American Journal of Analytical Chemistry
Vol.5 No.5(2014), Article ID:44584,7 pages DOI:10.4236/ajac.2014.55037
Electrical Properties and Phase Behavior of Proton Conducting Nanocomposites Based on the Polymer System (1 − x)[PVOH + H3PO2 + H2O]·x(Nb2O5)
Angélica María Mazuera, Rubén Antonio Vargas
TFnoM Group, Universidad del Valle, Cali, Colombia
Email: mariamtango@gmail.com, ruben.vargas@correounivalle.edu.co
Copyright © 2014 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/


Received 17 February 2014; revised 21 March 2014; accepted 29 March 2014
ABSTRACT
In the present work, novel blend polymer electrolyte membranes using poly(vinyl alcohol) (PVA), doped with hypophosphorous acid (H3PO2) and reinforced with porous niobium oxide (Nb2O5) microparticles in different compositions were prepared by the solution-casting technique. Their phase behavior and ionic conductivity were studied by differential scanning calorimetric (DSC), thermogravimetric analysis (TGA) and impedance spectroscopy (IS) in the radio-frequency range. Using a constant H3PO2/PVA weigh ratio of 0.25, it was found that the water content in the blended hydrogel membranes increased with increasing the filler Nb2O5 content, thus increasing the electrical conductivity. However, the suitable weight ratio of Nb2O5:(H3PO2/PVA) for the blend performance (both mechanically and electrically) was x = 0.075, with a maximum ionic conductivity of 2.7 ( 10−3 S·cm−1 at 120˚C. For all blends prepared, the lost tangent plots show asymmetrical peaks, as a consequence of correlations in the mobile ion diffusion as a function of frequency. Although this “universal dynamic response” was observed at all temperatures, variations in the tan(() relaxation peaks indicate a decrease of ionic correlation when the temperature is increased. Both the dc conductivity and tan(() peaks frequency dependency are thermally activated, following an Arrhenius-type behavior with activation energy of the same order, indicating that the corresponding ionic processes have the same origin, i.e., proton jump among available sites in the polymer matrix. The additions of oxide particles to the membranes improve their thermal and electrical properties, attributed to an approximation effect.
Keywords:Nanocomposite, Polymer Blend, Impedance Study, DSC/TGA, Ionic Conductivities, Polymer Electrolytes, Phase Behavior

1. Introduction
Polymers and polymeric composites have steadily gained growing importance in our daily life. Permanent electrical effects in both dielectrics and conductors have been utilized in a wide variety of applications. Among them, poly(vinyl alcohol) (PVA), a hydrophilic, biodegradable and non-toxic synthetic polymer, has been widely used in different areas of research, development and production. These extend from solid state electrochemistry applications (fuel cell, batteries, and electrochromic devices) [1] -[4] to the biological and medical fields. PVOH polymer molecule consists of monomer units, Figure 1.
Depending on the stereoregularity of OH groups along the backbone chain, it has been identified three distinct polymer configurations, identified as isotactic (i), syndiotactic (s) and atactic (a) [5] [6] . That is, in i-PVOH, all the OH groups lie in the same side of the plane of the extended chain backbone; in s-PVOH, the OH groups regularly alternate from one side of the plane to the other and in a-PVOH, no such specific OH distribution is established. All the three configurations co-exist, but in general, with s-PVA as the prominent phase (as much as 53% - 62% content [5] [7] ). It seems that, under favorable conditions, the sequential distribution of OH groups in either side to backbone in s-PVA can be explored to design a regular bridging with water molecule in a layer structure.
The swollen properties of PVOH-based membranes play an important role in their electrical conductivity performance and thermo-mechanical stability [8] . The proton transport properties of a membrane can be improved by enhancing the hydrophilicity of the membrane, since it raises the selectivity of water toward organic [9] and inorganic [10] aqueous solution. For example, PVOH interacts with inorganic salts and acid to form an ionic conductor in a polymer matrix. The membranes remain conducting even at high PVOH content. However, the thermo-mechanical PVOH is highly deteriorated with water content, being its volumetric size change of the membranes, one of its main effects [12] . In other words, the mechanical properties (e.g. Young modulus) and the physicochemical properties (e.g. electrical conductivity) of the PVA-based membranes are both directly linked to the to the presence of water in the polymer matrix, which might be located in water-filled ionic domains as a segregated liquid-like phase.
The addition of ceramic filler into polymer matrix is allowed to reduce the glass transition temperature (Tg) and the crystallinity of the polymer, and also it is allowed to increase the amorphous phases of polymer matrix, then increase the ionic conductivity. There are various ceramic fillers, such as Al2O3, TiO2, SiO2 [4] [11] [13] -[15] , which have been extensively studied. These experimental results indicated improvements in the ionic conductivity, thermal and mechanical properties as the different ceramic fillers were added into the solid polymer electrolyte (SPE). The enhancement of the ionic conductivity was explained by considering that ceramic particle fillers in the polymer matrix created some defects and free volume at interface between the ceramic particle and the polymer chain.
In this work, we attempted to disperse the nano-sized Nb2O5 particles into the PVA/H3PO2 matrix and study their ionic transport behavior as a function of temperature and electric field frequency to obtain information about the nature and types of molecular dynamics and their relations to chemical composition, molecular structure and morphology. The electrical responses of the membranes are compared with thermal analysis measurements to infer structure-properties relationship in the prepared films that might be relevant to the development of proton exchange membranes (PEMs).
2. Materials and Methods
Solid polymer blend electrolytes were prepared by solution casting technique to optimize the blend ratio on the basis ofionic conductivity and mechanical stability of the film. PVA (Aldrich granulated, 98% - 99% hydrolyzed, average Mw: 85000-146000) was poured in distilled water at 353 K with hypophosphorous acid (H3PO2) (aqueous solution at 50% density). The appropriate quantities of PVA and acid were chosen at constant concentration for a common matrix P/OH = 0.25. It was mixed with concentrations of oxide niobium in aqueous solu-

Figure 1. Monomer PVOH polymer molecule.
tion and stirred for several hours. The PVA/acid was then mixed with chosen weight ratio of Nb2O5 such as 0.02, 0.075, 0.1, and 0.25. The dissolved polymer solution and ceramic powder were mixed together, and the solution was stirred continuously for several hours to obtain homogeneous mixture. The solutions were allowed to evaporate under a dried atmosphere until a solid state was achieved. We obtained smooth, of white color, dry to the touch and thin films of 0.05mm thickness.
The thermal phase behavior of the samples was characterized using a differential scanning calorimetry (DSC Q100) and thermogravimetry (TG 2050) microbalance of TA Instruments. Each sample was scanned at a rate of 10˚C/min, and dry nitrogen was used as purge gas, in a temperature range 30˚C < T < 300˚C. Complex impedance measurement were done in the frequency range 20 Hz to 5 MHz using a WAYNE KERR 6420 analyzer, with amplitude of the voltage 500 mV, measured with electrodes of platinum of 1.45 mm, for temperature range by heating 30˚C - 200˚C and cooling 200˚C - 30˚C.
The Nyquist plots (Re Z, Im Z) showed a monotonically decreasing linear part in the low frequency limit the polymer/electrodes interfaces. The bulk resistance R of a membrane is obtained from the intercept of this linear part of the Z spectra with the real axis (Re Z). The DC conductivity 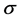 was calculed from
was calculed from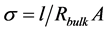 , where l is the thickness and A is the contact area of the sample [5] .
, where l is the thickness and A is the contact area of the sample [5] .
3. Results and Discussion
3.1. Thermal Analysis
The thermal measurements (DSC and TG) done on the composites with the concentrations prepared showed the decomposition temperature above 225˚C, shown as a huge DSC exothermic peak. Another wide endothermic peak appears at about 150˚C for some concentration. This peak is shifted to higher temperatures when the ceramic filler is increased due to the melting of the crystalline part of the polymer matrix whose proportion with regard to the amorphous part, increases with the addition of ceramics microparticle since the intensity of the pick increases.
For the case x = 0.02 the polymer is mainly amorphous due to not presents this event endothermic, Figure 2.
The slope negative of the offset, below 150˚C into the membranes with high content of oxide niobium is relatively identical, showed exit of water of the composites is the same rate. This behavior is shown in TG. The TG curves (Figure 3) show that the water content in these samples is released between 50˚C and 125˚C, for x = 0.02, with a total weight loss 12%. On increasing temperature, a relatively slow mass loss rate continues reaching a total weight loss of 44% up to approximately 223.9˚C, where the blend’s decomposition takes place.
Water contents in the membranes favor their proton transport [16] and promise a wide range for the composites to operate as PEM at higher temperatures [9] . Although a high water content in the membrane is desirable, in the fuel cell can block reactant access to the catalyst layer, reducing the overall cell performance [17] .
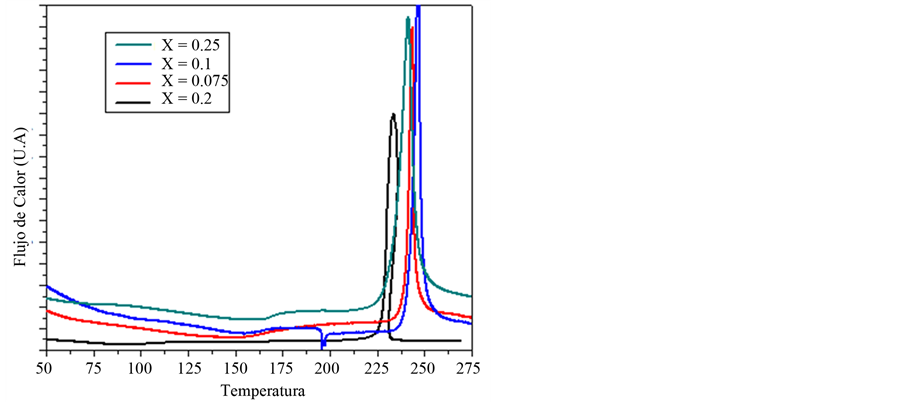
Figure 2. DSC thermograms, for system (1 − X)[PVOH + H3PO2 + H2O]·X(Nb2O5), with X = 0.02, 0.075, 0.1, 0.25.
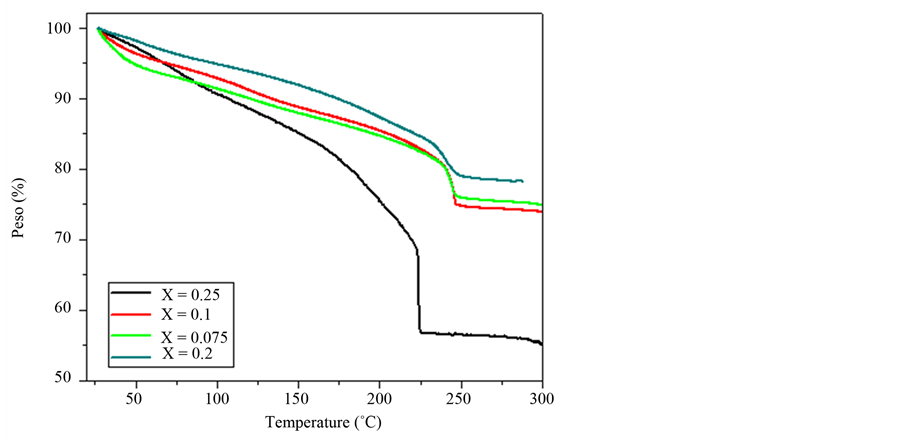
Figure 3. TGA thermograms, for the system (1 − x)[PVOH + H3PO2 + H2O]·x(Nb2O5), with x = 0.02, 0.075, 0.1, 0.25.
3.2. Electric Analysis
The impedance measurement was analyzed by means of conductivity DC, σ, calculated from the Nyquist plots as described above. The absorption of water of the membranes in general, allowed to reach a conduction of order 10−3 (W·cm)−1 to high temperatures up to 100˚C, for x = 0.1, reaching a conductivity of 1.48 ´ 10−3 (W·cm)−1, higher than that for x = 0. 25, which is of 1.0 ´ 10−4 (W·cm)−1 at 70˚C.
For Table 1, Ea is calculed from the linear fitting to the Arrhenius equation,
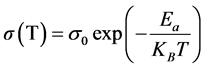 (1)
(1)
In Figure 4, is showed that for the DC conductivity of x = 0.075 presents higher thermal stability due to linear increase of conductivity up to 2.66 ´ 10−3 (W·cm)−1 at 120˚C, although in this temperatures of superficial water is evaporated decrement the free ions united to this.
However, when increase the temperature, increase mobility too, since occur a decrease the site for mobiles ion and an increase of mobility for flexibility of polymerics chain. In Figure 4, the membranes increase rapid the conductivity with content of water [15] [18] , so much internal as external which favor the transport of ions but decrease when liberate the superficial water, being different the behavior of X = 0.075, which retain more concentration of water that the other ones.
In Table 1, the energy activation correspond a good solid electrolyte in order the tenth of eV, being X = 0.1 the one that presents a value but it lowers of 0.0478 eV, in the wide range of temperature between 30˚C - 110˚C. On the average the range thermoactivation the samples are between 30˚C - 100˚C.
In Figure 5, the loss factor increase with frequency and with temperature too, arriving a maxim value for later decrease, since the high value of loss factor is generated by the free charge accumulations into interface between the electrodes and electrolyte [19] , while to high frequencies, the periodic reversal of the electric field occurs so fast that there is no excess ion diffusion in the direction of the field. The polar ionization due to the charge accumulation decreases, leading to a decrease in the value of the dielectric loss. The process relaxation in Figure 6, is showed, used to 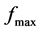 for the loss factor, correspond to each sample, is possible calculed time of relaxation,
for the loss factor, correspond to each sample, is possible calculed time of relaxation, 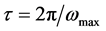 , showed in Table 2.
, showed in Table 2.
This times, into Table 2, indicate that exist a transition of mobility of short ions to large reach into the sample, which to more temperature the ions are moved into the composite a more velocity. The best time of relaxation τ is by X = 0.1 and 0.02 of 1.44 μs.
Table 1. Activation energy, Ea, for the doping samples in the 30˚C - 200˚C temperature range. Ea is calculed from the linear fitting to the Arrhenius equation,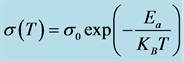 .
.
Table 2. Time relaxation, 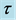 , for the doping samples in the 30˚C - 200˚C temperature range for heating X = 0.075 and 0.25 and cooling X = 0.02, 0.075 and 0.1. Ea is calculed from equation,
, for the doping samples in the 30˚C - 200˚C temperature range for heating X = 0.075 and 0.25 and cooling X = 0.02, 0.075 and 0.1. Ea is calculed from equation, 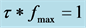 [5] [19] .
[5] [19] .
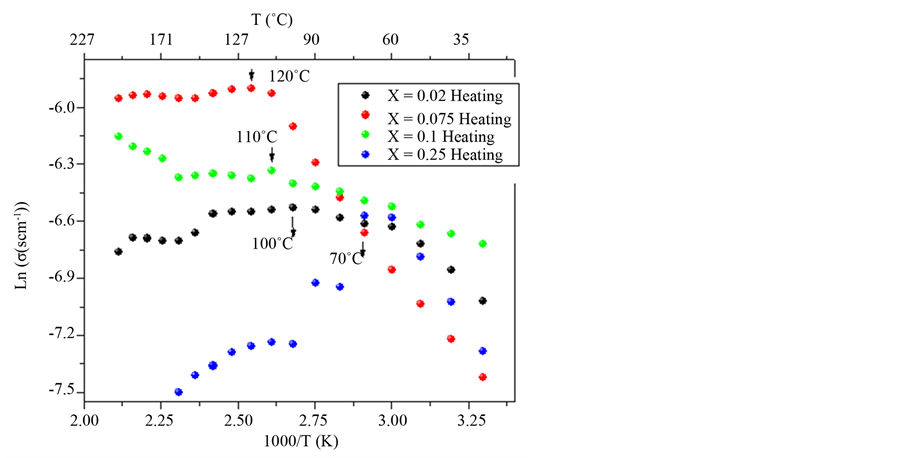
Figure 4. DC conductivity vs 1000/T during heating cycle between 30˚C and 200˚C, for the system (1−X)[PVOH + H3PO2 + H2O]·X(Nb2O5), with X = 0.02, 0.075, 0.1, 0.25.
4. Conclusions
Solid polymer electrolyte membranes using poly(vinyl alcohol) (PVA), doped with hypophosphorous acid (H3PO2) and reinforced with porous niobium oxide (Nb2O5) microparticles in different compositions were prepared by the solution-casting technique.
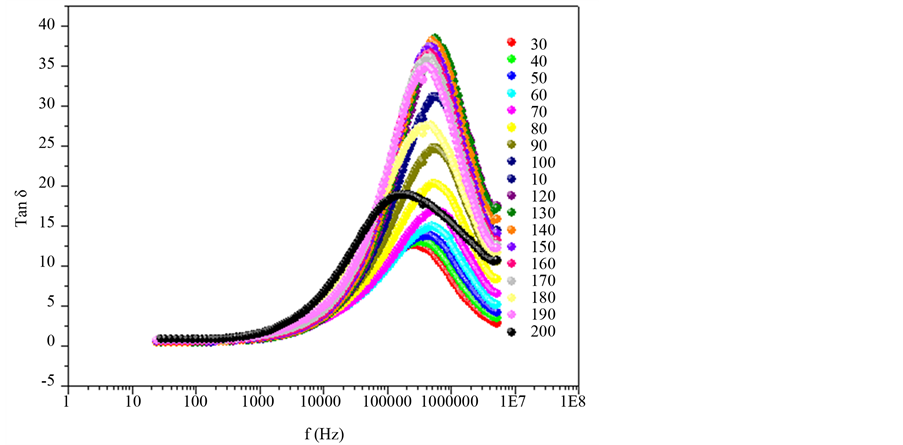
Figure 5. Variation of the loss factor, Tan δ, as a frequency at temperature range 30˚C - 200˚C for heating, by x = 0.075.
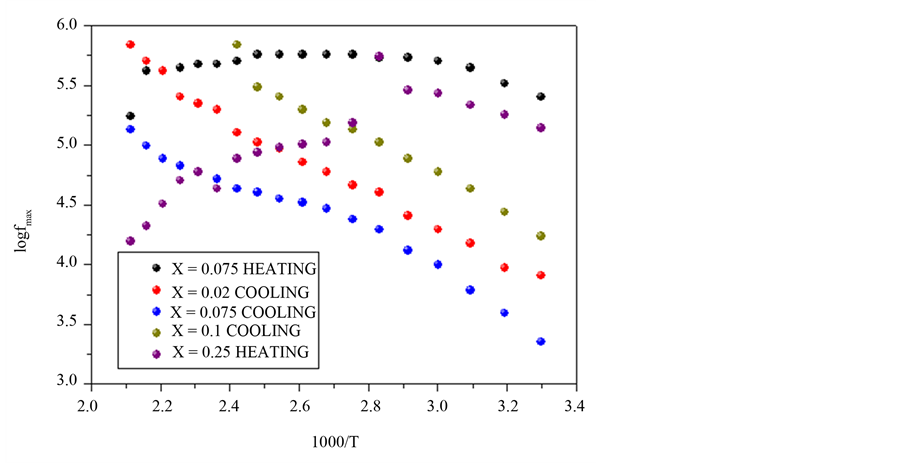
Figure 6. Logarithm of maxim frequency of curves the loss factor, vs. 1000/T at temperature range 30˚C - 200˚C for heating, by x = 0.075 and 0.25 and cooling for X = 0.02, 0.075 and 0.1.
The ionic conductivity of the developed films was measured by AC impedance technique. The maximum dc ion is obtained for PVA/H3PO2 molar ratio of P:OH = 0.25 and Nb2O5/(PVA/H3PO2) weight ratio of 0.075, reaching a value of 2.66 ´ 10−3 (W·cm)−1 at 120˚C, with an activation energy, Ea = 0.1863 eV, and relaxation time of 1.74 μs. The thermal stability 238˚C is observed for the film x = 0.075 from TG/DTA analysis.
The additions of oxide particles to the membranes improve their thermal and electrical properties, attributed to an approximation effect. The nanoparticles surrounding the polymeric chains establish bridges that promote a dynamic effect for ion mobility, as well as making room water absorption and retention at higher temperatures due to the refractive properties of niobium oxide. These membranes can potentially be used as PEMs for cells fuel applications due to their high ionic conductivity and good thermal and mechanical stabilities.
Acknowledgements
The authors express their sincere gratitude to University of Valle who made the development and publication of this study possible through access to the Phase Transition in not Metallic System laboratory and to the Administrative Department of Science, Technology and Innovation-Colciencias (Colombia) for financial support.
References
- Avellaneda, C.O., Alkahlout, A., Pawlicka, A., Leite, E.R. and Aegerter A. (2006) Solid State Electrochromic Devices with Nb2O5 Thin Film and Gelatin-Based Electrolyte. The 10th International Symposium on Polymer Electrolytes, Foz do Iguaçú, 15-19 October 2006, 22.
- De Carvalho, L.M., Tan, A.R., Da Costa, E.T. and De Souza, A. (2006) Nanostrutured Hybrid Materials for Fuel Cell Application. The 10th International Symposium on Polymer Electrolytes, Foz do Iguaçú, 15-19 October 2006, 33.
- Siracusano, S., Vaglio, B., Lufrano, F., Staiti, P. and Aricó, A.S. (2013) Electrochemical Characterization of a PEM Water Electrolyzer Based on a Sulfonated Polysulfone Membrane. Journal of Membrane Science, 448, 209-214. http://dx.doi.org/10.1016/j.memsci.2013.07.058
- Gao, H., Tian, O. and Lian, K. (2010) Polyvinyl Alcohol-Heteropoly Acidpolymer Electrolytes and Their Applications in Electrochemical Capacitors. Solid State Ionics, 181, 874-876. http://dx.doi.org/10.1016/j.ssi.2010.05.006
- Mazuera, A.M. and Vargas, R.A. (2012) Estudio del comportamiento térmico y eléctrico en nanocompositas polimé- ricas basadas en óxidos de nióbio micrométrico con poli(alcohol de vinilo), con composición (1 − x) [PVOH + H3PO2 + H2O] − x (Nb2O5). Bachelor Thesis, Universidad del Valle, Colombia.
- Gonzáles, Y.F. and Vargas, R.A. (2011) Estudio de las propiedades termodinámicas y eléctricas demateriales compuestos poliméricos basados enelpoli(vinilalcohol) (PVA) + H3PO2+ TiO2. Revista Iberoamerica dePolimeros, 12, 64-75. http://www.ehu.es/reviberpol/pdf/MAR11/gonzalez.pdf
- Martinelli, A., Fernicola, A., Matic, A., et al. (2006) Ionic Liquid Based Proton Conducting Membranes. The 10th International Symposium on Polymer Electrolytes, Foz do Iguaçú, 15-19 October 2006, 3.
- Umeda, J., Mariya, M., Sakomoto, W. and Yogo, T. (2009) Synthesis of Proton Conductive Membranes Based on Inorganic-Organic Hybridstructure Bound with Phosphonic Acid. Electrochemical Acta, 55, 298. http://dx.doi.org/10.1016/j.electacta.2009.08.054
- Vargas, R.A., Delgado, M.I. and Palacios, I. (2004) Effect of Water Vapor on the Ion Transport in Polymer Films of PVOH/LiH2PO4/H2O. Solid State Ionics, 175\1-4, 729-732.
- Zapata, V.H., Castro, W.A. and Vargas, R.A. (2011) Conductividad y relajación eléctrica en compositas poliméricasde PVOH + LiH2PO4 + Al2O3 analizadas mediante ajuste a losmodelos VTF, KWW y la ley de potencias de Jonscher. Revista Colombiana de física, 43, 1-5.
- Qiao, J., Okada, T. and Ono, H. (2009) High Molecular Weight PVA Modified PVA/PAMPS Proton Conducting Membranes with Increased Stability and Their Application in DMFCs. Solid State Ionics, 180, 1318-1323. http://dx.doi.org/10.1016/j.ssi.2009.08.010
- Zapata, V.H., Castro, W.A. and Vargas, R.A. (2008) Estudios de Conductividad y Relajación Eléctrica en el Sistema Polimérico PVOH + LIH2PO4 + H2O. Revista Colombiana de física, 40, 611-614.
- Wang, X., Yucel, T., Lu, Q., Hu, X. and Kaplan, D.L. (2010) Silk Nanospheres and Microspheres from Silk/PVA Blend Films for Drug Delivery. Biomaterials, 31, 1025-1035. http://dx.doi.org/10.1016/j.biomaterials.2009.11.002
- Castro, W.A., Zapata, V.H. and Vargas, R.A. (2006) Estudios de transporte iónico y equilibrio de fases en compositas poliméricas., Revista Colombiana de física, 38, 1491-1494.
- Zhang, W., Zhang, Z. and Wang, X. (2009) Investigation on Surface Molecular Conformations and Pre-Evaporation Performance of Thepoly(vinyl Alcohol) (PVA) Membrane. Journal Colloid Interface Science, 333, 346-353. http://dx.doi.org/10.1016/j.jcis.2009.01.058
- Ye, H., Huang, J., Xung, J.J., et al. (2008) New Membranes Based on Ionic Liquids for PEM Fuel Cells at Elevated Temperatures. Journal of Power Sources, 178, 651-660. http://dx.doi.org/10.1016/j.jpowsour.2007.07.074
- Baschuk, J.J. and Li, X. (2010) Modeling of Ion and Water Transport in the Polymer Electrolytemembrane of PEM Fuel Cells. International Journal of Hydrogen Energy, 35, 5095-5103. http://dx.doi.org/10.1016/j.ijhydene.2009.10.032
- Castro, W.A., Zapata, V.H. and Vargas, R.A. (2013) Electrical Conductivity Relaxation in PVOH-LiClO4-Al2O3. Ionics, 19, 83-89. http://dx.doi.org/10.1007/s11581-012-0710-3
- Mohan, V.M., Qiu, W., Shen, J. and Chen, W. (2009) Electrical Properties of Poly(vinyl alcohol) (PVA) Based on LiFePO4 Complex Polymer Electrolyte Films. Journal of Polymer Research, 17, 143-150. http://dx.doi.org/10.1007/s10965-009-9300-0


