH contained 79.2 g/L glucose, 17.2 g/L succinic acid and 4.0 g/L TN. As shown in Figure 1, pretreatment with 0.5% Ca(OH)2 caused a 10.1% decrease in glucose concentration, pretreatment with 0.5% H2O2 led to a slight decrease (3.9%) in glucose concentration, while pretreatment with acids had little influence on glucose concentration. In addition, all of the pretreatment methods led to a similar decrease in TN concentration, ranging from 11.4 to 15.2%. Fortunately, the succinic acid concentration in SLH was only slightly influenced by the pretreatment methods. Therefore, pretreatment under acidic conditions had a rather small affect on the composition of SLH.
3.2. Succinic Acid Production from SLH with Different Pretreatments in Anaerobic Bottle Fermentation
Use of SLH obtained from different pretreatment methods for succinic acid production was investigated in anaerobic bottle fermentation. As shown in Table 1, when
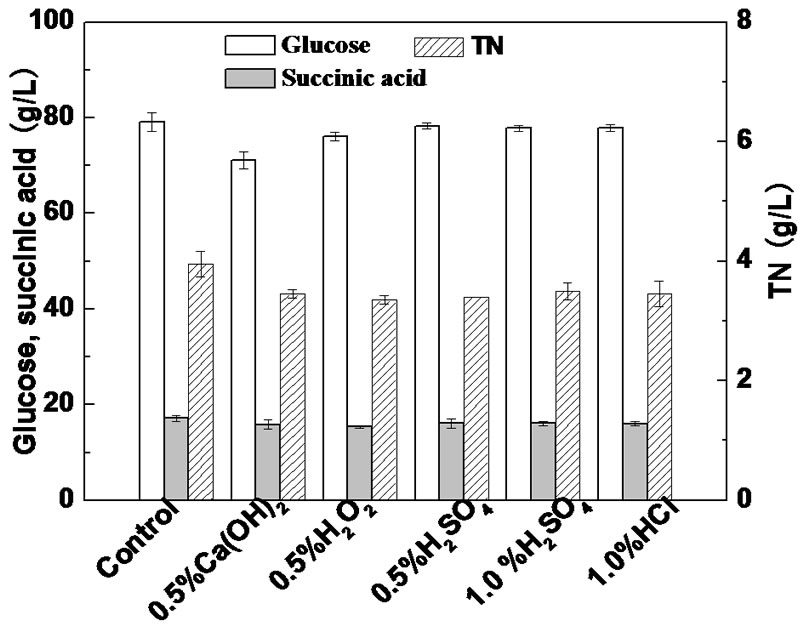
Figure 1. Effect of different pretreatment methods on the composition of SLH. The error bars in the figure indicate the standard deviations among three parallel replicates.
SLH with 0.5% H2O2 pretreatment was used, cell growth and succinic acid production were severely inhibited, while use of 0.5% Ca(OH)2 pretreatment had no significant influence on succinic acid fermentation. Among the pretreatment methods, H2SO4 had a positive influence on succinic acid fermentation. Compared with the control, using SLH with 0.5% H2SO4 pretreatment (PSLH) caused 14.2 and 20.0% increases in glucose consumption rate and succinic acid yield, respectively. Nevertheless, it is surprising that SLH with HCl pretreatment showed distinct differences from SLH with H2SO4 pretreatment, leading to poor cell growth and succinic acid production. Furthermore, increasing the H2SO4 concentration from 0.5% to 1% for SLH pretreatment had no significant influence on succinic acid fermentation. It has been reported that using sulfuric acid treatment can improve the fermentability of cane molasses for succinic acid production by A. succinogenes [16]. In this study, sulfuric acid was also successfully used for SLH pretreatment to enhance succinic acid production.
3.3. Batch Fermentation with PSLH and SLH
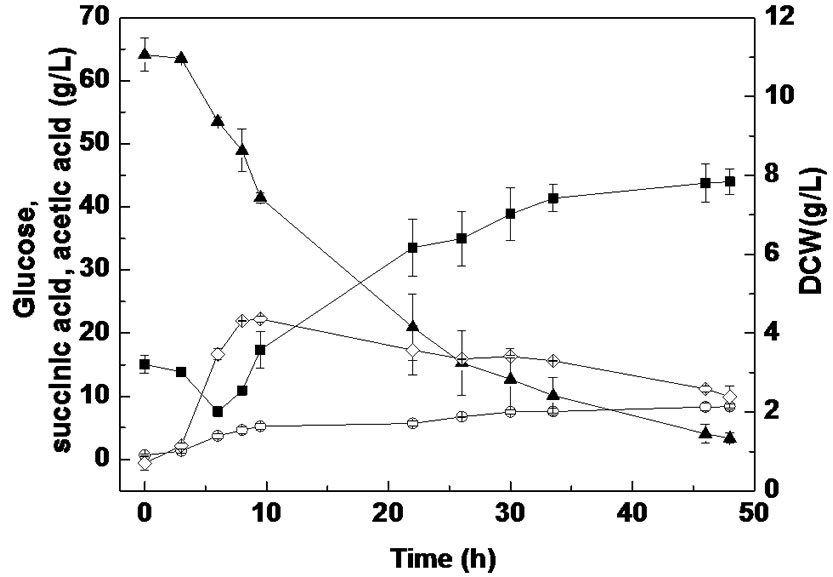
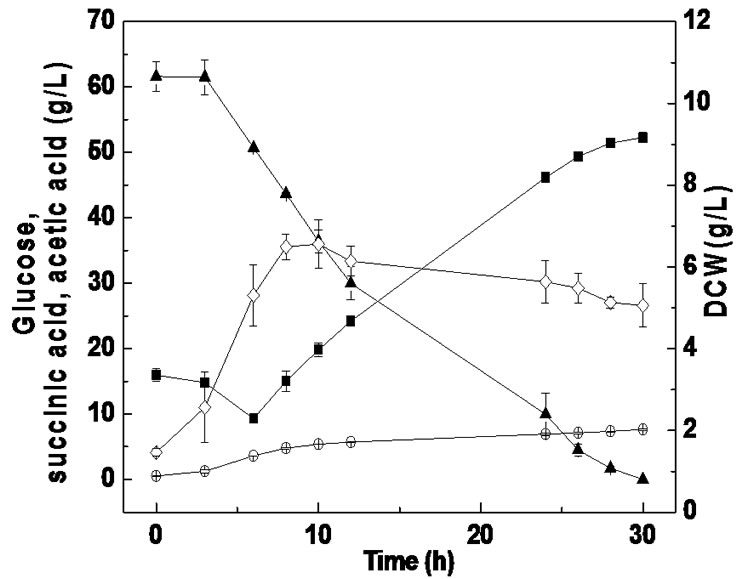
Batch fermentation was carried out to compare succinic acid fermentation with PSLH and SLH. As shown in Figure 2, when SLH was used as a substrate, a cell growth of 4.31 g/L was achieved at 8 h, which then linearly decreased to 2.38 g/L by the end of fermentation. However, when using PSLH as a substrate, cell growth remained above 4.64 g/L from 8 h to the final stage of fermentation. Therefore, it appears that the inhibition effect on cell growth using PSLH was decreased by sulfuric acid pretreatment. Nevertheless, the glucose consumption rates using SLH and PSLH were similar, which differed from anaerobic bottle fermentation. This might be due to the different culture conditions between anaerobic
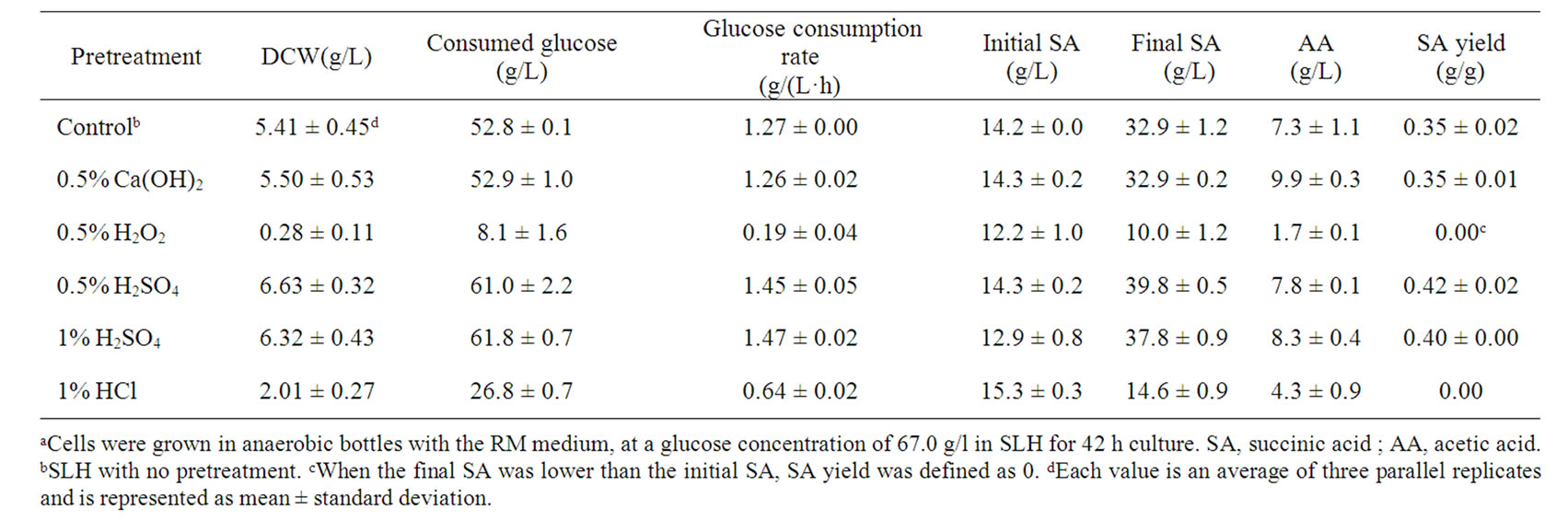
Table 1. Succinic acid production using SLH obtained from different pretreatment methodsa.
 (a)
(a)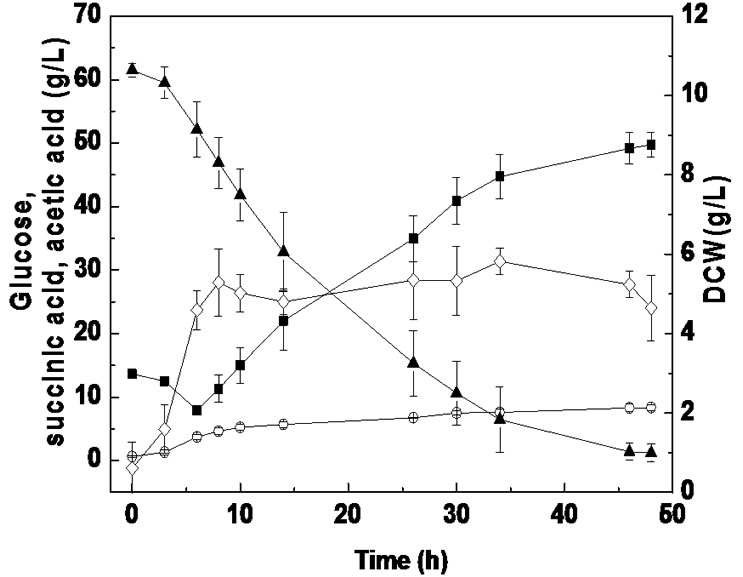 (b)
(b)
Figure 2. Batch fermentation from SLH (a) and PSLH (b) in the RM medium for 48 h culture (■, succinic acid; ○, acetic acid; ▲, glucose; ◇, dry cell weight). Values reported are averages from duplicate experiments.
bottle fermentation and batch fermentation. We suggest that one of reasons for this might be the difference in pH adjustment, where Na2CO3 was used in batch fermentation and MgCO3 in anaerobic bottle fermentation. It has been reported that sodium ion concentration markedly affects cell growth and succinic acid production by A. succinogenes, while magnesium ions barely have any effect [24].
Pretreatment of molasses with sulfuric acid was concluded to remove some metal ions [25]. However, many bioactive components were also present in sake lees besides metal ions. Previous studies have shown that some of the peptides in sake lees can inhibit tyrosinase activity and inhibit the catalytic activity of the angiotensin I-converting enzyme [26,27]. As shown in Figure 2, some components in sake lees can also influence the metabolism of A. succinogenes, which may cause the utilization of succinic acid as a substrate at the beginning of fermentation. The lowest succinic acid concentrations for SLH and PSLH fermentation were both obtained at 6 h. Furthermore, when SLH was used as a substrate, 10.6 g/L glucose and 7.5 g/L succinic acid were consumed within 6 h, whereas 9.3 g/L glucose and 5.2 g/L succinic acid were consumed with PLSH. It seems that the loss of glucose and succinic acid at the beginning of fermentation was decreased by sulfuric acid pretreatment. Thus, the mechanism of SLH pretreatment with sulfuric acid may be related to an influence on bioactive components in SLH.
As a result, when PSLH was used as a substrate in batch fermentation, a succinic acid yield of 0.60 g/g was achieved with consumption of 60.3 g/L glucose, which was 25.0% higher than with SLH. In addition, the accumulation of the main byproduct, acetic acid, was not influenced by the sulfuric acid pretreatment, showing a similar acetic acid yield of 0.14 g/g between PSLH and SLH.
3.4. Succinic Acid Production from PSLH with Biotin Supplementation
Production yield and productivity are two important process parameters that determine the economical viability of a bioprocess [14]. Although sulfuric acid pretreatment could improve succinic acid yield, glucose consumption rate is also important for industrial succinic acid fermentation and is directly related to succinic acid productivity. A previous study showed that biotin could enhance glucose consumption by A. succiniciproducens in a glucose-based medium [28] and improve the fermentability of spent yeast cell hydrolysate for succinic acid production by A. succinogenes [29]. To evaluate the effects of biotin on succinic acid production from PSLH by A. succinogenes, anaerobic bottle fermentation of PSLH supplemented with 0 - 1.0 mg/L biotin was carried out (Figure 3). It can be seen that when PSLH was supplemented with 0.2 mg/L biotin during a 24 h culture, the succinic acid concentration increased 25.0%, cell growth increased 34.4%, and glucose consumption was enhanced 28.6%. Further increasing biotin concentration from 0.2 to 1.0 mg/L had no significant influence on succinic acid fermentation from PSLH. Therefore, supplementation with 0.2 mg/L of biotin could be used to improve glucose consumption by A. succinogenes in the SLH-based medium.
Batch fermentation from PSLH supplemented with 0.2 mg/L of biotin was subsequently carried out in the RM medium, and succinic acid production from glucose supplemented with 10 g/L of YE and a salt mixture were also carried out for comparison (Figure 4). As shown in Figures 2(b) and 4(a) and Table 2, the cell growth for PSLH supplemented with 0.2 mg/L biotin was slight enhanced, the glucose consumption rate increased by 64.0% and succinic acid productivity was enhanced by 61.3%, compared with PSLH alone. However, biotin supplementation had no significant influence on succinic acid yield. These results are in agreement with those of a previous study that addition of 50 mg/L biotin during succinic acid fermentation by A. succiniciproducens caused a 16% increase in glucose utilization and succinic acid productivity but had little influence on the succinic acid yield [28]. Thus, the function of biotin in succinic acid fermentation by A. succiniciproducens and A. succinogenes is apparently similar. The function of biotin in cell metabolism is involved in fatty acid synthesis, amino acid catabolism, gluconeogenesis, and is also related to gene expression [30]. Unfortunately, we cannot further elucidate the role of biotin in succinic acid fermentation in this study.
When PSLH supplemented with 0.2 mg/L of biotin was used in batch fermentation during a 30 h culture, a succinic acid concentration of 52.3 g/L resulted from 61.6 g/L
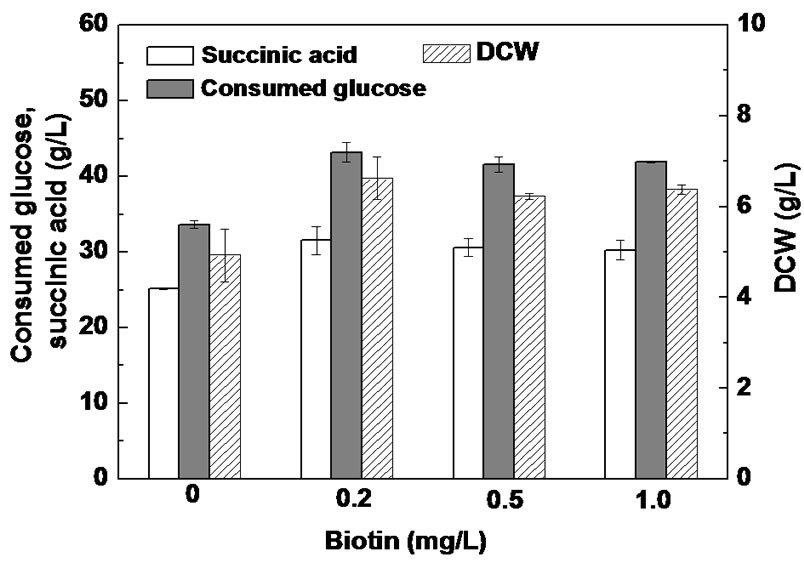
Figure 3. Effect of biotin supplementation on succinic acid production from PSLH. Cells were grown in anaerobic bottles with EHSL-based medium at a glucose concentration of 67.0 g/l for a 24 h culture. The error bars in the figure indicate the standard deviations among three parallel replicates.
 (a)
(a)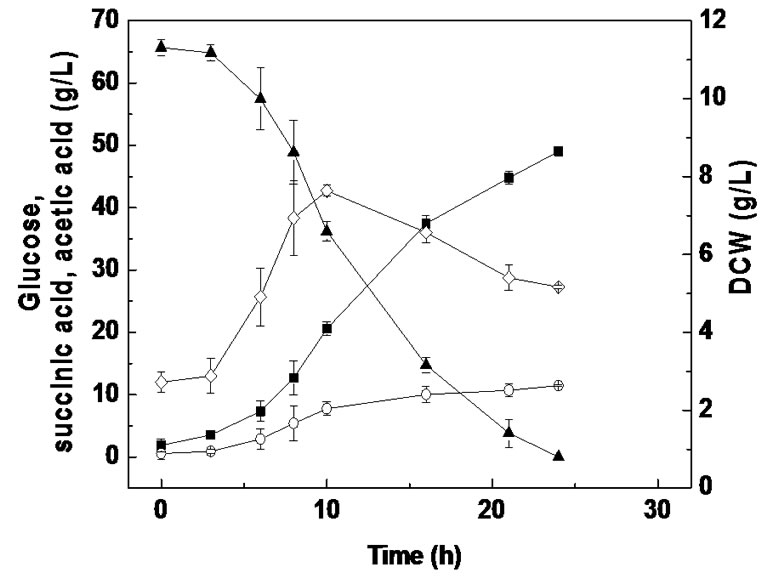 (b)
(b)
Figure 4. Batch fermentation from PSLH supplemented with 0.2 mg/L biotin for a 30 h culture in the RM medium (a), and glucose with 10 g/L YE and a salt mixture for a 24 h culture (b) (■, succinic acid; ○, acetic acid; ▲, glucose; ◇, dry cell weight). Values reported are averages from duplicate experiments.
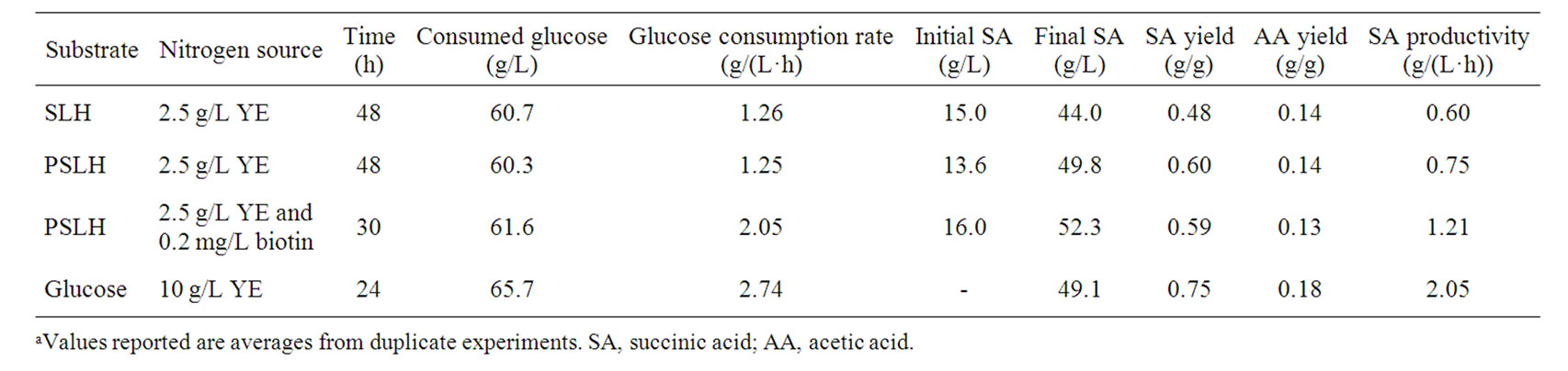
Table 2. Comparisons of succinic acid fermentation from PSLH, SLH and glucose in a 3 L fermentora.
of glucose, with a yield of 0.59 g/g and a productivity of 1.21 g/(L·h). These were a 22.9% increase in succinic acid yield and a 101.7% increase in succinic acid productivity compared with SLH. Neglecting succinic acid present at the outset of fermentation (contained in SLH), the succinic acid yield and productivity using PSLH with 0.2 mg/L of biotin supplementation was 0.85 g/g and 1.74 g/(L·h). Therefore, there was a 13.3% increase in succinic acid yield and a 15.1% decrease in succinic acid productivity compared with glucose containing 10 g/L YE and salts as the culture medium (Figure 4(b) and Table 2). Therefore, the results obtained by using PSLH with biotin supplementation and 2.5 g/L YE were comparable with using glucose and 10 g/L YE.
4. Conclusion
A method for enhancing succinic acid production from sake lees hydrolysate was developed in this study. Pretreatment of sake lees hydrolysate with sulfuric acid was an effective method for enhancing succinic acid yield, while biotin supplementation could further increase succinic acid productivity. Thus, combined pretreatment with sulfuric acid and biotin supplementation gave a succinic acid concentration of 52.3 g/L, with consumption of 61.6 g/L glucose in PSLH during a 30 h batch fermentation. The results suggest that dilute sulfuric acid pretreatment combined with biotin supplementation can improve the fermentability of sake lees hydrolysate, as an alternative substrate for the efficient production of succinic acid by A. succinogenes.
REFERENCES
- J. G. Zeikus, M. K. Jain and P. Elankovan, “Biotechnology of Succinic Acid Production and Markets for Derived Industrial Products,” Applied Microbiology and Biotechnology, Vol. 51, No. 5, 1999, pp. 545-552. doi:10.1007/s002530051431
- I. Bechthold, K. Bretz, S. Kabasci and R. Kopitzky, “Succinic Acid: A New Platform Chemical for Biobased Polymers from Renewable Resources,” Chemical Engineering & Technology, Vol. 31, No. 5, 2008, pp. 647-654. doi:10.1002/ceat.200800063
- J. B. McKinlay, C. Vieille and J. G. Zeikus, “Prospects for a Bio-Based Succinate Industry,” Applied Microbiology and Biotechnology, Vol. 76, No. 4, 2007, pp. 727-740.
- H. Song and S. Y. Lee, “Production of Succinic Acid by Bacterial Fermentation,” Enzyme and Microbial Technology, Vol. 39, No. 3, 2006, pp. 352-361. doi:10.1016/j.enzmictec.2005.11.043
- P. C. Lee, S. Y. Lee, S. H. Hong and H. N. Chang, “Isolation and Characterization of a New Succinic Acid-Producing Bacterium, Mannheimia Succiniciproducens MBEL55E, from Bovine Rumen,” Applied Microbiology and Biotechnology, Vol. 58, No. 5, 2002, pp. 663-668. doi:10.1007/s00253-002-0935-6
- H. Song, T.Y. Kim, B. Choi, S. J. Choi, L. K. Nielsen, H. N. Chang and S. Y. Lee, “Development of Chemically Defined Medium for Mannheimia Succiniciproducens Based on Its Genome Sequence,” Applied Microbiology and Biotechnology, Vol. 79, No. 2, 2008, pp. 263-272. doi:10.1007/s00253-008-1425-2
- M. V. Guettler, D. Rumler and M. K. Jain, “Actinobacillus succinogenes sp. nov., a Novel Succinic-Acid-Producing Strain from the Bovine Rumen,” International Journal of Systematic and Evolutionary Microbiology, Vol. 49, No. 1, 1999, pp. 207-216. doi:10.1099/00207713-49-1-207
- M. J. Van Der Werf, M. V. Guettler, M. K. Jain and J. G. Zeikus, “Environmental and Physiological Factors Affecting the Succinate Product Ratio during Carbohydrate Fermentation by Actinobacillus sp. 130Z,” Archives of Microbiology, Vol. 167, No. 6, 1997, pp. 332-342. doi:10.1007/s002030050452
- P. C. Lee, S. Y. Lee, S. H. Hong, H. N. Chang and S. C. Park, “Biological Conversion of Wood Hydrolysate to Succinic Acid by Anaerobiospirillum succiniciproducens,” Biotechnology Letters, Vol. 25, No. 2, 2003, pp. 111-114. doi:10.1023/A:1021907116361
- I. Meynial-Salles, S. Dorotyn and P. Soucaille, “A New Process for the Continuous Production of Succinic Acid from Glucose at High Yield, Titer, and Productivity,” Biotechnology and Bioengineering, Vol. 99, No. 1, 2008, pp. 129-135. doi:10.1002/bit.21521
- D. B. Hodge, C. Andersson, K. A. Berglund and U. Rova, “Detoxification Requirements for Bioconversion of Softwood Dilute Acid Hydrolyzates to Succinic Acid,” Enzyme and Microbial Technology, Vol. 44, No. 5, 2009, pp. 309-316. doi:10.1016/j.enzmictec.2008.11.007
- M. Jiang, S. W. Liu, J. F. Ma, K. Q. Chen, L. Yu, F. F. Yue, B. Xu and P. Wei, “Effect of Growth Phase Feeding Strategies on Succinate Production by Metabolically Engineered Escherichia coli,” Applied and Environmental Microbiology, Vol. 76, No. 4, 2010, pp. 1298-1300. doi:10.1128/AEM.02190-09
- Y. Saito, K. Wanezaki, A. Kawato and S. Imayasu, “Structure and Activity of Angiotensin I Converting Enzyme Inhibitory Peptides from Sake and Sake Lees,” Bioscience, Biotechnology, and Biochemistry, Vol. 58, No. 10, 1994, pp. 1767-1771. doi:10.1271/bbb.58.1767
- J. J. Beauprez, M. De Mey and W. K. Soetaert, “Microbial Succinic Acid Production: Natural Versus Metabolic Engineered Producers,” Process Biochemistry, Vol. 45, No. 6, 2010, pp. 1103-1114. doi:10.1016/j.procbio.2010.03.035
- C. X. Wan, Y. B. Li, A. Shahbazi and S. N. Xiu, “Succinic Acid Production from Cheese Whey Using Actinobacillus succinogenes 130 Z,” Applied Biochemistry and Biotechnology, Vol. 145, No. 1-3, 2008, pp. 111-119. doi:10.1007/s12010-007-8031-0
- Y. P. Liu, P. Zheng, Z. H. Sun, Y. Ni, J. J. Dong and L. L. Zhu, “Economical Succinic Acid Production from Cane Molasses by Actinobacillus succinogenes,” Bioresource Technology, Vol. 99, No. 6, 2008, pp. 1736-1742. doi:10.1016/j.biortech.2007.03.044
- P. Zheng, J. J. Dong, Z. H. Sun, Y. Ni and L. Fang, “Fermentative Production of Succinic Acid from Straw Hydrolysate by Actinobacillus succinogenes,” Bioresource Technology, Vol. 100, No. 8, 2009, pp. 2425-2429. doi:10.1016/j.biortech.2008.11.043
- Q. Li, M. H. Yang, D. Wang, W. L. Li, Y. Wu, Y. J. Zhang, J. M. Xing and Z. G. Su, “Efficient Conversion of Crop Stalk Wastes into Succinic Acid Production by Actinobacillus succinogenes,” Bioresource Technology, Vol. 101, No. 9, 2010, pp. 3292-3295. doi:10.1016/j.biortech.2009.12.064
- J. Yu, Z. M. Li, Q. Ye, Y. Yang and S. L. Chen, “Development of Succinic Acid Production from Corncob Hydrolysate by Actinobacillus succinogenes,” Journal of Industrial Microbiology and Biotechnology, Vol. 37, No. 10, 2010, pp. 1033-1040. doi:10.1007/s10295-010-0750-5
- J. B. McKinlay, J. G. Zeikus and C. Vieille, “Insights into Actinobacillus succinogenes Fermentative Metabolism in a Chemically Defined Growth Medium,” Applied and Environmental Microbiology, Vol. 71, No. 11, 2005, pp. 6651-6656. doi:10.1128/AEM.71.11.6651-6656.2005
- N. Tsutsui, Y. Yamamoto and K. Iwami, “Protein-Nutritive Assessment of Sake Lees Obtained by Brewing from Liquefied Rice,” Journal of Nutritional Science and Vitaminology, Vol. 4, No. 1, 1998, pp. 177-186. doi:10.3177/jnsv.44.177
- K. Q. Chen, H. Zhang, Y. L. Miao, M. Jiang and J. Y. Chen, “Succinic Acid Production from Enzymatic Hydrolysate of Sake Lees Using Actinobacillus succinogenes 130Z,” Enzyme and Microbial Technology, Vol. 47, No. 5, 2010, pp. 236-240. doi:10.1016/j.enzmictec.2010.06.011
- AOAC International, “Official Methods of Analysis Method 988.05,” Gaithersburg, 1999.
- Y. P. Liu, P. Zheng, Z. H. Sun, Y. Ni, J. J. Dong and P. Wei, “Strategies of pH Control and Glucose-Fed Batch Fermentation for Production of Succinic Acid by Actinobacillus succinogenes CGMCC1593,” Journal of Chemical Technology & Biotechnology, Vol. 83, No. 5, 2008, pp. 722-729. doi:10.1002/jctb.1862
- T. Roukas, “Pretreatment of Beet Molasses to Increase Pullulan Production,” Process Biochemistry, Vol. 33, No. 8, 1998, pp. 805-810. doi:10.1016/S0032-9592(98)00048-X
- H. J. Jeon, M. Noda, M. Maruyama, Y. Matoba, T. Kumagai and M. Sugiyama, “Identification and Kinetic Study of Tyrosinase Inhibitors Found in Sake Lees,” Journal of Agricultural and Food Chemistry, Vol. 54, No. 26, pp. 9827-9833.
- S. Okino, R. Noburyu, M. Suda, T. Jojima, M. Inui and H. Yukawa, “An Efficient Succinic Acid Production Process in a Metabolically Engineered Corynebacterium glutamicum Strain,” Applied Microbiology and Biotechnology, Vol. 81, No. 3, 2008, pp. 459-464. doi:10.1007/s00253-008-1668-y
- N. P. Nghiem, B. H. Davison, J. E. Thompson, B. E. Suttle and G. R. Richardson, “The Effect of Biotin on the Production of Succinic Acid by Anaerobiospirillum succiniciproducens,” Applied Biochemistry and Biotechnology, Vol. 57-58, No. 1, 1996, pp. 633-638. doi:10.1007/BF02941744
- K. Q. Chen, J. Li, J. F. Ma, M. Jiang, P. Wei, Z. M. Liu and H. J. Ying, “Succinic Acid Production by Actinobacillus succinogenes Using Hydrolysates of Spent Yeast Cells and Corn Fiber,” Bioresource Technology, Vol. 160, No. 1, 2010, pp. 244-254.
- R. J. McMahon, “Biotin in Metabolism and Molecular Biology,” Annual Review of Nutrition, Vol. 22, No. 4, 2002, pp. 221-239. doi:10.1146/annurev.nutr.22.121101.112819
NOTES
*Corresponding author.

