American Journal of Analytical Chemistry
Vol.07 No.05(2016), Article ID:66272,13 pages
10.4236/ajac.2016.75039
Better Refined Adsorption Isotherm than BET Equation
Daekyoum Kim
Kwongmyoung-si Neobudae-ro, 32 Beon-kil, South Korea

Copyright © 2016 by author and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 9 March 2016; accepted 3 May 2016; published 6 May 2016
ABSTRACT
During studying the heat capacity of metals and brightening more than the original
Keywords:
Refined BET, Binomial, Transforming, Adsorption Increasing Term, Surface Area, Inflection Point

1. Introduction
We derived the heat capacity equations of metals and then used consistent step multiplication of the appropriate binomial equations [1] . They are fitted to experimental data well [2] . The heat capacity equation (type V) and the adsorption equation (type II) draw sigmoid (S character) lines all together. And they are symmetrical with each other. The measurement gases of heat capacity are hydrogen and helium. The adsorption gases are vapor and nitrogen. The movements of their measurement gases are different. The formers are expansion and the later contraction.
The most important term in the derivation of heat capacity equation was the temperature increasing term, . In case of adsorption it becomes
. In case of adsorption it becomes . Here
. Here  and
and 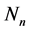 are the total molecules adsorbed at the layers of
are the total molecules adsorbed at the layers of  and
and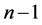 . Let us put
. Let us put  which is unit in data fitting as the constant. The constant affects the adsorption equation from starting to ending like other constants. If
which is unit in data fitting as the constant. The constant affects the adsorption equation from starting to ending like other constants. If 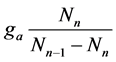 is transformed into
is transformed into 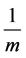 power of
power of 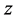 in every derivation of adsorption such as
in every derivation of adsorption such as 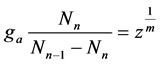 [3] , the more advanced adsorption equation than BET eq. comes out. Hence we get the surface area.
[3] , the more advanced adsorption equation than BET eq. comes out. Hence we get the surface area.
2. Statistical Modeling of Adsorption Isotherm
Suppose each layer has one binomial equation. And suppose  molecules are adsorbed on
molecules are adsorbed on  localized sites of the unit surface layers of the adsorbent and
localized sites of the unit surface layers of the adsorbent and 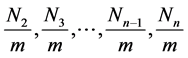 molecules on
molecules on 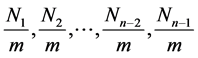 sites made of 1st to
sites made of 1st to 














Next the adsorption probability of from 






Let us multiply Equation (1) and Equation (2) side by side. Then for

In the above 
In Equation (3) the largest term dominates the equation. So the total differential of Equation (3) becomes the zero which requires that the coefficients of all terms should be zero. Hence by using


In [4] 







It is possible that 
Figure 1. Bonding constant and non-bonding constant in statistic quantization.
The next equations are

In Equations (4) and (5) let us put


In Equation (6) add side by side and rearrange

In Equation (6) multiple side by side and rearrange

We solve Equation (8) with 

We solve Equation (7) with Equation (9) in order to eliminate

Equation (10) represents the adsorption amount of the first layer. It is Langmuir equation. The quantization values (








value of 




Equation (11) is the same as Equation (10) obtained by using chemical potential in [1] . Therefore the total adsorption amount per unit surface (


In the above

And the total adsorption rate is a linear function of 






In the above

If the measurement gas is nitrogen, the general empirical formulae, 



cular weight of nitrogen and 




3. Result and Discussion
The base of Equations ((10) and (12)) is










Figure 2. Theoretical adsorption isotherm curve, Equation (12) (m = 0.7, n = 4, ga = 0.5) with respect to βa values.
Figure 3. Theoretical adsorption isotherm curve, Equation (12) (n = 4, βa = 0.00901, ga = 1.0) with respect to m values.
Less 









Figure 4. Theoretical adsorption isotherm curves, Equation (12) (βa = 0.009, ga = 0.5, m = 0.7) with respect to n values.
Figure 5. Theoretical adsorption isotherm curve, Equation (12) (m = 1.7, n = 8, βa = 0.009) with respect to ga values.
Figure 6. Theoretical adsorption isotherm of the first layer using Equation (10) including experimental constants of Figure 7 and Figure 8.
Figure 7. Theoretical adsorption isotherm, Equation (12) (m = 0.9, n = 3.28, βa = 0.000011, ga = 1.0) with BET equation (c = 150) and experimental nitrogen adsorption at −196˚ on non-porous samples of silica and aluminna [6] .
experimental data are fitted to Equation (12) well. As we see in Figure 7 and Figure 8, BET isotherms there can’t imitate the experimental data except for beginning.
Equation (13) can be used in the data fitting with 

What is the catalyst? As we see Figure 9, 1, 2, 3, 4, 5, 6 and 7 molecules can function as the catalyst. That is, the molecules of the surface adsorption layer can’t function like the catalyst. Because they use much
Figure 8. Theoretical adsorption isotherm curve, Equation (12) (m = 2.4, n = 4.5, βa = 0.12, ga = 1.0) with BET equation (c = 130) curve and experimental water adsorption at 25˚ on cross-linked polystyrene sulfuric acid resin [7] .
Figure 9. Real adsorption molecules to get the surface area (numbered rings).
energy in order to hold the surface. So they are not active. The molecules which lie on the surface layer adsorption molecules which hold the surface, can function as the real catalyst. Therefore in Equation (12)


If this equation is integrated from the inflection point (



Table 1. 

Table 2. 

the surface area for the catalyst obtained from the equation becomes 


tituting


and 



Then the inflection points are obtained by Secant method [8] through the program showed in Appendix 1. Specific surface areas are changed according to the relative pressures. These are showed in Table 1 and Table 2 precisely. The integrations with respect to z values give the total adsorption site numbers of the adsorbate. Before the inflection point the specific surface area of the adsorbent is not counted as a catalyst since it makes the strong surface film [9] . The adsorption rate increases consistently after the inflection point. The values of 



We have felt intimately that the adsorption molecules of more than 2nd layers lie down the first layer molecules softly since the small quantity of adsorption molecules control the drawing and the surface area of numerical number.
Our study have realized the saying that “After considerable work on the theory, Hill (1946) formed the opinion that any future improvement on it must be in the form of refinement rather than a modification on the basic theory” [11] [12] .
4. Conclusion
The total adsorption rate equations closely related with the past references are derived correctly and the figures according to four constants (
Acknowledgements
The author thanks for the encouragements of Yongduk Kim, an emeritus professor of Sogang University.
Cite this paper
Daekyoum Kim, (2016) Better Refined Adsorption Isotherm than BET Equation. American Journal of Analytical Chemistry,07,421-433. doi: 10.4236/ajac.2016.75039
References
- 1. Reif, F. (1985) Fundamentals of Statistical and Thermal Physics. McGraw-Hill Inc Ed. 1965, Chapter 1, 10-46.
- 2. Kim, D., Lee, Y. and Kim, T.-W. (2010) Article Title. New Physics: Sae Mulli (The Korean Physical Society), 60, 729-740.
- 3. Mathews, J.H. and Howell, R.W. (2001) Complex Analysis for Mathematics and Engineering. 4th Edition, Jones and Bartlett Publishers, Inc., Section 2-3, 62-69.
- 4. Kim, D. (2000) Statistical Condensation Adsorption Isotherms of Gas Molecules Adsorbed on Porous Adsorbents, Surface Monolayer Adsorption Isotherms and Hysteresis Phenomena. Korean Journal of Chemical Engineering, 17, 600-612.
http://dx.doi.org/10.1007/BF02707174 - 5. Kim, D. and Choi, Y.S. (2011) Brightness of Lena’s Image for Various γ Values by Using Differentials of the Geometric Mean Heat Capacity Equations at Constant Volume. New Physics: Sae Mulli (The Korean Physical Society), 61, 248-255.
http://dx.doi.org/10.3938/NPSM.61.248 - 6. Gregg, S.J. and Sing, K.S.W. (1969) Adorption, Surface Area and Porosity. Academic Press Inc. (Ltd.), Chapter 2, 53, 77.
- 7. Sundheim, B.R., Waxman, M.H. and Gregor, H.P. (1953) J Phys Chem., 57, 974-978.
- 8. Ji, Y., Kim, H. and Heo, J. (2002) C-Roguhyouhan Numerical Analysis (Korean Language). Nopigipi Publishing Company, 24-27.
- 9. Jura, G. and Harkins, W.D. (1944) Surfaces of Solids. XI. Determination of the Decrease (π) of Free Surface Energy of a Solid by an Adsorbed Film. Journal of the American Chemical Society, 67, 1356-1362.
http://dx.doi.org/10.1021/ja01236a046 - 10. Brunauer, S., Emmett, P.H. and Teller, E. (1938) J. of Amer. Chem. Soc., 90, 309-319.
- 11. Caurie, M. (The Year after 1979) J. Food Sci.
- 12. Caurie, M. (The year after 1979) J. Food Sci., 63.
Appendix 1
/*--Finding zero by Secant method [8] to get inflection point from Eq. (12). with c++ and c languages */
#include
using namespace std;
#include
#include
#include
float eval_f(float x); //evaluation of f(x)
float fprime(float x1,float x2); //diff. of f(a)
void main()
{ float x1,x2,xn,e;
int i=0;
printf(“\nType initial point: “);
scanf(“%f”, &x1);
printf(“\nType second point: “);
scanf(“%f”,&x2);
printf(“\nType acceptable error interval in y: “);
scanf(“%f”, &e);
i=0;
do
{ xn=x1-eva;_f(x1)/fprime(x1,x2);
x1=x2;
x2=xn;
printf(“\n%3dth Iteration Root :%f
++i,xn,fabs(eval_f(xn)));
getch();
} while(fabs(eval_f(xn))> e);
}
float eval_f(float x)
{ float b;
//cout<<”x=”<
double bc;
double bb,bb1,bb2,cc,dd,dd1,dd2,dd3,ee,ee1,ee2,ff,ff1,ff2;
double aa,aa1,aa2,ba1,ba2,ba3,ccc1,ccc2,cc1,cc2,cc3,cc4,cc11,cc12;
const double b1=.000091,ga=.983,an=10.0,am=.64;
double ad21,ad22,ad23;
bb=pow(x,(1./am))-pow(x,(an/am));
bb1=(1./am)*pow(x,(1./am)-1.))-(an/am)*pow(x,(an/am-1.));
bb2=(1./am)*(1./am-1.)*pow(x,(1./am)-2.))-(an/am)*(an/am-1.)*pow(x,(an/am-2.));
ccc1=-(1./am)*pow(x,,1./am-1.);
ccc2=-(1./am)*(1./am-1.)*pow(x,1./am-2.);
cc1=pow((1.-pow(x,1./am)),-1.);
cc2=pow((1.-pow(x,1./am)),-2.);
cc3=pow((1.-pow(x,1./am)),-3.);
cc4=pow((1.-pow(x,1./am)),-4.);
dd=pow(x,(an/am))/ga;
dd1=(an/am)*pow(x,((an/am)-1.))/ga;
dd2=(an/am)*(an/am-1.)*pow(x,((an/am)-2.))/ga;
ee=pow(x,2./am)-pow(x,an/am);
ee1=(2./am)*pow(x,(2./am)-1.)-(an/am)*pow(x,(an/am)-1.);
ee2=((2./am)*(2./am-1.)*pow(x,(2./am)-2.)-(an/am)*(an/am-1.)*pow(x,(an/am-2.);
ff=((an-1.)/ga)*pow(x,an/am);
ff1=((an-1.)/ga)*(an/am)*pow(x,(an/am)-1.);
ff2=((an-1.)/ga)*(an/am)*(an/am-1.)*pow(x,(an/am)-2.);
aa=bb*cc1+dd;
aa1=bb1*cc1+(-1)*cc2*ccc1*bb+dd1
aa2=bb2*cc1+(-1)*cc2*ccc1*bb1+(-1)*(-2)*cc3*ccc1*ccc1*bb+(-1)*ccc2*cc2*bb+(-1)*bb1*cc2*ccc1+dd2;
ba1=pow((b1+aa),-1);
ba2=pow((b1+aa),-2);
ba3=pow((b1+aa),-3);
ad21=aa2*ba1+(-1)*ba2*aa1*aa1+(-1)*(-2)*ba3*aa1*aa1*aa+(-1)*aa2*ba2*aa+(-1)*aa1*aa1*ba2;
ad22=ee2*cc2*ba1+(-2)*cc3*ccc1*ee1*ba1+(-1)*ba2*aa1*ee*cc2+(-2)*(-3)*cc4*ccc1*ccc1*ee*ba1+(-2)*ccc2*cc2*ee*ba1+(-2)*ee1*cc3*ccc1*ba1+(-2)*(-1)*ba2*aa1*cc3*ccc1*ee+(-1)*(-2)*ba3*aa1*aa1*ee*cc2+(-1)*aa2*ba2*ee*cc2+(-1)*ee1*ba2*aa1*cc2+(-1)*(-2)*cc3*ccc1*ba2*aa1*ee1;
ad23=ff2*ba1+(-1)*ba2*aa1*ff1+(-1)*(-2)*ba3*aa1*aa1*ff+(-1)*aa2*ff*ba2+(-1)*ff1*ba2*aa1;
b=ad21+ad22+ad23;
return(b);
}
float fprime (float x1,float x2)
{ float b6;
b6=(eval_f(x2)-eval_f(x1))/(x2-x1);
return(b6);
}














