American Journal of Analytical Chemistry
Vol.4 No.10B(2013), Article ID:38719,7 pages DOI:10.4236/ajac.2013.410A2002
Optimization and Validation of a Method for Heavy Metals Quantification in Soil Samples by Inductively Coupled Plasma Sector Field Mass Spectrometry (ICP-SFMS)
1Instituto Nacional de Investigaciones Nucleares, Carretera México-Toluca, La Marquesa Ocoyoacac, México
2Departamento de Medio Ambiente, Centro de Investigaciones Energéticas, Medioambientales y Tecnológicas (CIEMAT), Avda, Madrid, España
Email: *hector.hernandezm@inin.gob.mx
Copyright © 2013 Héctor Hernández-Mendoza et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received June 19, 2013; revised July 20, 2013; accepted August 19, 2013
Keywords: Soil; Heavy Metal; ICP-SFMS
ABSTRACT
In this work, a method for quantification of heavy metals Inductively Coupled Plasma Sector Field Mass Spectrometry (ICP-SFMS) in soil samples of El Bierzo district (Spain) has been optimized and validated. Optimization was carried out for elements: Cr, Mn, Co, Ni, Cu, Zn, Se, As, Cd, Hg, Pb and U. Validation of the method was performed with Certified and Standard Reference Materials (CRMs and SRMs); SRM2709, CRM020-051 and CRM050-051. Results obtained under optimized conditions can be summarized as follows: a) the Limits of Detection (LODs) were in the order of sub fg·g−1 for Cr, Mn, Cu, Co, As, Cd, Hg, Pb and U, and few fg·g−1 for Ni, Zn and Se; b) precision measurement, in terms of relative standard deviation (RSD), was being below 5%; c) the average recovery of CRM was between 81.3% and 98%. In conclusion, the method offers several advantages: fast, good accuracy, very low values of Limits of Quantification (LOQs) and high sensitivity on measurement of heavy metal.
1. Introduction
Heavy metals are of concern to human health and environment due to their toxic effects. Heavy metals are present in soils in high concentrations due to geological anomalies or as a result of contamination, mainly caused by human activities. Depending on environmental conditions, these high concentrations of heavy metals can be accumulated in agricultural soil or groundwater leachate, causing contamination of the soil and water resources. Furthermore, these metals can be taken directly by humans or animals following several pathways: inhalation of re-suspended soil dust, incorporation onto the food chain as a result of uptake by edible plants and animals [1-3] and the ingestion of contaminated water. In all cases, it constitutes a significant risk to the health of humans and animals [4]. Therefore, the progress on environmental and biological matrices analysis of heavy metals is affected by three major factors: 1) the demand for quantification of an increasing number of analytes at lower concentration levels; 2) the interest in elemental speciation due to issues of bioavailability and toxicity; and 3) the need of minimizing contamination and sample manipulation.
For many years, the analytical technique to quantify metal ions in soil samples has been atomic spectroscopy, or more specifically, Atomic Absorption Spectrometry (AAS), Atomic Emission Spectrometry (ICP-AES) and Inductively Coupled Plasma Mass Spectrometry (ICPQMS) [5].
ICP-QMS technique has been the key of the most successful methods in Atomic Spectrometry (AS) [6-9], due to its high sensitivity, good precision and accuracy, multi-element detection and faster preparation of sample. However, ICP-QMS still has some limitations, mainly by interferences that can seriously affect its analytical performance and these would lead to spectroscopic and nonspectroscopic interferences. Spectroscopic interferences are caused by atomic or molecular ions with same nominal mass of our particular isotope. They may disturb or even obscure the true analytical signal, so that the accuracy cannot be a reliable determination. Non-spectroscopic interferences are caused by polyatomic ions that are formed from precursors having numerous sources, such as the sample matrix, reagents used for preparation, plasma gases or entrained atmospheric gases. To obtain a good precision and accuracy signal in ICP-QMS, it is necessary to analyze these factors, in order to reduce as much as possible or even eliminate spectroscopic interferences [10]. Nonspectral interference refers to matrix-induced change in signal intensity that is unrelated to the presence or absence of spectral components. The detail of this type of interferences is summarized in the literature [11]. External calibration or standard addition methods, coupled with internal calibration, have been used normally to correct the non-spectroscopic interfereences. Another alternative to avoid interferences is by using a reaction/collision cell, resulting in a capability to partially remove interferences, depending on the operating conditions [12-14]. The development of more powerful ICP Mass Spectrometers has been mainly based on the union of a magnetic sector analyzer to the plasma source, plus a second electrostatic sector analyzer, which allows focusing both the angle at which ions deflect as their energy (double focusing). In this way, it is possible to avoid interferences of atomic or molecular ions in complex samples [15,16]. This is because its resolutions, approximately ~300 for Low-Resolution Mode (LRM), ~3500 - 4000 for Medium-Resolution Mode (MRM) and ~7500 - 10.000 for High-Resolution Mode (HRM). For this reason, ICP-SFMS is an excellent choice for the analysis of heavy metals, due to its extremely high sensitivity, the capability for interference-free analysis and the significantly improved isotopic ratio precision when compared with ICP-QMS [17].
El Bierzo District is located in the Province of Leon (Spain) and has a carboniferous basin across 310 km2 that has been exploited since the middle of XIX century [18]. The intense activity of coal mining has generated 571 sites with mine tailings, covering an area of 52.51 km2 over the original soils. The tailings were dumped for many years and have been exposed at natural alterations that include several physical, chemical and biological processes. This alteration generates many impacts on air, water and soils, as the spontaneous combustion of coal or like acid mine drainage, which releases heavy metals in the environment. The mine tailings also cause a serious visual impact on the landscape.
The objectives of the present study have been: 1) develop, optimize and validate a method to determinate heavy metals in soil samples by ICP-SFMS; 2) applied this method for the analysis of heavy metals in soil samples of the El Bierzo coal mining district (Spain); and 3) evaluation of contamination impact by heavy metal in place.
2. Experimental
2.1. Instrumentation
Determinations of heavy metals were carried out with an Element XR Mass Spectrometer (Thermo Fisher Scientific, Germany). Aqueous samples were introduced with an auto sampler CETAC ASX-520 (CETAC Technologies, Inc. USA). The ICP torch was shielded with a grounded platinum electrode (GuardElectrodeTM, Thermo Scientific, Germany).
2.2. Materials and Reagents
Instrumental mass calibration of ICP-SFMS was performed by using a certified multi-element solution XXIII (Ba, B, Fe, Co, Ga, In, K, Li, Lu, Na, Rh, Sc, Y, Tl, and U) from Merck (Germany). Optimization of the method was made with a standard solution of approximately 10 μg·L−1 of each metals of interest (Cr, Mn, Co, Ni, Cu, Zn, Se, As, Cd, Hg, Pb and U) coming from a standard dissolution that contained nearly 1 mg·L−1 (Merck, Germany). A 1.0 ± 0.2 μg·L−1 of In external standard solution (Merck, Germany) and a Lu internal standard dissolution (Merck, Germany) of 1.1 ± 0.2 μg·L−1 were used during the measurements to monitor instrumental stability. Standard and Certified Reference Materials (SRMs and CRMs) were supplied for validation of method; CRM020-051 and CRM050-051 (Resource Technology Corporation, Sigma-Aldrich, United Kingdom), and SRM2709 (National Institute of Standards & Technology, USA). A microwave-assisted acid digestion was used for mineralization of soil samples (ETHOS 1, Milestone S.r.l., Italy). Filtration of sample after acidic digestion was carried out through filter papers of 2.5 µm (Millipore Ibérica, Spain). High purity water (>18 MΩ·cm−1) was obtained from a Milli-Q Element A10 Century (Millipore Ibérica, Spain). HNO3 and HCl were purified by distillation in a Milestone Duopur (Milestone s.r.l., Italy) subboiling system. Certified Ar gas (99.999%) was supplied by Air Liquide España. Measurements of elements were carried out in a clean room laboratory (ISO 6 class) at 24˚C ± 1˚C.
2.3. Preparation of Samples
Sample points analyzed in this paper were collected in El Bierzo district (Spain). The methodology followed during soil sample recollection and characterization has been described elsewhere [18,19]. In the framework of a larger study, the soil points were sampled biannually for 2006- 2008 period and were physicochemical characterized in its inter-annual and intra-annual variation [19]. Soil samples were air dried and sieved to 2 mm and this fraction was used for the heavy metal analysis [20]. Studies were performed in nine points. Figure 1 shows the localization of El Bierzo district and the locations where the soil samples were collected.
A microwave-assisted acid digestion was used for mineralization of soil samples, based on recommendations given in EPA method 3051A [21]. The method was used for a fast extraction of heavy metals from soil samples. The soil samples were prepared by accurately weighing of 0.1 g of sample into the Teflon microwave digestion vessels. Acid conditions of digestion were: 9 mL of HNO3 (10 mol·L−1) and 3 mL of HCl (10 mol·L−1). Lu was added as internal standard. Following digestion (Table 1), the dissolutions were cooled, and then the samples were filtered, transferred to 25 mL volumetric flasks and filled up with Mill-Q water. Before the ICPSFMS measurements, the samples were diluted 1:50 with Milli-Q water and in tracer was added as external standard. Analysis of each sample was carried out in triplicate.
3. Results and Discussion
3.1. Validation of Method Abbreviations and Acronyms
Several parameters have been taken into account and evaluated for the validation of the analytical methods for quantitative determination of heavy metals in soils, such as: optimization of measurement, dynamic range and linearity, LODs and LOQs, repeatability, precision and accuracy.
Instrumental settings were optimized for the quantification of elements; Cr, Mn, Co, Ni, Cu, Zn, Se, As, Cd,
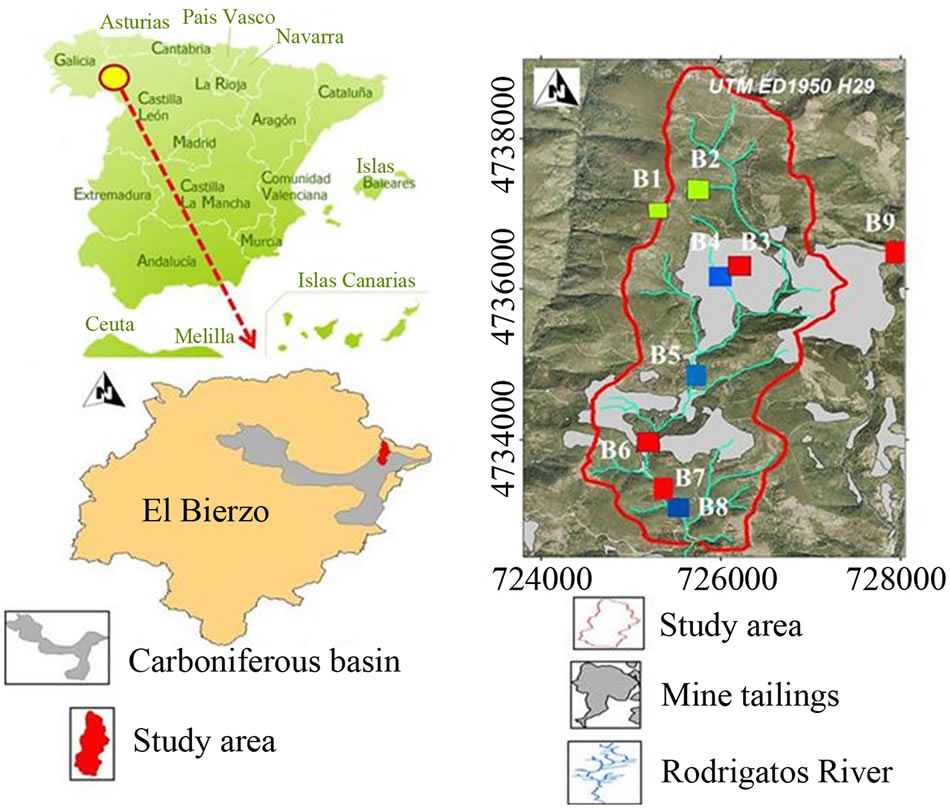
Figure 1. The studies were performed in nine points, localization of El Bierzo, Spain and locations where soil samples were collected.
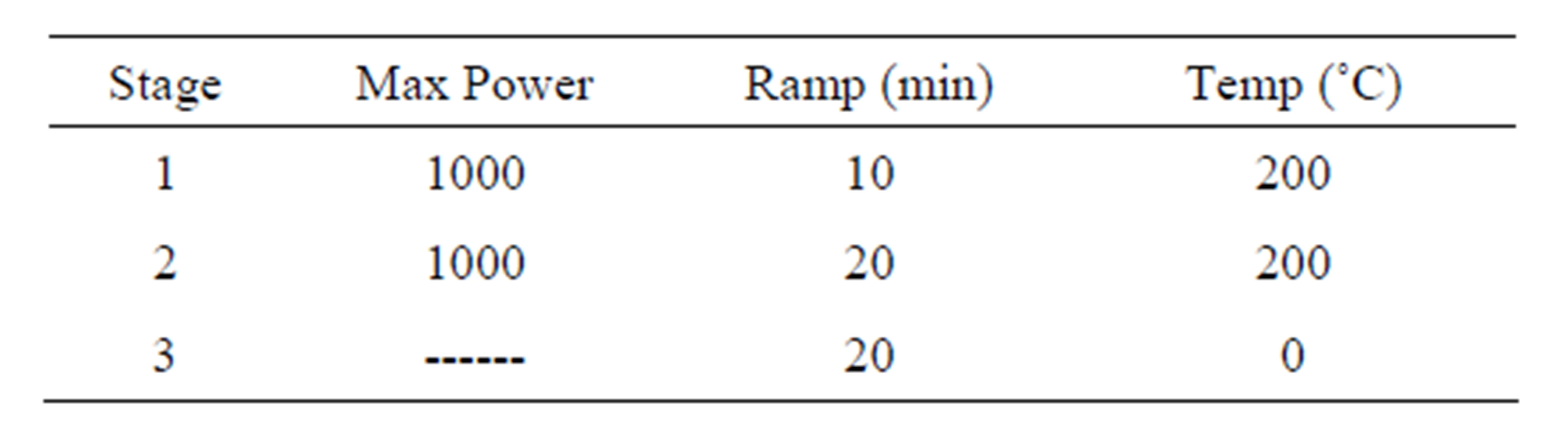
Table 1. Settings used in microwave assisted digestion.
In, Lu, Hg, Pb and U. The optimization was performed in three steps:
a) An instrumental mass calibration in LRM, MRM and HRM using the same dissolution certified multielement solution XXIII was performed. Then, a tuning of the ICP-SFMS parameters was carried out with the same multi-element solution, to obtain the maximum intensities for 115In and 238U isotopes in the LRM. The signal intensity for these isotopes was about 1.3 × 106 cps and 1.8 × 106, respectively. The oxides content in the plasma was below 1%, b) Optimization of signal intensity for the analytes of interest in MRM (52Cr, 55Mn, 59Co, 60Ni, 63Cu, 64Zn, 114Cd, 115In, 176Lu, 200,202Hg, 206,207,208Pb, and 238U) and HRM (75As, 78Se, 115In and 176Lu). This was achieved by adjusting the respective mass windows, centering of mass in peak maximum and using integration windows of 10%, in order to avoid interferences. A stability test was carried out for monitoring the signal and correct the mass offset for these isotopes and c) stability test was performed by monitoring the signal of 115In (1.0 ± 0.2 μg·L−1) and 176Lu (1.1 ± 0.2 μg·L−1) in standard solution samples during 15 min, the operating conditions for ICP-SFMS are summarized in Table 2.
To determination of LODs and LOQs, calibration curves were used with the following concentrations (µg·L−1) of each element: 0.25, 0.5, 1, 5, 10, 50, and 100. The calibration curves exhibited good linearity for each isotope (Table 3).
The calculation of LODs was performed by measuring a set of blank samples (n = 15) from 5% (v/v) HNO3. A standard solution containing about 10 μg·L−1 of each element was used to obtain the standard intensities signal. Then, the LOD was calculated using the following formula:
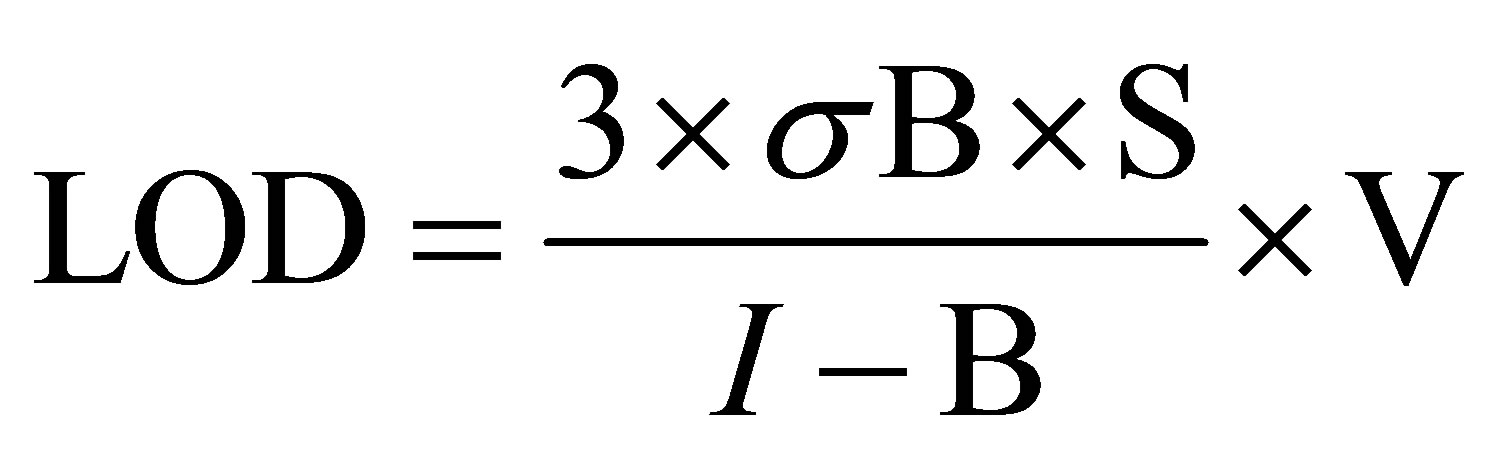
where: LOD is the limit of detection (fg per sample), σB corresponds to the standard deviation of the blanks signal (cps), S is the concentration of the standard (fg·mL−1), I is the signal intensity of the standard (cps), B is the average intensity of blank signal (cps) and V is final volume of the sample (V= 10 mL).
The limit of quantification (LOQ) was calculated considering the region when the signal owing to the analyte is ten times the standard deviation of the background signal and, in general, it can be considered to be ap
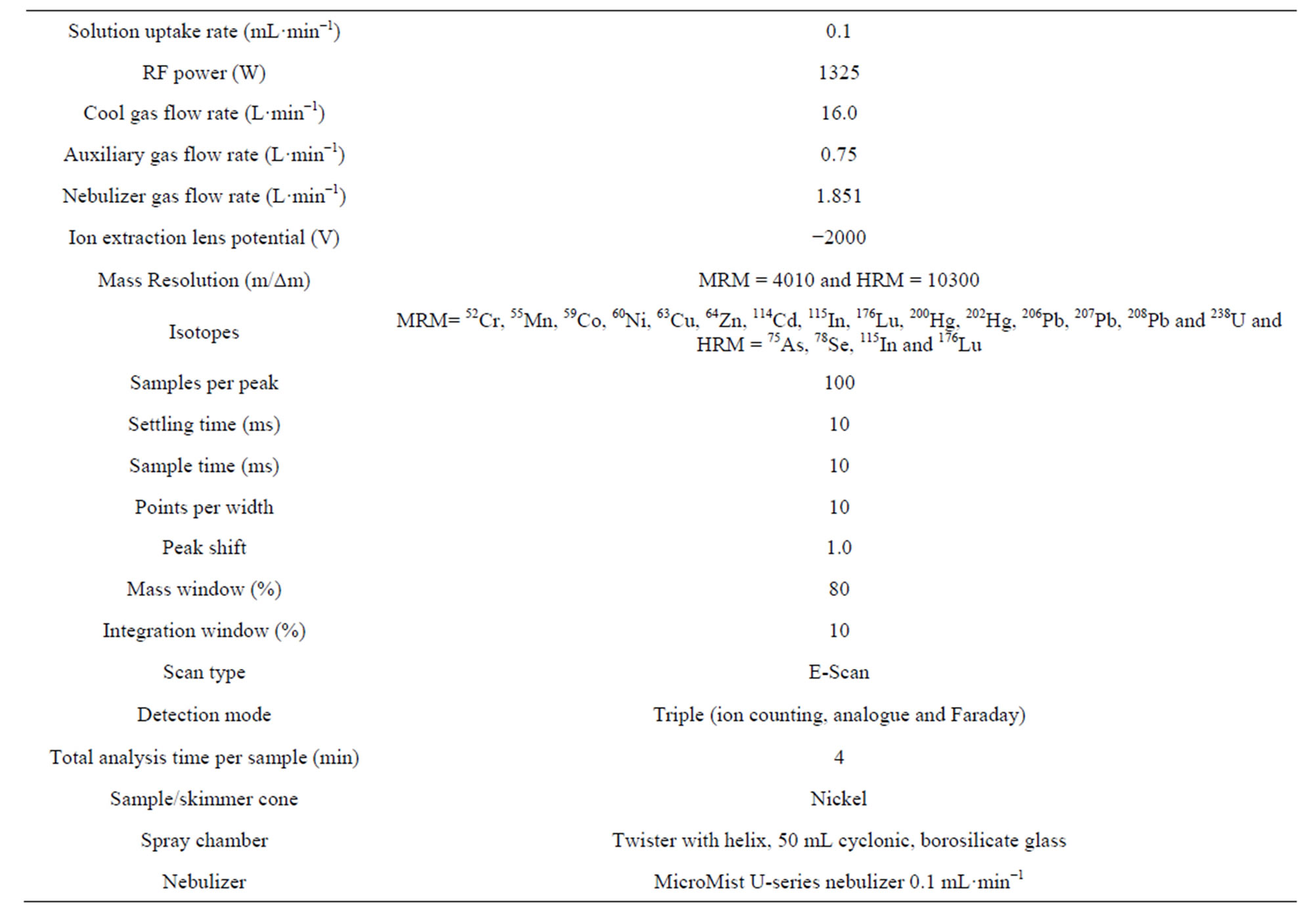
Table 2. Instrumental conditions optimized for the measurement of heavy metals in soil samples by HR-ICP-MS.
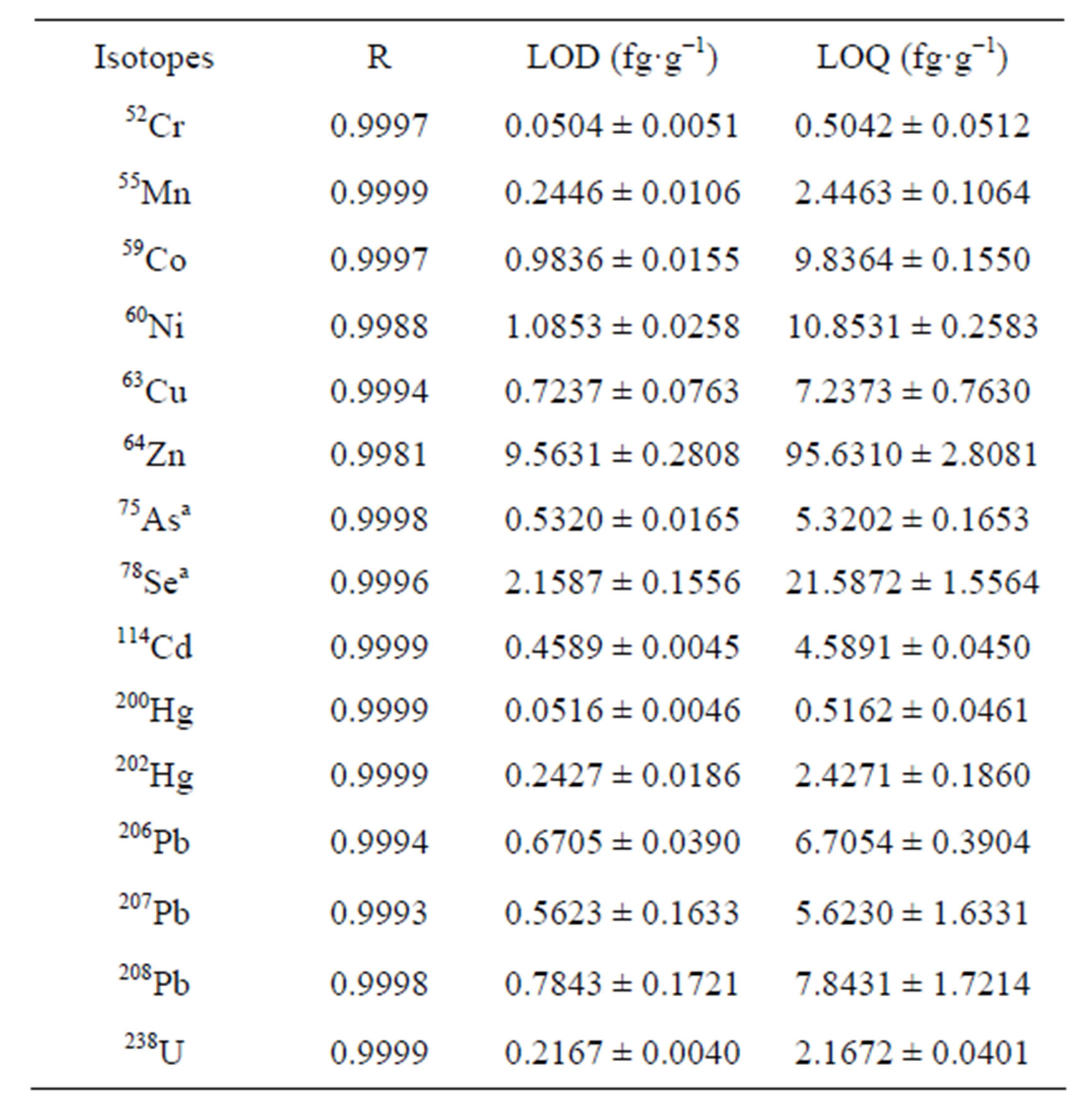
Table 3. Calibration curves, LOD and LOQ of each isotope by HR-ICP-MS.
aThe calibration curves of As and Se corresponds to the measurement by HRM.
proximately ten times the LOD (Table 3).
The determination of repeatability was carried out with SRM2709 and the precision or RSD was calculated with percentage recovery and using the standard deviation divided by the mean of replicated samples. Table 4 shows the results of precision and repeatability test. Finally, accuracy of method was determined by comparing the measured concentration of CRM020-051; CRM050- 051 and SRM2709 expressed as recovery yield. The average recovery of CRM was between 81.3% and 98% (Table 5).
3.2. Analysis of Heavy Metals in Soil Samples from El Bierzo District
The monitoring of heavy metals in soil samples has been the principal aim of this study. This information allows having a more precise knowledge of the real state of the soils and so, to evaluate the contamination degree dues to mine activities. These aspects have been considered in the starting point of the potential restoration activities. In this work eight zones that represent the spatial variability within the upper basin of the river Rodrigatos were chosen, which include reference areas not affected by mining activities (see Figure 1, B8 sample). The results obtained in analysis of heavy metals were show in the Table 6.
The reference levels are established with B8 sample, because this point is an area that is not affected by mine activities and has the same parent material than the other soil points sampled. The high Mn concentration in B4 sample is in accord with high concentrations of Mn oxides found in this sample [19].
These Mn oxides have a great affinity to adsorb heavy metals on its surface and this is in accord with the highest concentrations of Co, Zn, Ni, Cu, Cr and Cd measured in B4 sample. The great adsorption capacity of Mn oxides has been studied in several works [22-24].
B6 sample distinguishes because is the second soil sample with heavy metal values higher than reference levels for Cu, Pb, As and Hg. B3 sample exceeds the Cu reference value. In summary, the results obtained by analysis of heavy metal in soil were not consistent for evaluation of contamination in El Bierzo district.
4. Conclusions
It has been proved the power of HR-ICP-MS technique for the analysis of heavy metals in complex matrices (soil samples), due to its ability to avoid or eliminate spectroscopic interferences. This study was carried out to establish a validation method for quantitative multi-elemental analysis of heavy metals (Cr, Mn, Co, Ni, Cu, Zn, Se, As, Cd, Hg, Pb and U) in soil samples by using ICP-SFMS. It has been applied with success in monitoring of toxic metals at El Bierzo District.
The results showed that LODs ranged between sub fg·g−1 for nine of the elements studied (Cr, Mn, Co, Cu, As, Cd, Hg, Pb and U) and few fg·g−1 for the other three elements (Ni, Zn and Se). This ensures that the LOQs are adequate for quantitative determinations in soil samples with poor concentration of heavy metals. Additionally, the method gave good precision values, below 5%, and an average recovery between 81.3% and 98%. In conclu-
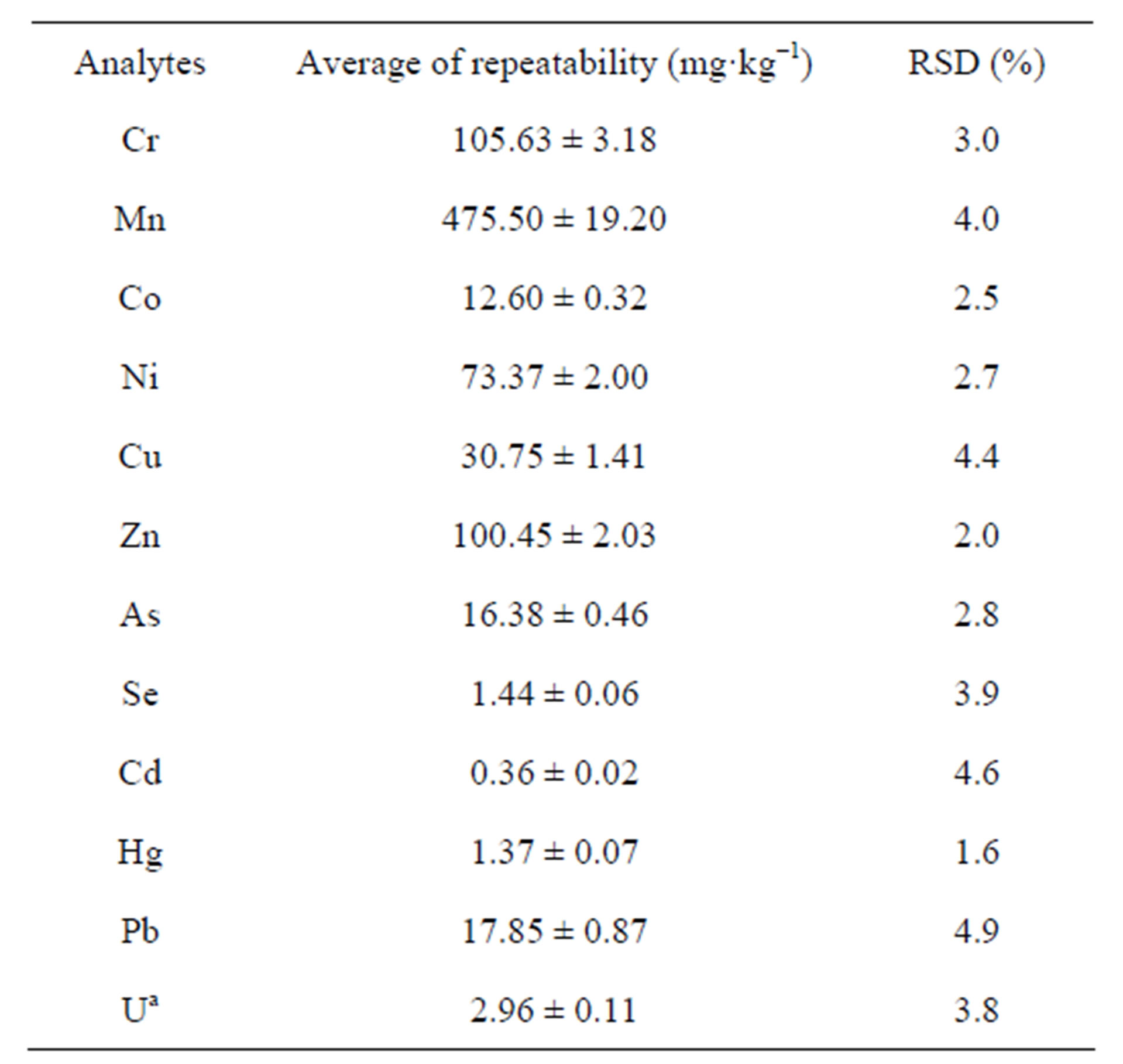
Table 4. Results of precision (RSD) and repeatability test in SRM 2709 by HR-ICP-MS.
aU content is unknown in SRM.
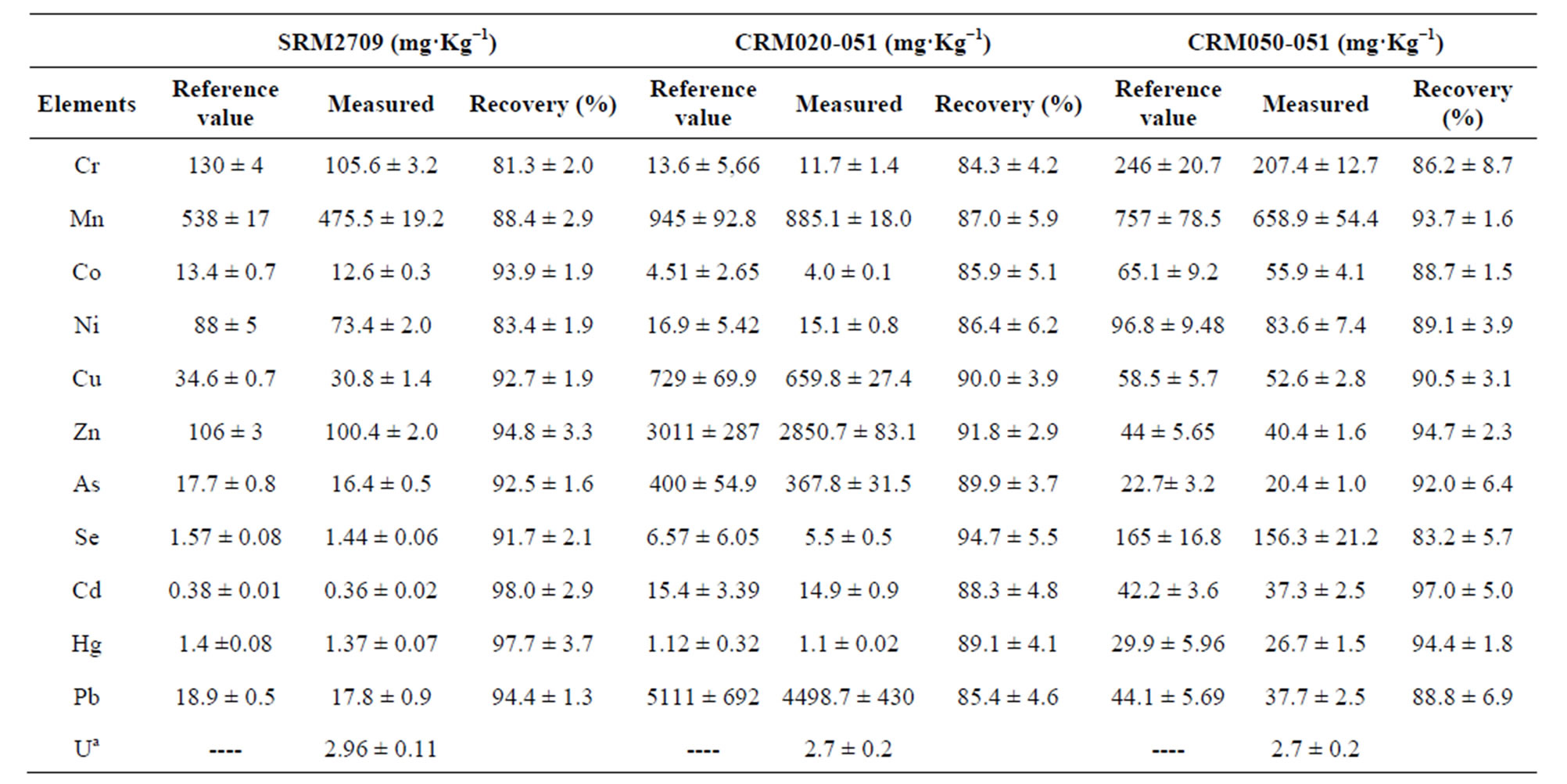
Table 5. Results of the quantification of elements in Certified Reference Materials (CRMs).
aU content is unknown in the three CRM.
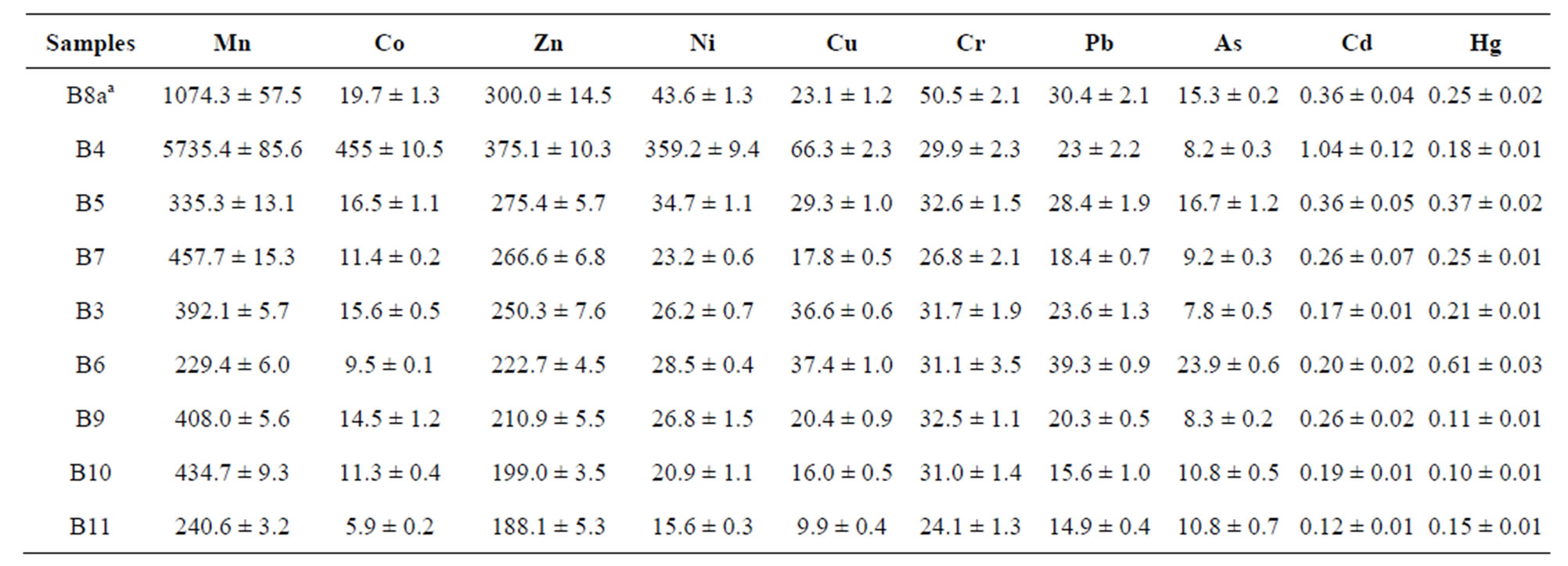
Table 6. Results of analysis of soil samples coming of El Bierzo, Spain. The average concentration level of each element is given in mg·Kg−1.
aReference value.
sion, the method presented several advantages against other analytical techniques, such as the rapidity of analysis, good accuracy and very low LOQs.
REFERENCES
- Z. J. Xue, S. Q. Liu, Y. L. Liu and Y. L. Yan, “Health Risk Assessment of Heavy Metals for Edible Parts of Vegetables Grown in Sewage-Irrigated Soils in Suburbs of Baoding City, China,” Environmental Monitoring and Assessment, Vol. 184, No. 6, 2012, pp. 3503-3513. http://dx.doi.org/10.1007/s10661-011-2204-6
- M. Wang, B. Markert, W. Chen, C. Peng and Z. J. Ouyang, “Identification of Heavy Metal Pollutants Using Multivariate Analysis and Effects of Land Uses on Their Accumulation in Urban Soils in Beijing, China,” Environmental Monitoring and Assessment, Vol. 184 No. 10, 2012, pp. 5889-5897. http://dx.doi.org/10.1007/s10661-011-2388-9
- E. Oyoo-Okoth, W. Admiraal, O. Osano, V. Ngure, M. H. Kraak and E. S. Omutange, “Monitoring Exposure to Heavy Metals among Children in Lake Victoria, Kenya: Environmental and Fish Matrix,” Ecotoxicology and Environmental Safety, Vol. 73 No. 7, 2010, pp. 1797-1803. http://dx.doi.org/10.1016/j.ecoenv.2010.07.040
- M. Klavins, O. Potapovics and V. Rodinov, “Heavy Metals in Fish from Lakes in Latvia: Concentrations and trends of Changes,” Bulletin of Environmental Contamination and Toxicology, Vol. 82, No. 1, 2009, pp. 96-100. http://dx.doi.org/10.1007/s00128-008-9510-x
- S. J. Hill, T. A. Arowolo, O. T. Butler, J. M. Cook, M. S. Cresser, C. Harringtone and D. L. Miles, “Atomic Spectrometry Update. Environmental Analysis,” Journal of Analytical Atomic Spectrometry, Vol. 18, No. 2, 2003, pp. 170-202. http://dx.doi.org/10.1039/b212655a
- J. P. Goulle, L. Mahieu, J. Castermant, N. Neveu, L. Bonneau, G. Laine, D. Bouige and C. Lacroix, “Metal and Metalloid Multi-Elementary ICP-MS Validation in Whole —Blood, Plasma, Urine and Hair. Reference Values,” Forensic Science International, Vol. 153, No. 1, 2005, pp. 39-44.
- N. Jakubowski, T. Prohaska, L. Rottmann and F. Vanhaecke, “Inductively Coupled Plasmaand Glow Discharge Plasma-Sector Field Mass Spectrometry Part I. Tutorial: Fundamentals and Instrumentation,” Journal of Analytical Atomic Spectrometry, Vol. 26, No. 4, 2011, pp. 693-726. http://dx.doi.org/10.1039/c0ja00161a
- N. Jakubowski, T. Prohaska, F. Vanhaecke, P. H. Roos and T. Lindemann, “Inductively Coupled Plasmaand Glow Discharge Plasma-Sector Field Mass Spectrometry Part II. Applications,” Journal of Analytical Atomic Spectrometry, Vol. 26, No. 4, 2011, pp. 727-757. http://dx.doi.org/10.1039/c0ja00007h
- K. G. Heumann, S. M. Gallus, G. Rädlinger and J. Vogl, “Precision and Accuracy in Isotope Ratio Measurements by Plasma Source Mass Spectrometry,” Journal of Analytical Atomic Spectrometry, Vol. 13, No. 9, 1998, pp. 1001-1008. http://dx.doi.org/10.1039/a801965g
- W. T. May and H. R. Wiedmeyer, “A Table of Polyatomic Interferences in ICP-MS,” Journal of Analytical Atomic Spectrometry, Vol. 19, 1998, pp. 150-155.
- E. H. Evans and J. J. Giglio, “Interferences in Inductively Coupled Plasma Mass Spectrometry. A Review,” Journal of Analytical Atomic Spectrometry, Vol. 8, No. 1, 1993, pp. 1-18. http://dx.doi.org/10.1039/ja9930800001
- M. Iglesias, N. Gilon, E. Poussel and J-M. Mermet, “Evaluation of an ICP-Collision/Reaction Cell-MS System for the Sensitive Determination of Spectrally Interfered and Non-Interfered Elements Using the Same Gas Conditions,” Journal of Analytical Atomic Spectrometry, Vol. 17, No. 10, 2002, pp. 1240-1247. http://dx.doi.org/10.1039/b204786c
- R. Wahlen, L. Evans, J. Turner and R. Hearn, “The Use of Collision/Reaction Cell ICP-MS for the Determination of Elements in Blood and Serum Samples,” Spectroscopy, Vol. 20, No. 12, 2005, p. 84.
- I. Feldmann, N. Jakubowski, C. Thomas and D. Stuewer, “Application of Hexapole Collision and Reaction Cell in ICP-MS. Part II Analytical Figures of Merit and First Applications,” Fresenius’ Journal of Analytical Chemistry, Vol. 365, No. 5, 1999, pp. 422-428. http://dx.doi.org/10.1007/s002160051634
- M. Moldovan, E. M. Krupp, A. E. Holliday and O. X. F. Donard, “High Resolution Sector field ICP-MS and Multicollector ICP-MS as Tools for Trace Metal Speciation in Environmental Studies: A Review,” Journal of Analytical Atomic Spectrometry, Vol.19, No. 7, 2004, pp. 815-822. http://dx.doi.org/10.1039/b403128h
- D. Beauchemin, “Environmental Analysis by Inductively Coupled Plasma Mass Spectrometry,” Mass Spectrometry Reviews, Vol. 29, No. 4, 2010, pp. 560-92. http://dx.doi.org/10.1002/mas.20257
- J. S. Becker, “Recent Developments in Isotope Analysis by Advanced Mass Spectrometric Techniques Plenary Lecture,” Journal of Analytical Atomic Spectrometry, Vol. 20, No. 11, 2005, pp. 1173-1184. http://dx.doi.org/10.1039/b508895j
- F. J. Díaz, M. Mejuto, A. I. Cardona, V. Rodríguez and A. García-Álvarez, “Soil Eco-Physiological Indicators from a Coal Mining Area in El Bierzo District (Spain),” EGU General Assembly, Vienna, Vol. 12, 2-7 May 2010, p. 11350.
- M. Mejuto, “Afectación de la Minería del Carbón en las Propiedades Físicas y Químicas de los Suelos de la Cuenca Hidrográfica del río Rodrigatos (El Bierzo, León),” Centro de Investigaciones Energéticas, Medio Ambientales y Tecnológicas (CIEMAT) Press, Madrid España, 2012.
- Normas Españolas Certificadas, “Preparación de muestras para ensayos de suelo,” UNE 103.100, Madrid España, 1995.
- D. D. Link, P. J. Walter and H. M. Kingston, “Development and Validation of the New EPA Microwave-Assisted LeachMethod 3051A,” Environmental Science & Technology, Vol. 32, No. 22, 1998, pp. 3628-3632. http://dx.doi.org/10.1021/es980559n
- X. H. Feng, L. M. Zhai, W. F. Tan, F. Liu and J. Z. He, “Adsorption and Redox Reactions of Heavy Metals on Synthesized Mn Oxide Minerals,” Environmental Pollution, Vol. 147, No. 2, 2007, pp. 366-73. http://dx.doi.org/10.1016/j.envpol.2006.05.028
- A. Neaman F. Mouélé, F. Trolard and G. Bourrié, “Improved Methods for Selective Dissolution of Mn Oxides: Applications for Studying Trace Element Associations,” Applied Geochemistry, Vol. 19, No. 6, 2004, pp. 973-979. http://dx.doi.org/10.1016/j.apgeochem.2003.12.002
- C. H. Green, D. M. Heil, G. E. Cardon, G. L. Butters and E. F. Kelly, “Solubilization of Manganese and Trace Metals in Soils Affected by Acid Mine Runoff,” Journal of Environmental Quality, Vol. 32, No. 4, 2003, pp. 1323- 1334. http://dx.doi.org/10.2134/jeq2003.1323
NOTES
*Corresponding author.

