American Journal of Analytical Chemistry
Vol.3 No.11(2012), Article ID:24467,8 pages DOI:10.4236/ajac.2012.311096
Development of a Highly Sensitive Extractive Spectrophotometric Method for the Determination of Nickel(II) from Environmental Matrices Using 2-Acetylpyridine-4-methyl-3-thiosemicarbazone
1Department of Chemistry, Analytical Division, College of Natural and Computational Sciences, Mekelle University, Mekelle, Ethiopia
2Department of Chemistry, Government Degree College for Women, Chintamani, Karnataka
3Department of Chemistry, Analytical Division, Sri Krishnadevaraya University, Anantapur, India
Email: *dndnrchem@gmail.com
Received April 19, 2012; revised May 24, 2012; accepted June 5, 2012
Keywords: Nickel(II); Environmental Matrices; Extractive Spectrophotometry; 2-Acetylpyridine-4-methyl-3-thiosemicarbazone and Atomic Absorption Spectrometry
ABSTRACT
Nickel(II) reacts with 2-acetylpyridine-4-methyl-3-thiosemicarbazone (APMT) and forms a yellow colored complex, which was extracted into n-hexanol from sodium acetate and acetic acid buffer at pH 6.0. The absorbance value of the Ni(II)-APMT complex was measured at different intervals of time at 375 nm to ascertain the time stability of the complex. The extraction of the complex into the solvent was instantaneous and stable for more than 72 hrs. The system obeyed Beer’s law in the concentration range of 0.235 - 2.43 µg×ml–1 of nickel(II), with an excellent linearity and a correlation coefficient of 0.999. The molar absorptivity and Sandell’s sensitivity of the extracted species were found to be 2.16 × 104 L mol–1×cm–1 and 0.003 µg×cm–2 at 375 nm, respectively. Hence a detailed study of the extraction of nickel(II) with APMT has been undertaken with a view to developing a rapid and sensitive extractive spectrophotometric method for the determination of nickel(II) when present alone or in the presence of diverse ions which are usually associated with nickel(II) in environmental matrices like soil and industrial effluents. Various standard alloy samples (CM 247 LC, IN 718, BCS 233, 266, 253 and 251) have been tested for the determination of nickel for the purpose of validation of the present method. The results of the proposed method are comparable with those from atomic absorption spectrometry and were found to be in good agreement.
1. Introduction
An important use of nickel in the food industry as a catalyst is evident from its use in the hydrogenation of oils. Nickel is an important element due to its high strength and resistance to corrosion in many media [1]. It is used in nickel plating and also in the manufacture of alloys along with iron, copper, aluminum, chromium, zinc and molybdenum. Nickel containing steels are highly resistant to corrosion. Because of its high melting point (1453˚C), nickel is also used in the production of heatresistant steels and cast iron. Nickel-plated steels are used in the manufacture of some food processing vessels and many other pieces of equipment. Nickel(II) is present in small amounts in most of the soils, plants and animal tissues. The interest in the determination of nickel has grown considerably in recent years, owing to its involvement in some essential metabolic processes [2]. Nickel is relatively non-toxic and does not cause any serious human health hazard, despite the fact that acid foods take up nickel during cooking. The nickel deposited in the human body from nickel vessels is not readily absorbed and causes no detectable hazard. However, a high incidence of respiratory tract neoplasia among workers in nickel refineries and carcinogenic properties of this metal have been reported [1]. Thioand phenylthiosemicarbazones have a wide range of applications in medicine and agriculture. Owing to the ability of these reagents to form intense colored complexes with various metal ions [3-5] they are widely employed in spectrophotometric and extractive spectrophotometric analysis, atomic absorption spectrometry and solid-liquid separation. The nickel(II)- thiosemicarbazone complexes have intense colors and high molar absorptivities when compared with the analogous thiosemicarbazone complexes. A literature survey indicated that only a few thiosemicarbazones [6-13] have been explored for the extractive spectrophotometric determination of nickel(II) and 2-acetylpyridine-4-methyl- 3-thiosemicarbazone (APMT) has so far not been used as an analytical reagent for the extraction of nickel(II). In the present work, APMT has been examined in order to evaluate its usefulness as an extractive spectrophotometric reagent for nickel(II). Further, this method has been applied successfully for the analysis of nickel(II) in environmental matrices like soil and industrial effluents. For the determination of nickel at micro levels there are several frequently adopted methods using analytical techniques such as AAS, ICP-OES, ICP-AES, ICP-MS, X-ray fluorescence spectroscopy, spectrophotometry, spectrofluorometry and other such techniques. Among these, the spectrophotometric methods are preferred as they are cheaper and easier to handle and have comparable sensitivity.
2. Experimental
2.1. Instrumentation
A Shimadzu 240 UV-VIS spectrophotometer with a 1.0 cm quartz cell was used for absorbance studies. An Elico LI-120 digital pH meter was used for pH adjustment. A Perkin-Elmer 2380 atomic absorption spectrometer was used for the comparison of results.
2.2. Reagents and Samples
2-acetylpyridine amounting 0.5 g was dissolved in 25.0 ml ethanol and mixed in a flask containing 1.5 g 4- methyl 3-thiosemicarbazide dissolved in 25.0 ml of a 1:1 ethanol-water mixture. The resulting reaction mixture was refluxed in a water bath for 30 min. It was allowed to stand at room temperature until pale yellow crystals were formed [14]. These were separated and recrystallized from ethanol (Scheme 1).
The molecular formula and molecular weight of APMT are C9H12N4S and 250 respectively. The compound was characterized by IR and 1H-NMR spectral data. Infra red spectrum of APMT shows bands at (3288 (s), 3239 (s)3043 (m), 1577 (s), 1537 (s), 1497 (s), 1364 (w), 1147 (m), 832 (s) and 681 (s) cm–1 corresponding to n(N-H) (asymmetric and symmetric), n(C-H)aromatic stretch, n(C=N) stretching(Schiff base), n(C-H) aromatic ring, n(C-H) of pyridine ring, n(N-H) stretch(primary amide), n(C=S), n(C-H)-oop bend (aromatic) and n(C-C)-oop bend aromatic ring vibrations. 1H-NMR spectrum of APMT (CDCl3+DMSO-d6) showed signals at 2.39 (3H,S), 7.37 - 8.58 (m) due to C5H4N (pyridine), and 3.24 (s) corresponds to CH3 group attached to nitrogen atom of thiosemicarbazone.
2.3. Preparation of Standard Solution of Nickel(II)
6.73 g of ammonium nickel sulfate hexahydrate [(NH4)2×Ni(SO4)2×6H2O)] was weighed, dissolved in double-distilled water containing a few drops of concentrated sulfuric acid and made up to one liter. The stock solution was then standardized gravimetrically using dimethylglyoxime[15]. The required dilute solutions of nickel(II) were prepared by diluting the stock solution with doubledistilled water. All reagents used were of analytical reagent grade unless otherwise stated.
2.3.1. Buffer Solutions
1.0 M Hydrochloric acid and 1M sodium acetate (pH 0.5 - 3.0), 0.2 M of NaOAc and 0.2 M AcOH (pH 4.0 - 7.0) and 2.0 M NH4Cl - 2.0 M NH4OH (7.0 - 10.0) buffer solutions were prepared in distilled water. Suitable portions of these solutions are mixed to get the desired pH.
2.3.2. Collection of Environmental Matrices, Preparation of Solutions and Analytical Procedure
The environmental matrices (soil and industrial effluents) to be analyzed were collected from around Kadapa, Andhra Pradesh, South India. The dried sample was pulverized in a mortar for the purpose of analysis, to a convenient size. An aliquot of 500 mg of soil in100 ml of industrial effluent was digested with 5 ml of HNO3 (65%) in a Teflon vessel. The sample was digested for 3 hrs at 80˚C, and again digested at 160˚C for 45 min. After treating with double-distilled water the supernatant liquid was made up to the mark in a 25 ml standard flask.
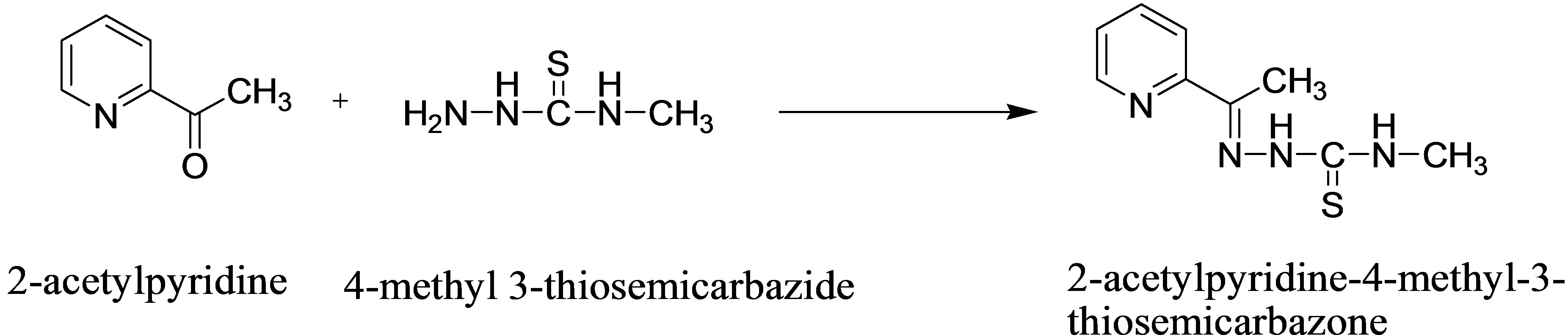
Scheme 1. Synthesis of 2-acetylpyridine-4-methyl-3-thiosemicarbazone (APMT).
2.3.3. Analytical Procedure for Standard Alloy Samples
A total of 0.1 g of each oven-dried (110˚C) alloy sample was dissolved in 15 ml of aqua regia. The solution was heated to near dryness and nitrate was expelled from the residue using 5ml of concentrated hydrochloric acid. Each residue was extracted into double-distilled water separately and made up to 100 ml.
2.3.4. General Procedure
To an aliquot of a working standard solution containing 0.1 - 10 µg×ml–1 nickel(II) were added pH 6.0 buffer (3 ml), 1 × 10–3 M reagent solution (1 ml) and a salting-out agent, 0.1 M magnesium sulfate (1 ml). The mixture was shaken two times with 10 ml portions of n-hexanol each time for 1 min and allowed to stand for a few minutes. The two organic phases were collected into a 25.0 ml volumetric flask and made up to the mark with n-hexanol. The absorbance for all the organic phases was measured at 375 nm against the reagent blank.
3. Results and Discussion
3.1. Absorption Spectra of the Reagent and Ni(II)-APMT Complex
An aliquot of 1.0 ml of 4 × 10–5 M nickel(II) solution was transferred into a 25 ml separating funnel and to it; 3.0 ml of buffer (pH 6.0) and 1.0 ml of 4 × 10–4 M APMT solutions were added. The absorption spectrum of the reagent solution against the solvent blank, and the absorption spec- trum of the solution containing nickel(II) complex against the reagent blank, were recorded. From the spectra, it is clear that the Ni(II)-APMT complex and the reagent have maximum absorbance at 375 and 340 nm, respectively. The reagent has a minimum absorbance at the maximum absorbance of the complex and does not interfere in the determination of nickel(II). Hence, further absorbance measurements of the complex were carried out at 375 nm.
3.2. Effect of pH on the Extraction of Ni(II)-APMT Complex
A preliminary study showed that the formation of Ni(II)- APMT complex was affected by the hydrogen ion concentration. The optimum pH range for the absorbance was determined by using buffers such as potassium chloride-hydrochloric acid (pH 1.0 - 2.6), sodium formateformic acid (pH 2.6 - 3.4), sodium acetate–acetic acid (pH 3.4 - 6.5) and ammonium chloride–ammonium hydroxide (pH 7.0 - 11.0). In each case, a mixture containing 1.0 ml of 4 × 10–5 M nickel(II) solution, 3.0 ml of the suitable buffer and 1.0 ml of 4 × 10–4 M of APMT solution were taken into a 25 ml separating funnel and the volume of the aqueous phase adjusted to 10.0 ml with double distilled water. The absorbance for all the organic extracts was measured at 375 nm using their respective reagent blanks. A graph between pH and absorbance is given in Figure 1. This indicates that the complex shows maximum absorbance in the pH range 5.5 - 6.5. so in subsequent studies buffer having pH 6.0 was used.
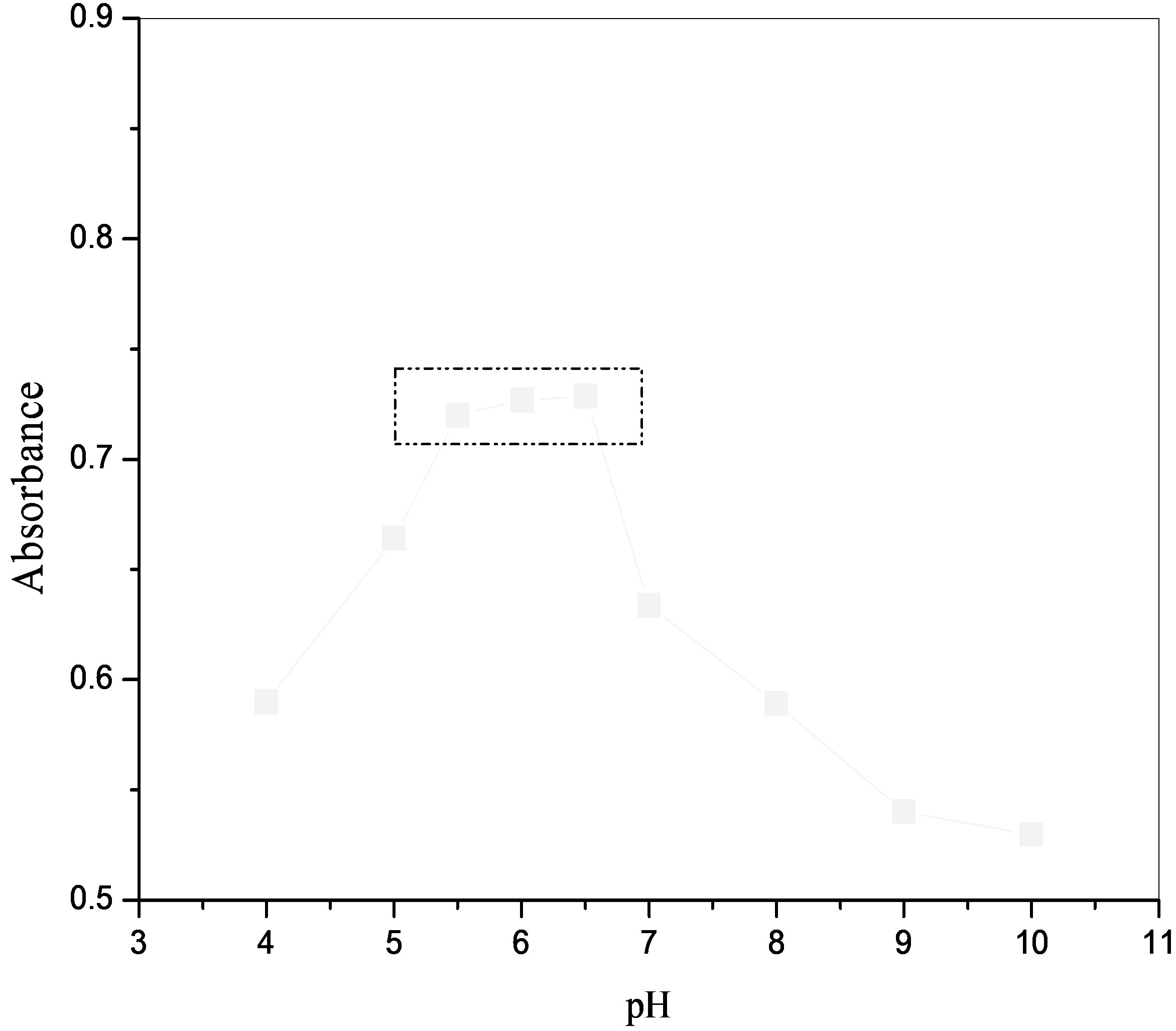
Figure 1. Effect of pH on the absorbance of Ni(II)-APMT complex; Ni(II) 1.0 ml of 4 × 10–5 M; APMT 1.0 ml of 4 × 10–4M.
3.3. Effect of Reagent Concentration on the Absorbance of Ni(II)-APMT Complex
The effect of reagent concentration on the formation of the Ni(II)-APMT complex was studied using 1.0 ml of 4 × 10–5 M metal ion solution, 3.0 ml of pH 6.0 buffer and 1.0 ml of APMT solution containing different concentrations ranging from 4 × 10–4 M to 12.03 × 10–4 M. The total volumes of the aqueous phases were brought up to 10.0 ml with double-distilled water. The aqueous phases were shaken separately with 10.0 ml of n-hexanol, and the organic phases were collected into 25 ml standard flasks. The organic phases were made up with n-hexanol and the absorbances of these phases were measured at 375 nm, against their corresponding reagent blanks. This study has revealed that a ten-fold molar excess of the APMT to that of nickel is necessary for maximum extraction of the metal ion. Hence, a tenfold molar excess of the reagent was maintained for maximum extraction of nickel(II).
3.4. Effect of Solvents on the Extraction of Ni(II)-APMT Complex
Solvents such as isoamylalcohol, n-amylalcohol, n-butanol, n-hexanol, benzene, xylene, chloroform, carbon tetrachloride, cyclohexane, cyclohexanol and methylisobutylketone were examined as extractants. As per the results reported in Table 1, n-butanol was found to be a suitable solvent for the effective extraction of Ni(II)- APMT complex. Hence, n-hexanol was chosen for all further studies.
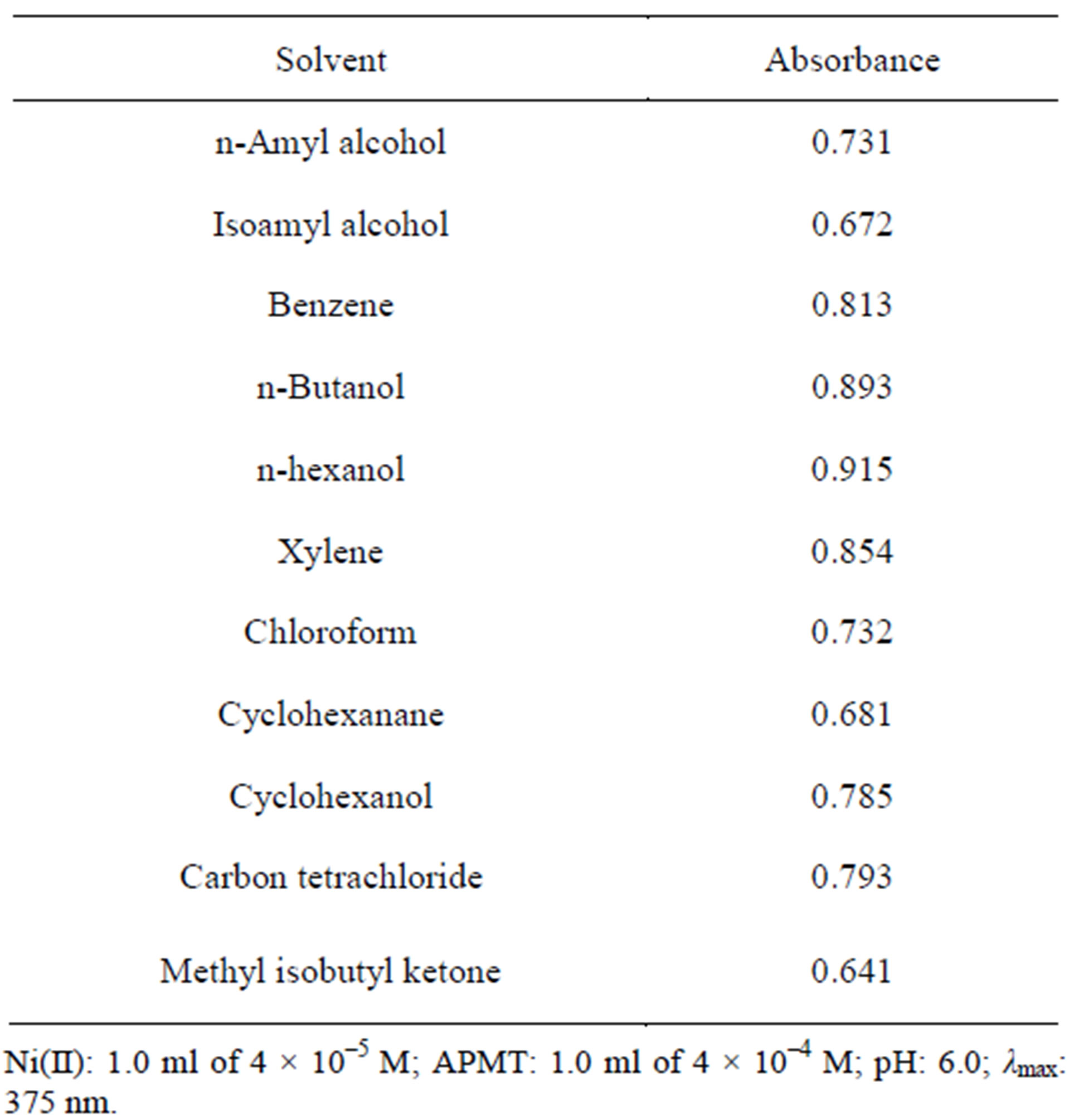
Table 1. Effect of solvents on the extraction of Ni(II)-APMT complex.
3.5. Effect of Salting-Out Agents on the Extraction of Ni(II)-APMT Complex
Various salting-out agents, such as magnesium sulfate, magnesium nitrate, lithium acetate, lithium sulfate, lithium nitrate and ammonium sulfate were employed to enhance the metal complex extraction into organic solvent in a single step. After the studies, it was observed that the presence of 0.1 M magnesium nitrate solution enhanced the extraction. The presence of nickel(II) in the aqueous phase after extraction was tested gravimetrically by using dimethylglyoxime. It was found that with magnesium nitrate as a salting-out agent, the complex was extracted quantitatively into n-hexanol.
3.6. Time Stability of the Color Reaction
The absorbance value of the Ni(II)-APMT complex was measured at different intervals of time at 375 nm to ascertain the time stability of the color of the complex. It was observed that the color remained constant for more than 72 hrs. Physico-chemical and analytical properties of nickel (II) complex of APMT are summarized in Table 2.
3.7. Applicability of Beer’s Law to the Ni(II)-APMT Complex System
Known aliquots of 10.0 ml solutions containing constant volumes of 3.0 ml of buffer (pH 6.0), 1.0 ml of 4 × 10–4 M APMT, 1.0 ml of 0.1 M magnesium nitrate solution and varying amounts of nickel(II) ranging from 0.1 - 10.0 µg×ml–1 were prepared. Each solution was shaken with 10.0 ml of n-hexanol for 2 min and then allowed to settle.
The organic phases were collected in different 25.0 ml standard flasks and then made up with n-hexanol. The absorbance for all the organic phases was measured at 375 nm, against their corresponding reagent blanks. From the experimental data, it was found that the complex system obeys Beer’s law in the concentration range 0.235 - 2.43 µg×ml–1 of nickel(II). The straight line obeys the equation A375 = 0.3403 C + 0.0054. The molar absorptivity and Sandell’s sensitivity of the method are 2.16 × 104 L×mol<–1×cm–1 and 0.003 µg×cm–2 of Ni(II) respectively. The specific absorptivity of the system is found to be 0.368 ml×g–1×cm–1. The standard deviation in the determination of 1.17 µg×ml–1 of Ni(II) is 0.008 for ten determinations. The relative standard deviation and the mean absorbance are 0.67 percent and 0.4137 ± 0.0013 respectively.
3.8. Determination of the Composition of Ni(II)-APMT Complex
The composition of the Ni(II) complex with APMT was studied using Job’s method of continuous variation, and

Table 2. Physico-chemical and analytical characteristics of Ni-(APMT)2 complex at glance.
the mole ratio method.
Extractive spectrophotometric investigation of the metal complex was conducted to obtain the composition of the complex. The composition of the complex was established by Job’s method of continuous variation. Equimolar solutions of nickel(II) and APMT (2 × 10–4 M) were prepared. The metal and reagent solutions were mixed in different proportions, keeping the total volume constant at 1.0 ml. To each solution, 3.0 ml of buffer (pH 6.0) solution and 1.0 ml of 0.1 M magnesium nitration solution as salting-out agent were added and the volumes of the aqueous phases brought to 10.0 ml with double-distilled water. Each of the aqueous phases was shaken with 10.0 ml of n-hexanol for 2 min and allowed to settle. The organic phase was collected into a 25.0 ml standard flask and made up to the mark with n-hexanol. The absorbance values of the organic phases were recorded at 375 nm, against their respective reagent blanks. From the above experimental results, it is evident that one mole of nickel(II) reacts with two mole of APMT, showing the composition of the complex to be 1:2. This composition was verified using the molar ratio method. From jobs continuous variation method the stability constant of the complex has been found to be 8.3 × 1011.
3.9. Effect of Foreign Ions on the Extraction of Ni(II)-APMT Complex
Known amounts of various cations and anions were added to a fixed amount of nickel(II) in order to study the effect of interference of these ions on the extraction and determination of nickel(II), using the analytical procedure described in the Experimental section. An error of 72% in the absorbance reading was considered to be tolerable. The results are given in Table 3. Cations like aluminum(III), uranium(VI), indium(III) and iron(II) do not interfere, even when present up to 4000 µg/ml. Manganese(II), tungsten(VI), barium(II) and magnesium(VI) do not have any effect up to 2500 µg/ml, whereas zinc(II), lead(II), selenium(IV), cerium(IV), copper(II) and palladium(II) interfere seriously with the extraction of nickel(II). Anions like fluoride, chloride, bromide, tartrate, thiosulphate and oxalate do not interfere when present up to 4000 µg/ml or more. Thiocynate, thiourea and phosphate interfere seriously, whereas EDTA masks nickel(II) completely due to the higher stability of the Ni(II)-EDTA complex. Sulfate, acetate and citrate do not interfere when present up to 2000 µg/ml. The interference of zinc(II), lead(II) selenium(IV), cerium(IV), copper(II), cadmium(II) and palladium(II) can be eliminated
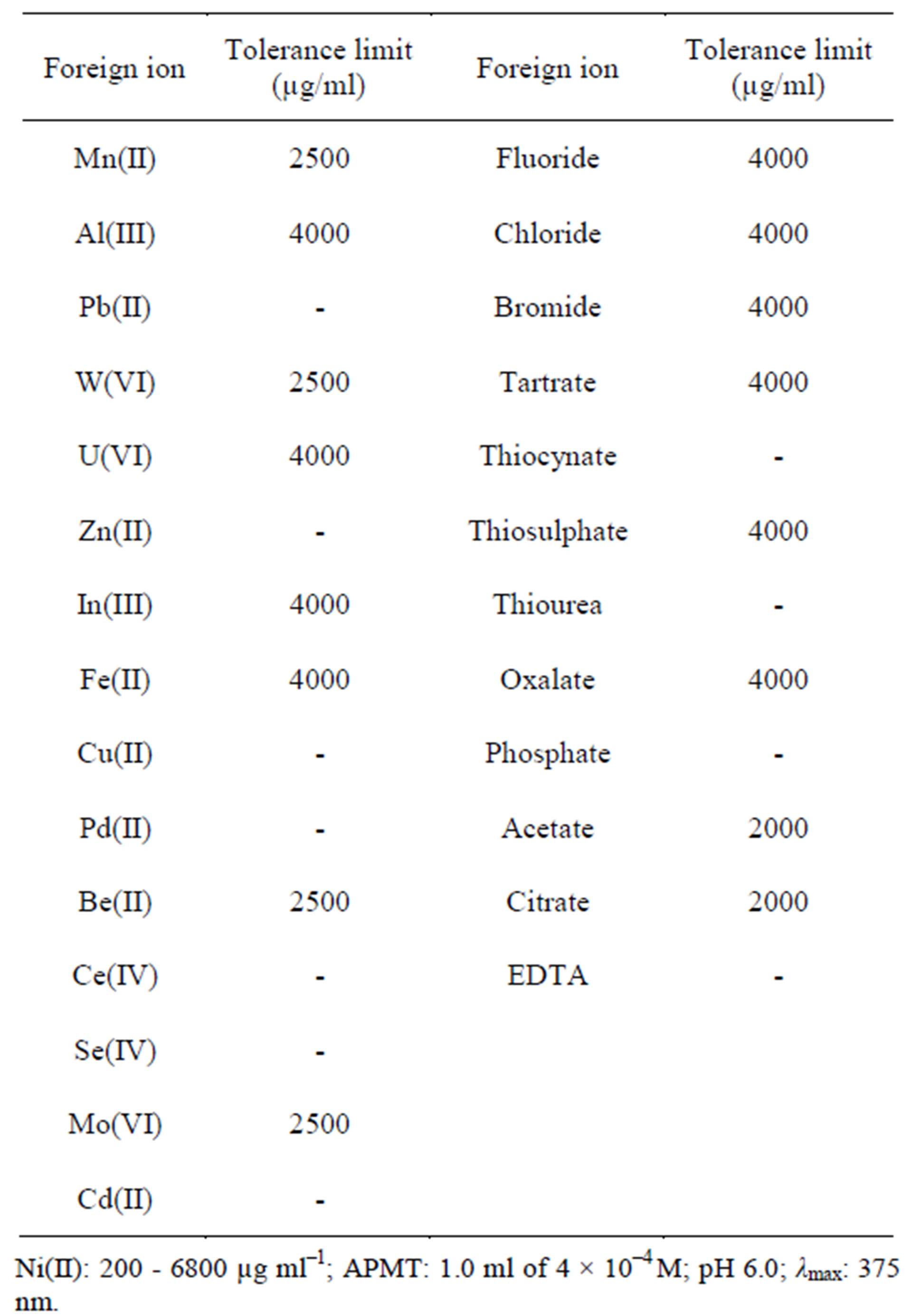
Table 3. Effect of foreign ions on the extraction of Ni(II)- APMT complex.
by using 1.0 ml of 0.5% thiosulphate solution.
The present method when compared with other existing spectrophotometric methods Table 4 shows more sensitive and selective. It also offers advantages like reliability and reproducibility in addition to its simplicity, instant color development and low interference. APMT is cheap, stable at high temperatures, and easy to dispense and store. The reagent APMT was more stable when complexed with Ni(II) and the color of the complex was stable for more than 48 hrs. APMT extracts nickel selectively when associated with the following metal ions: Al(III), U(VI), In(III), Fe(II), Mn(II), W(VI), Ba(II), Mg(VI), fluoride, chloride, bromide, tartrate, thiosulphate and oxalate. APMT is a stable reagent for extractive determination of nickel(II).
3.10. Applications
The developed extractive spectrophotometric method for nickel(II) has been successfully applied for its determination in environmental matrices and standard alloy samples.
3.10.1. Determination of Nickel(II) in Soil Samples
Soil samples were collected from in and around Kadapa, Ananthapur, AP, India. Each aliquot was analyzed for nickel(II) by the general procedure which was given in the experimental section. Nickel(II) present in soil samples was determined from the calibrated plot (Beer’s law plot) using APMT and the results checked by atomic absorption spectrometry Table 5.
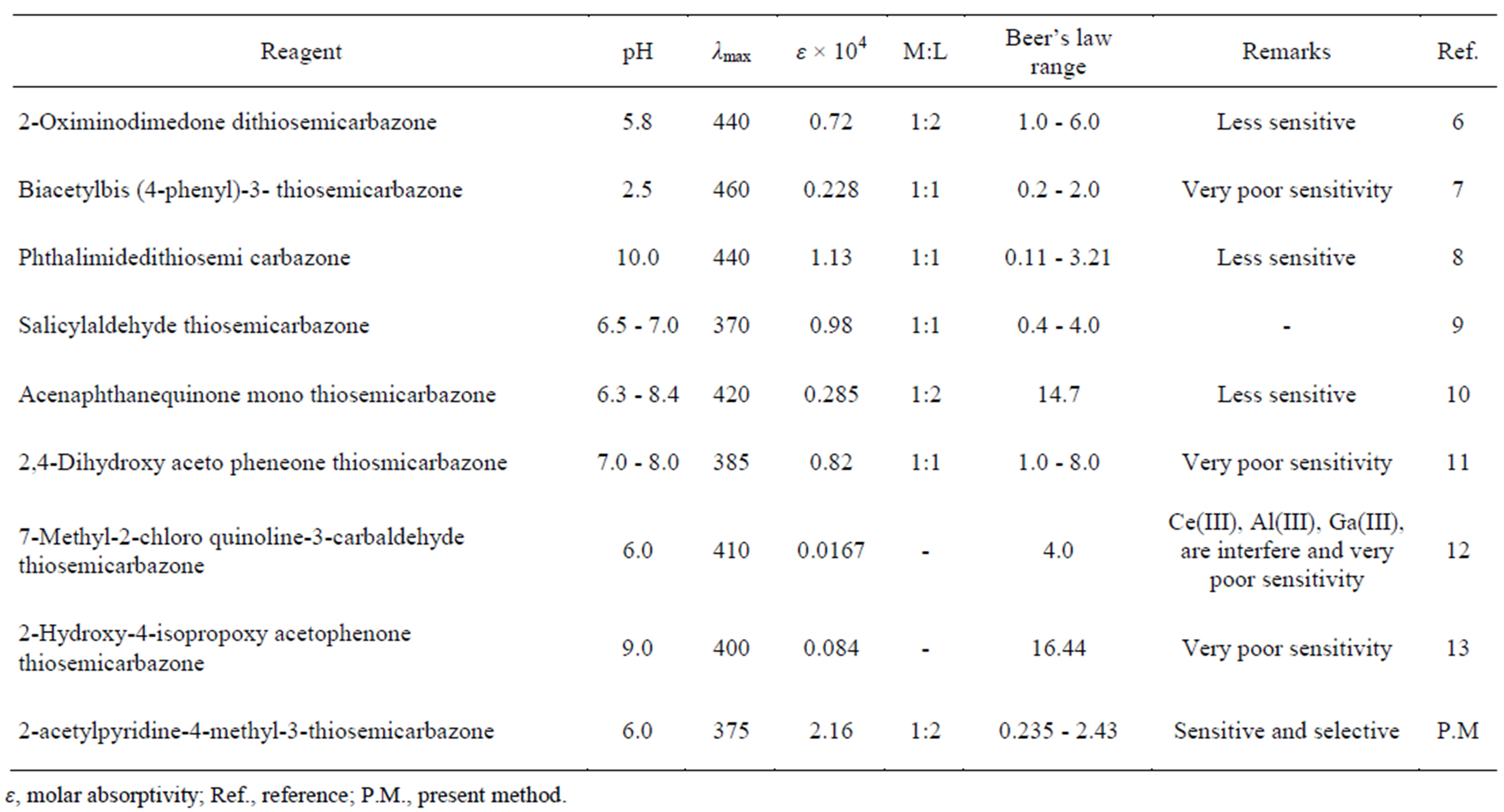
Table 4. Comparison of present method with other reported spectrophotometric methods.
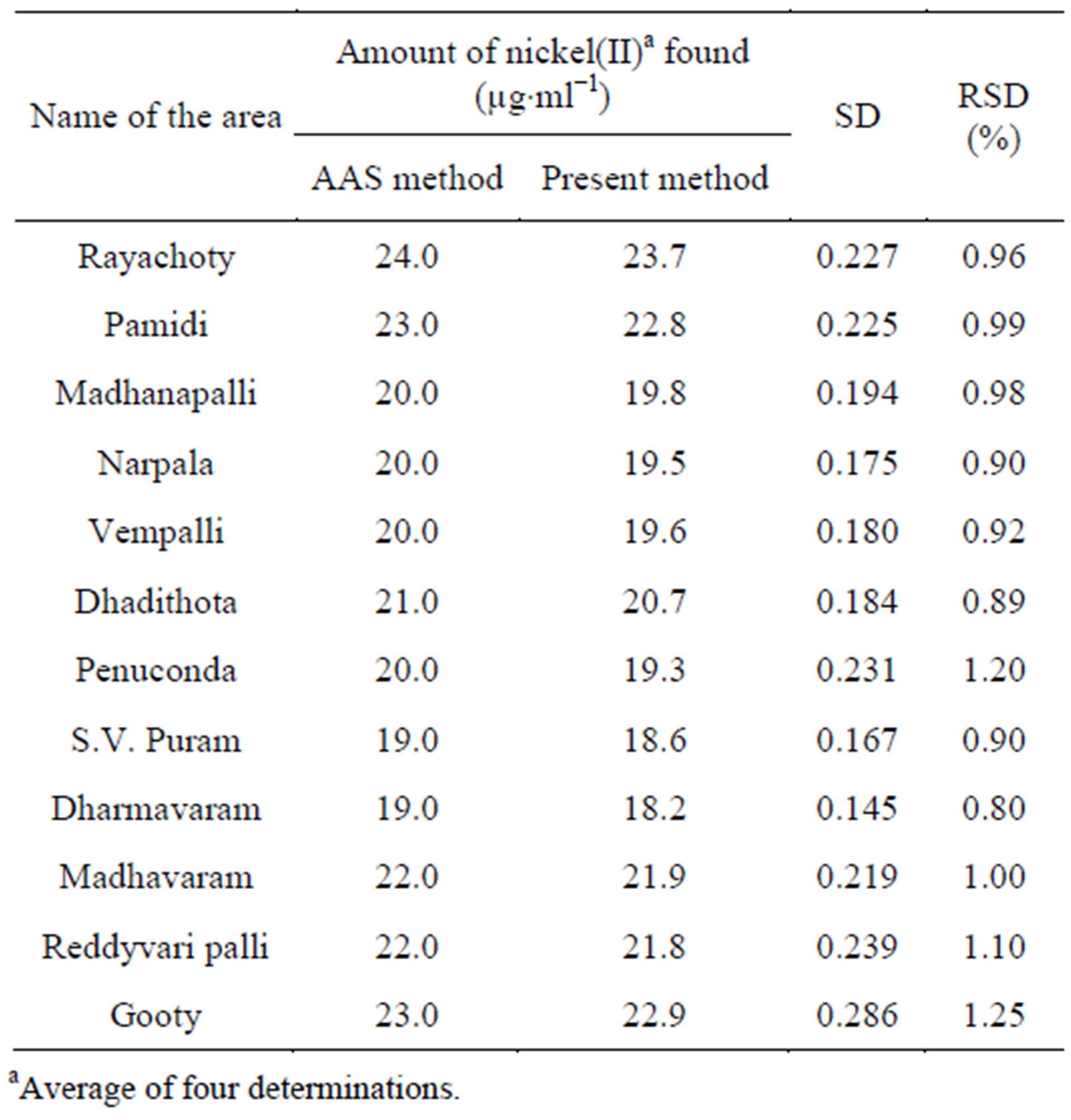
Table 5. Determination of nickel(II) in soil samples.
3.10.2. Determination of Nickel(II) in Industrial Effluents
Industrial effluents were collected from industrial areas in and around Kadapa, Andhra Pradesh, India. An appropriate amount of aliquot was taken for extractive spectrophotometric determination of nickel(II) by the method developed in the present work. The results obtained were confirmed by direct atomic absorption spectrometry Table 6.
3.10.3. Determination of Nickel(II) in Standard Alloy Samples
The present method was also applied for the determination of nickel(II) content in standard alloy samples such as nickel base super alloys (CM 247 LC and IN 718), alloy steels (BCS 233 and 266) and low alloy steels (BCS 253 and 251). An appropriate aliquot of each solution was analyzed for nickel(II) employing the recommended procedure given in Materials and methods, using APMT, and the obtained results were checked by direct atomic absorption spectrometry as shown in Table 7.
4. Conclusion
Thioand methylthiosemicarbazones are used in the extractive spectrophotometric determination of nickel(II). In the present investigation, the authors introduced a new reagent, 2-acetylpyridine-4-methyl-3-thiosemicarbazone (APMT) to the field of extractive spectrophotometric determination of nickel(II). The reagent was found to be sensitive when compared to earlier reported reagents. The selectivity of the reagent was further improved by
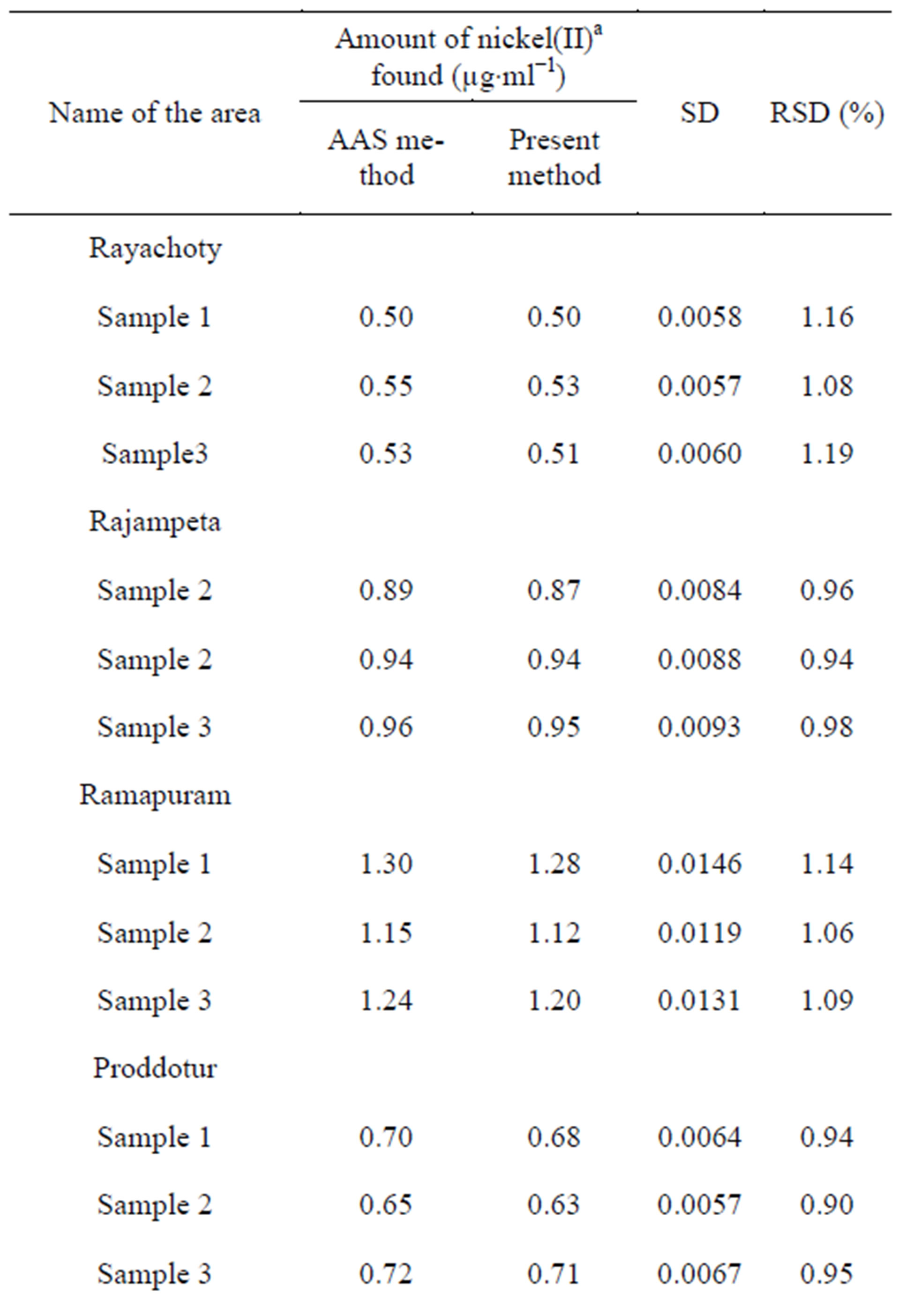
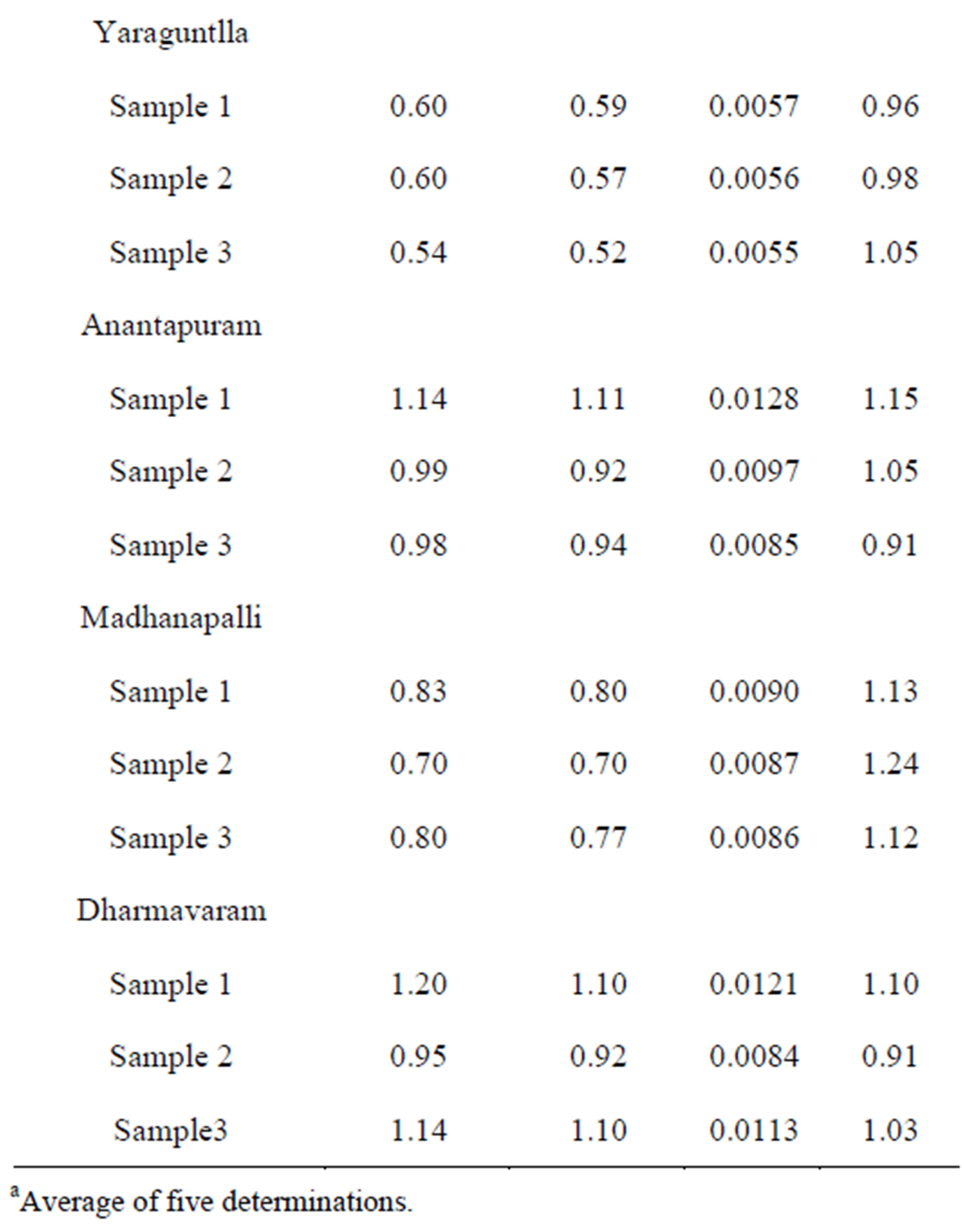
Table 6. Determination of nickel(II) in industrial effluents.
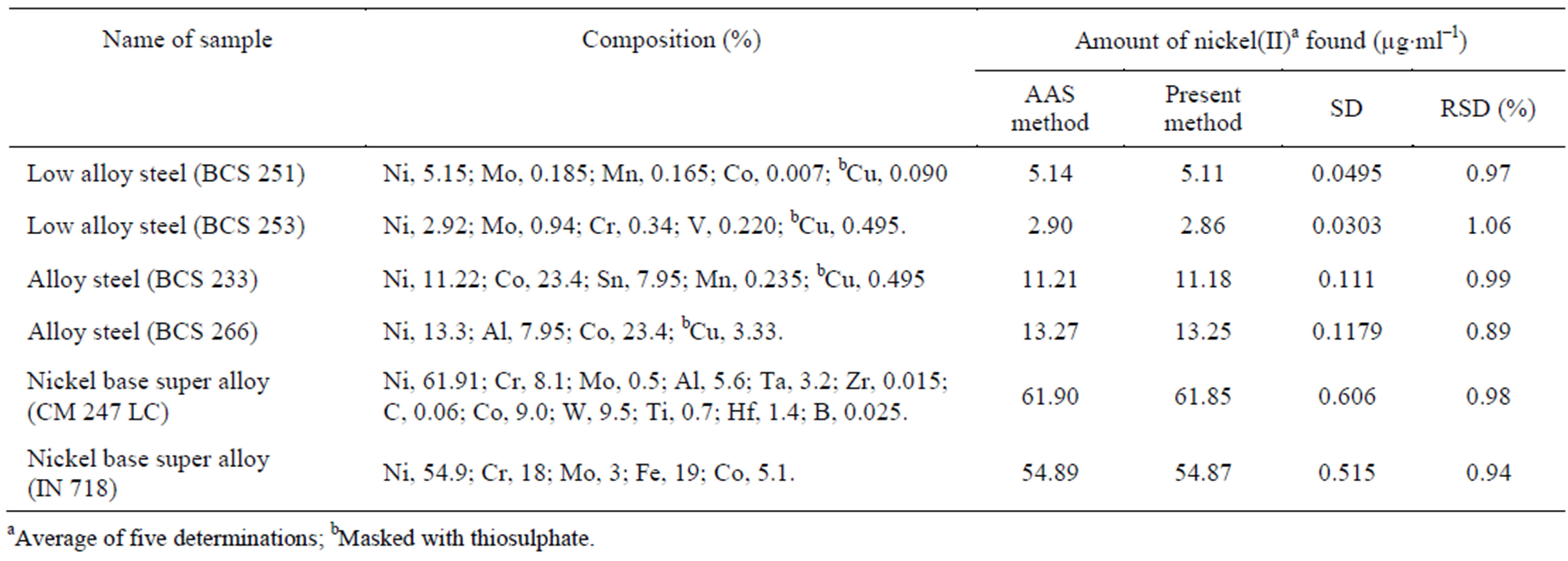
Table 7. Determination of nickel(II) in soil samples.
the use of proper masking agents to suppress the interference of diverse metal ions. The results from the present developed method clearly demonstrate the usefulness of APMT as an extracting agent for the determination of nickel(II) in environmental matrices and standard alloy samples.
5. Acknowledgments
The authors G. Ramachandra Reddy, A. Babul Reddy and P. Haribabu are highly grateful to University grants commission with special assistant programme (UGCSAP), Government of India, New Delhi for financial assistance in the form of Meritorious Research Fellowship.
REFERENCES
- B. K. Sharma, “Environmental Chemistry,” Goel Publishing House, Meerut, 1997.
- T. Z. Judith, T. Peter and T. Thomas, “Immunotoxicology of Environment Occupational Metals,” School of Medicine, New York, 1998.
- K. H. Reddy and D. V. Reddy, “Analytical Data and Comparison of Analytical Potentialities of Thiosemicarbazones and Semicarbazones,” Quarterly Chemistry Reviews, Vol. 1, 1985, pp. 47-98.
- B. S. Garg and V. K. Jain, “Analytical Applications of Thiosemicarbazones and Semicarbazones,” Microchemical Journal, Vol. 38, No. 2, 1988, pp. 144-169.
- R. B. Singh and H. Ishii, “Analytical Potentialities of Thiosemicarbazones and Semicarbazones,” Critical Reviews in Analytical Chemistry, Vol. 22, No. 5, 1991, pp. 381- 409. Hdoi:10.1080/10408349108051640
- F. Salinas, J. C. J. Sanchez and J. L. Lemus, “Spectrophotometric Study of 2-Oximinodimedone Dithiosemicarbazone-ni(II) Complex. Determination of Ni(II),” Bulletin des Sociétés Chimiques Belges, Vol. 95, No. 4, 1986, pp. 293-294. Hdoi:10.1002/bscb.19860950411
- A. G. Asuero, “Spectrophotometric Determination of Nickel with Biacetyl Bis(4-phenyl-3-thiosemicarbazone),” Microchemical Journal, Vol. 28, No. 2, 1983, pp. 198-202.
- K. G. Reddy, K. M. M. S. Prakash, K. H. Reddy and D. V. Reddy, “Rapid Spectrophotometric Determination of Nickel(II) Using Phthalimide Dithiosemicarbazone,” Acta Ciencia Indica, Series Chemistry, Vol. 10, 1984, pp. 175- 177.
- S. K. Singh, M. Kamini and S. K. Sindhwani, “Spectrophotometric Determination of Cobalt(II), Nickel(II) and Copper(II) with Acenaphthenequinone Monosemicarbazone (AQSC),” Journal of the Chinese Chemical Society (Taipei), Vol. 29, No. 2, 1982, pp. 131-134.
- K. H. Reddy and D. V. Reddy, “Analytical Data and Comparison of Analytical Potentialities of Thiosemicarbazones and Semicarbazones,” Quarterly Chemistry Reviews, Vol. 1, 1985, pp. 47-98.
- A. V. Reddy and Y. K. Reddy, “Sequential Extraction and Determination of Copper and Nickel with 2,4-Ihydroxy Acetophenone Thiosemicarbazone,” Talanta, Vol. 33, No. 7, 1986, pp. 617-619. doi:10.1016/0039-9140(86)80141-2
- V. A. Jadhav and M. U. Kulkarni, “7-Methl-2-chloroquinoline-3-carbaldehyde Thiosemicarbazone as Analytical Reagent for Copper, Cobalt and Nickel(II),” Journal of the Indian Chemical Society, Vol. 69, No. 5, 1992, pp. 287- 288.
- D. K. Desai and K. K. Desai, “2-Hdroxy-4-isopropoxyacetophenone Thiosemicarbazone as a Spectrophotometric Reagent for Nickel(II),” Oriental Journal of Chemistry, Vol. 12, 1996, pp. 203-205.
- E. Cristofol, F. Sanchez Rojas and J. M. C. Pavon, “Evaluations of Various N-Phenylthiosemicarbazones as Chromogenic Reagents in Spectrophotometric Analysis,” Talanta, Vol. 38, No. 4, 1991, pp. 445-448. Hdoi:10.1016/0039-9140(91)80084-D
- A. I. Vogel, “A Text Book of Quantitative Inorganic Analysis,” Longmans, Green and Co., London, 1961.
NOTES
*Corresponding author.

