American Journal of Analytical Chemistry
Vol. 3 No. 7 (2012) , Article ID: 21035 , 8 pages DOI:10.4236/ajac.2012.37061
Determination of Apparent Formation Constants of Eu(III) with Humic Substances by Ion Selective Liquid Membrane Electrode
1Department of Nuclear Engineering, Kyoto University, Kyoto, Japan
2Research Reactor Institute, Kyoto University, Osaka, Japan
Email: *sasaki@nucleng.kyoto-u.ac.jp
Received April 15, 2012; revised May 10, 2012; accepted June 7, 2012
Keywords: Apparent Formation Constant; Humic Substance; Eu(III); Ion Selective Electrode; Titration
ABSTRACT
This is the novel measurement of the apparent formation constants of trivalent lanthanide with humic substances by potentiometric titration using an ion selective electrode (ISE) consisting of bis(diphenylphosphoryl)methane as an ionophore. The ISE prepared exhibi-ted a Nernstian response to the Eu3+ concentration in the test solutions in the presence of humic acid and fulvic acid. The effect of the solution conditions, including the pH, initial metal and humic substance concentrations, and ionic strength, on the formation constants was examined. The present formation constants were compared with the reported data using solvent extraction method.
1. Introduction
Naturally occurring humic substances (fulvic and humic acids) exist in colloidal, suspended, and dissolved forms in groundwater. The molecular framework of these substances consists of aromatic rings and hydrocarbon chains. The negatively charged major functional groups—carboxyl and phenol groups—are distributed heterogeneously and coordinate with numerous radionuclides, including those in the trivalent ions of Am, Cm, and lanthanides [1,2] as radioactive wastes, which arise from the reprocessing of the spent fuel used in nuclear power plants. Thus, understanding the impact of humic substances on the migration behavior of trivalent radionuclides has been essential for safety assessments related to the disposal of such radioactive waste. The migration behavior of the trivalent metal ions is predominantly controlled by the solubility products, along with the sorption onto an artificial barrier and host rock in groundwater. Therefore, there is an intrinsic need for the formation constants of water-soluble organometallic species from the chemical thermo-dynamics point of view.
The apparent formation constants of metal-humate complexes under a variety of aquatic conditions have been determined using several traditional methods such as solvent extraction [3], spectrophotometry, conductimetry, ion exchange methods such as Schubert’s method [4- 6], ultra filtration [7,8], equilibrium dialysis method [9], and titration method using a pHand/or ion selective electrodes (ISE) [10-12]. Potentiometric titration using an ISE is a very conventional approach that is used to directly and easily measure the activity of free (uncomplexed) metal ions. Although a few ion-selective electrodes have been developed for the potentiometric determination of trivalent lanthanide and actinide ions [13-16], no application to determine the formation constant has been reported. Recently, we developed a novel technique to determine the formation constants of trivalent metal ions with carboxylic acid electrochemically, using a liquid membrane electrode [17,18]. The ISE exhibited a Nernstian response to a wide range of pH values, as well as various concentrations of Eu3+ as an analogue of Am3+ and Cm3+, along with a fairly good response time. In the present study, a titration technique using an ISE was utilized for the systematic measurement of the formation constants of europium with three humic substances. The constants were also determined using the traditional batch solvent extraction method for comparison.
2. Experimental
2.1. Reagents
The studied humic substance materials, humic acid (HA) and fulvic acid (FA), are commercial powders that are available from the International Humic Substances Society (IHSS) and Sigma-Aldrich Co. The Sigma-Aldrich humic acid in sodium form was purified prior to use following the known protocol (abbreviated as ALHA) [19], and has been characterized [20,21]. The IHSS HA (as SHA, Eliot soil humic acid, code 1S102H) and FA (as SFA, Suwanee river fulvic acid, code 1S101F), which have also been well characterized as standards for environmental organic matter [22], were used without further purification. Additional aqueous solutions were prepared using ultrapure Milli-Q water (Millipore) with a resistivity of 18.2 MΩ/cm. All of the chemicals were of analytical grade.
2.2. Titration with ISE
Prior to the titration to determine the formation constants of Eu(III) with the humic substances, the applicability of the present ISE was checked. A fresh nitrobenzene solution containing Eu(III)-bis(diphenylphosphoryl)methane (BDPPM) complex was prepared using an ion-pair solvent extraction with Na tetraphenylborate (TPhB) according to the procedure reported previously [17]. The concentrations of the extracted species, Eu(BDPPM)3·3TPhB and BDPPM, were 1 × 10–3 mol/dm3 (M) and 1 × 10–2 M, respectively. As shown in Scheme 1, the inner solution of ISE was 1 × 10–3 M Eu3+ at pH 2, and the identical supporting electrolyte, 0.1 M LiCl, was adopted in the inner solution for the silver-silver chloride electrode (SSE1) and reference electrode (SSE2). The pH combination electrode was calibrated to measure the hydrogen ion concentration ([H+] = 10–pHc), based on the reference method [23,24]. A combination glass electrode (Kyoto Electronics, 98-100-C171) was used to measure the pH, and the reference electrode was filled with 3.6 M NaCl + 0.4 M NaClO4.
The Eu-ISE measurements were performed using an automatic potentiometric titrator (AT-510, Kyoto Electro-nics) equipped with a 50-mL polypropylene vessel in a water bath at 298 ± 1 K, a magnetic Teflon stirrer, and a nitrogen gas purge system (10-mL/min). The potential, denoted as ISE potential (ΔVISE), generated between SSE1 and SSE2, after a constant potential was attained, was measured using a potentiostat (Hokuto-denko, HA- 151), and recorded by an X-Y recorder (Riken-denshi, SP-K2V).
0.1-mL portions of the titrants of the humic substances (ALHA, SHA, SFA) were added to 10 ml of the 10–5 and 3 × 10–5 M Eu3+ solutions (I = 0.1 and 0.01 M (NaCl)) at intervals of 300 sec. The pHc was automatically kept constant by adding NaOH solution. The ionic strength of the test solution was controlled to be 0.1 and 0.01 using the Na+-Cl– system throughout the experiment.
2.3. Solvent Extraction
The procedure for determining the formation constants by solvent extraction was described previously in detail

Scheme 1. ISE cell configuration.
[25]. In brief, in order to obtain data about distribution ratio D, an aqueous phase, containing various concentrations (10–6 - 10–3 M) of the humic substances and Eu (10–6 - 3 × 10–5 M), was adjusted to the desired pHc using sodium hydroxide, perchloric acid. The organic phase of xylene contained TTA (2-thenoyltrifluoroacetone) as an extractant and TBP (tri-n-butyl phosphate) as a synergist. The aqueous and organic phases were shaken for 6 h at 298 ± 1 K. After the phase separation, the pHc of the aqueous phase was measured and taken as the equilibrium pH. The Eu(III) concentrations in both phases were measured using inductively coupled plasma mass spectrometry, where the concentration in the organic phase was determined after stripping with 0.1 M HNO3. Because the recovery of Eu(III) was usually more than 95%, no serious unexpected reactions such as precipitation or a third phase formation were observed.
3. Theoretical
3.1. Determination of Apparent Formation Constants by Titration with ISE
Because of the heterogeneous chemical property of humic substances, the number of functional groups per molecule and the molecular weight cannot be clearly defined. Therefore, by analogy with Langmuir’s adsorption isotherm, the apparent formation constant, βapp, is defined as follows [10],
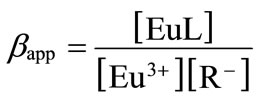 (1)
(1)
where [Eu3+] and [EuL] are the concentrations of free metal ions and metal ions bound with humic substance L, and [R–] is the concentration of the dissociated functional group in eq/dm3. Here, in the absence of metal ions, [R–] is given on the basis of the charge balance, [H+] + [Na+] = [R–] + [OH–] +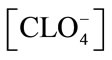 , and
, and
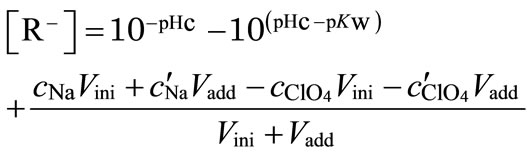 (2)
(2)
where cNa and 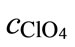 are the concentrations of sodium and perchlorate ions in the initial test solution,
are the concentrations of sodium and perchlorate ions in the initial test solution,  and
and  are the concentrations of the titrant solutions, Vini is the initial volume, Vadd is the added titrant volume, and pHc is the negative logarithm of the absolute hydrogen ion concentration (−log[H+]). The ion product (pKw) was used from the literature [26]. By using the apparent protonation constant, Kapp, as
are the concentrations of the titrant solutions, Vini is the initial volume, Vadd is the added titrant volume, and pHc is the negative logarithm of the absolute hydrogen ion concentration (−log[H+]). The ion product (pKw) was used from the literature [26]. By using the apparent protonation constant, Kapp, as
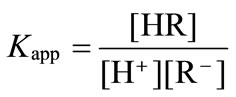 (3)
(3)
the degree of dissociation, α, can be written as
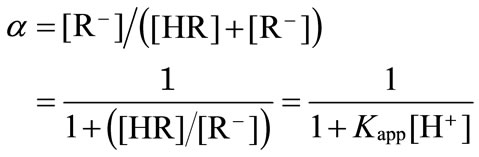 (4)
(4)
Similar to previous works [20,27], two major protondissociable functional groups—carboxylic and phenolic groups—are considered. Then, the two apparent protonation constants, Kapp,i (i = 1,2), can be rewritten as a function of pH and [Na+],
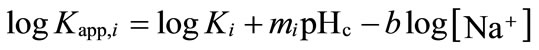 (5)
(5)
where mi, b, and logKi are the characteristic constant parameters of the humic substance [28].
At a given I, the [R–] is defined by the sum of two dissociated functional groups of the humic substance (R–) in meq/g, where j g of the humic substance is dissolved in V dm3 of the solution,
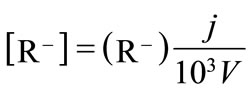 (6)
(6)
The (R−) is given by
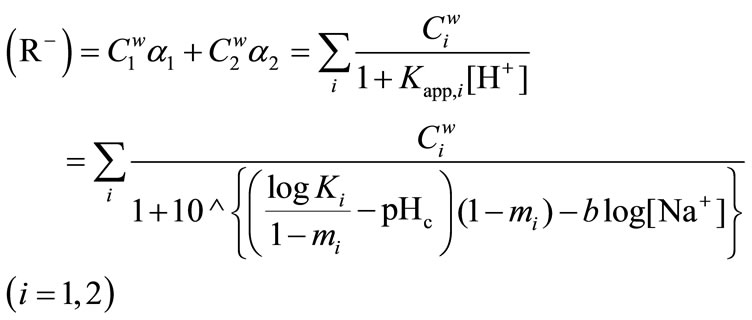 (7)
(7)
where 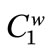 and
and  mean the total carboxylic and phenolic group concentrations, respectively, and
mean the total carboxylic and phenolic group concentrations, respectively, and  +
+ 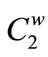 =
=  in meq/g. These parameters,
in meq/g. These parameters, 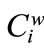 , logKi/(1–mi), and mi, were determined using a fitting analysis with Equations (2) and (6) [21,29], and are summarized in Table 1. The values are used for the calculation of the apparent formation constants, as described below.
, logKi/(1–mi), and mi, were determined using a fitting analysis with Equations (2) and (6) [21,29], and are summarized in Table 1. The values are used for the calculation of the apparent formation constants, as described below.
The total concentration of the proton-exchanging sites, CR, and metal ion [Eu]tot in mol/dm3 are described as
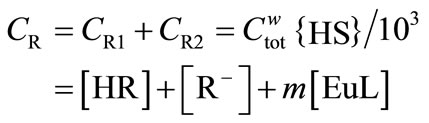 (8)
(8)
 (9)
(9)
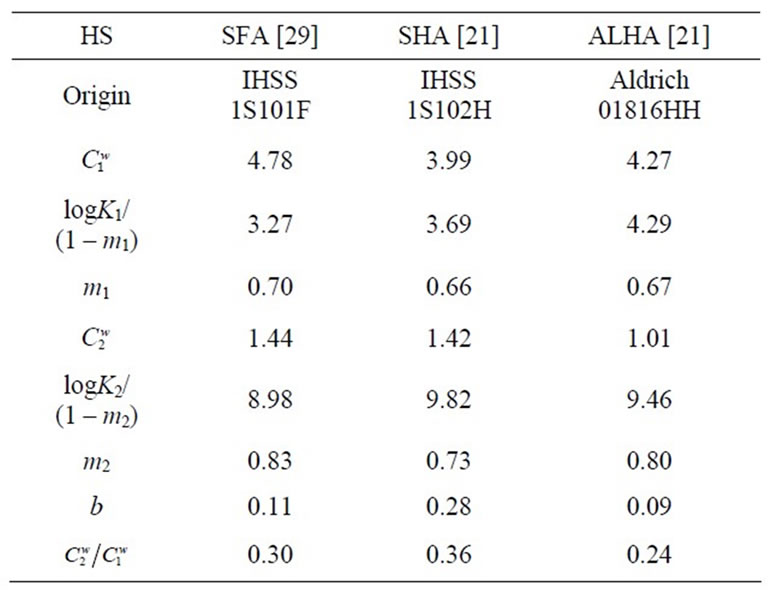
Table 1. Characteristics of humic substances.
where {HS} is the mass concentration of the humic substance in g/dm3, and m is the number of functional groups binding a europium ion and is assumed to be 3, which is equal to the charge number of Eu3+ in the present study. When complex EuL is formed with each binding site, the three proton-exchanging groups are assigned to be formally satisfied with a charge-neutralized complex. Then, Equation (8) can be replaced by
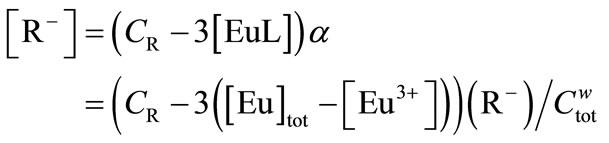 (10)
(10)
In the titration experiment with ISE, the concentration of free metal ion [Eu3+] can be measured directly. The observed ISE potential, ΔVISE, in mV, which arises from the different activities, aEu,SSE, of the free Eu3+ ions between SSE1 and SSE2 in Scheme 1, is described by the following Nernstian response,
 (11)
(11)
where R is a gas constant equal to 8.314 J/K/mol, T is the temperature (K), and F is the Faraday constant, which is equal to 96500 C/mol. When aEu ≈ [Eu3+] can be assumed in the present experimental condition of low [Eu3+], the linear relationship between the potential and concentration, with a slope of ca. 20 mV/decade, is shown as,
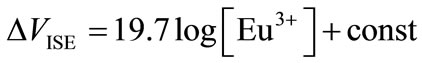 (12)
(12)
In the previous work, this linearity was observed in the presence of carboxylic acids such as malonic acid [18]. While interference from a coexisting trivalent ion might occur [17], this indicates that BDPPM is a highly sensitive ionophore for a single trivalent ion of the lanthanide or actinide series. Thus, according to Equations (9), (10), and (12), the apparent formation constant, βapp, in Equation (1) is determined.
3.2. Determination of βapp by Solvent Extraction
The extraction equilibrium constant, Kex, of europium for
 (13)
(13)
is described as
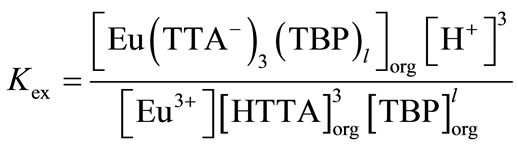 (14)
(14)
where the subscript org denotes the species in the organic phase. When the complex formation of Eu3+ by TTA– and the hydrolysis reaction by OH– are negligible in the aqueous phase at pHc < 5, the distribution ratio of Eu(III), D0, in the absence of a complexing ligand can be simply expressed by
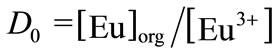 (15)
(15)
Then, distribution ratio D in the presence of a humic substance is given by
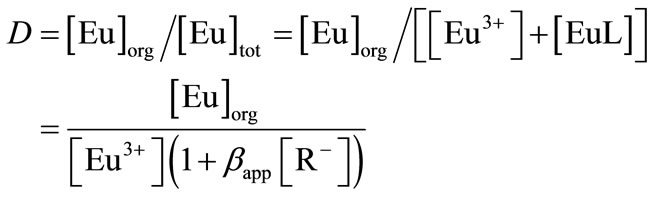 (16)
(16)
where the “initial” concentration of Eu is defined as
 (17)
(17)
Finally, from the difference between the presence (D) and absence (D0) of humic substances,
 (18)
(18)
The βapp values in Equation (18) can be determined, where the [R–] is described by Equations (4) and (10), as follows,
 (19)
(19)
4. Results and Discussion
4.1. Electrochemical Response of ISE
The electrochemical properties of the present ISE were characterized prior to use. Figure 1 shows the variation in ΔVISE as a function of the logarithm of [Eu3+] at different ionic strengths in the absence of humic substances. Although deviations from the Nernstian behavior (apparently as a result of the NaCl background) were observed in solutions containing lower europium concentrations, the ISE gave a good linear response with a slope of ca. 20 mV/decade for Eu3+ in the log[Eu3+] range of –6 - –3 at I = 0.01 and –5.3 - –3 at I = 0.1. The interference with the potential response by competitive ions is not
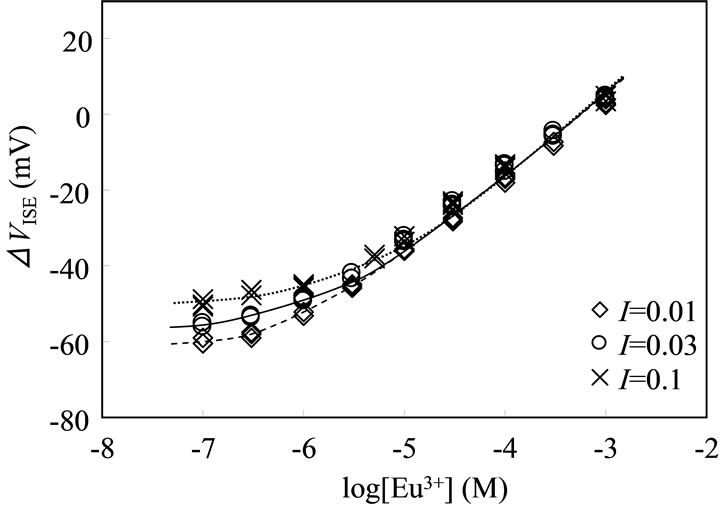
Figure 1. Potential response of ISE for Eu3+ at different ionic strengths of NaCl (pHc 5, 298 K).
considered here, because the quantification of the selectivity of the present ISE among the actinide series, Pu3+ vs.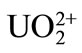 , U4+,
, U4+,  , and Pu4+, has been investigated, and showed good selectivity for the different valence state ions [17]. Indeed, no other metal ions, except Na and anions, would be present in this experiment.
, and Pu4+, has been investigated, and showed good selectivity for the different valence state ions [17]. Indeed, no other metal ions, except Na and anions, would be present in this experiment.
The electrode response on the pHc of the test solutions was investigated as shown in Figure 2. The reading potential value remained constant at the pHc range of 3 - 6,while the values at pHc 5 decreased slightly because of the competitive response for protons. In addition, a flat ISE response was found in a wide range of [Eu3+].
At europium concentrations of 10–3 - 5 × 10–6 M, the potential remained sufficiently stable over 6 h, and good reproducible readings in several sets of titration experiments in the system of the carboxylic acid-Eu complexation were obtained [18]. Similarly, the reading potential values remained constant after repetitive use of ISE in the present humic substance system, suggesting an undesirable reaction such as the sorption and the dissolution of humic substance for the ISE did not occur. Here, in order to investigate the dynamic response time of the ISE, the potential change in the test solution before and after the addition of Eu3+ was recorded with time. As shown in Figure 3, the addition of 10–2 M EuCl3 to the initial europium solution induced a sudden increase in the reading potential. The occurrence of this phenomenon just after the addition (tc ~ 0) must have been caused by a local increase in the Eu concentration near the electrode. In fact, the potential became stable within 30 sec, suggesting a homogeneous condition.
A similar behavior was observed in the presence of a humic substance. The [Eu3+] evaluated according to Equation (12) over time is shown in Figure 4. When the ALHA portion was dropped into the test solution to attain CR = 0 to 10–4 eq/dm3, the free Eu3+ ions were rapidly consumed as a result of the complexation with the humic acid, and a constant steady state was achieved
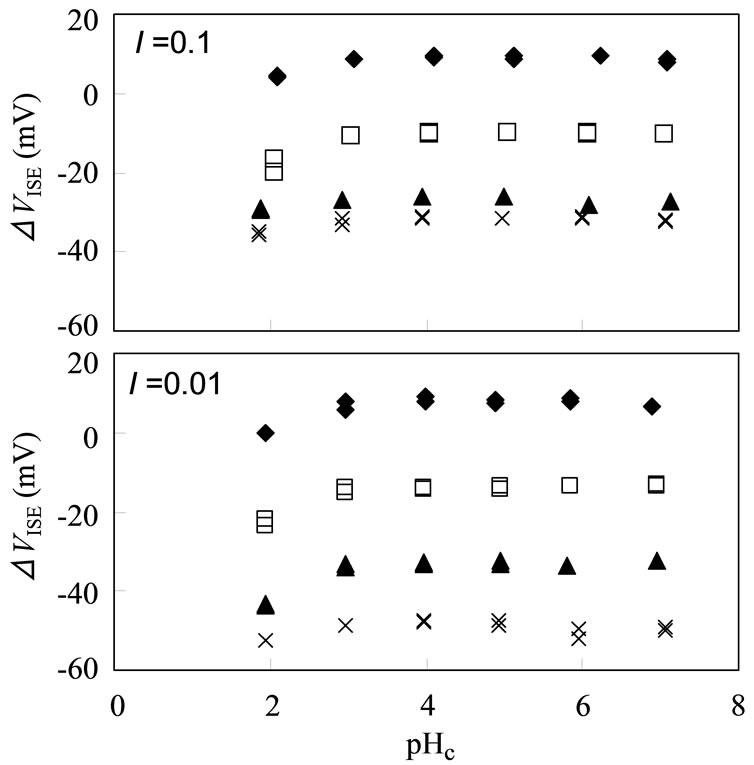
Figure 2. pH dependence of ISE response for different concentrations of Eu3+ at I = 0.1 (top) and 0.01 (bottom); log[Eu3+] = –3 (♦),–4(□), –5 (▲), and –5.3 for I = 0.1 and –6 for 0.01 (×).
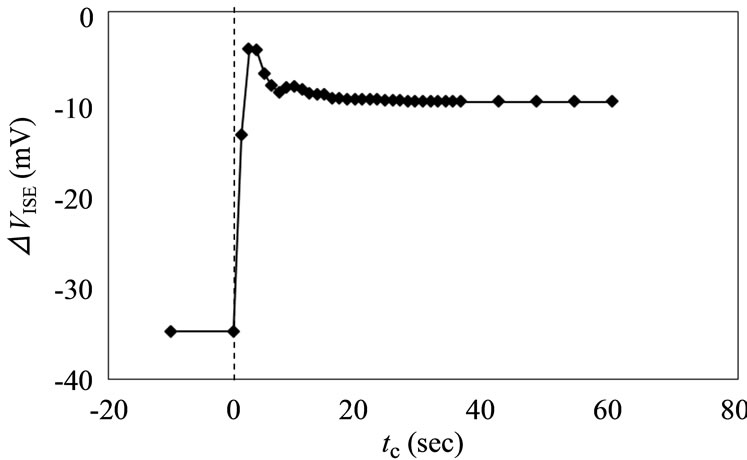
Figure 3. Potential response change from log[Eu3+] = –5.3 to –4 at pHc = 5 and I = 0.1.
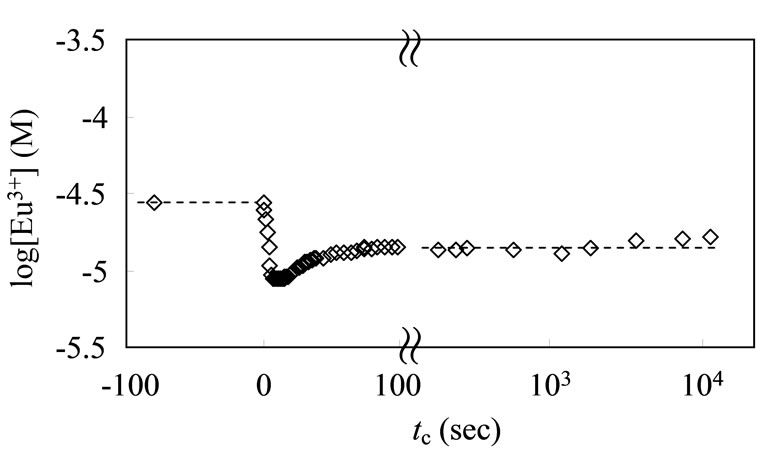
Figure 4. Time dependence of free Eu ion concentration with addition of ALHA solution; 20 ml of [Eu]tot = 3 × 10–5 M solution (pHc = 5, I = 0.1), and the titrant of 1 ml ALHA is CR = 2 × 10–3 eq/dm3.
after 100 sec. In the following experiment, the expected CR change for each drop was <10–5 eq/dm3 and the dropping interval was 300 sec. Thus, it is possible to conclude that this good property of ISE meets the basic requirements of the present study.
4.2. Titration Plot
Potentiometric titrations were made under the controlled constant pHc. The typical result of the logarithm of the europium concentration as a function of CR is shown in Figure 5. Because of the complexation of Eu with humic substances, the free europium ion concentration decreased with an increase in the amount of humic substances. The apparent formation constants, βapp, at each titration point were calculated based on the determined free [Eu3+].
4.3. Apparent Formation Constants
The dependency of CR on the apparent formation constants, βapp, is shown in Figure 6. Because of the heterogeneous property of HA, the βapp value varied with the pH, metal ion and humic substance concentrations, and ionic strength. In the presence of excess CR to the [Eu3+], the Eu3+ ions preferentially interacted with the stronger coordination sites [30]. Then, the βapp values would increase. On the other hand, the weaker coordination sites need to be used for complexation under the condition of a smaller amount of CR. A similar explanation is available from the viewpoints of the Eu concentration and ionic strength. At a given logCR condition, the series of βapp at [Eu]tot = 3 × 10–5 M are lower than that at 10–5 M, and the higher amount of Na+ (I = 0.1) effectively interferes with the occupation of Eu3+ on the binding sites of HA. Such an ionic strength effect on the metal complexation has been discussed by several researchers. Some model concepts have been represented by a linear relationship between the apparent formation constants and ionic strength with an empirical parameter [10,31].
The βapp data for SHA determined using the solvent extraction method, are also plotted in Figure 6. Although the CR range examined was different from each method, the increasing tendency of βapp with an increase of CR can be observed, suggesting the heterogeneous property of SHA. Thus, the data will be useful for constructing a semi-empirical model of the formation constants of metal ion-humic substance complexation [30]. On the other hand, Figure 7 shows the lower impact of I and [Eu] on the βapp for the SFA system. Even at the higher logCR above –3.5, the βapp value did not increase with increasing CR.
A comparison of the βapp data for the three humic substances under similar solution conditions is shown in Figure 8. The relatively high βapp values for SHA and
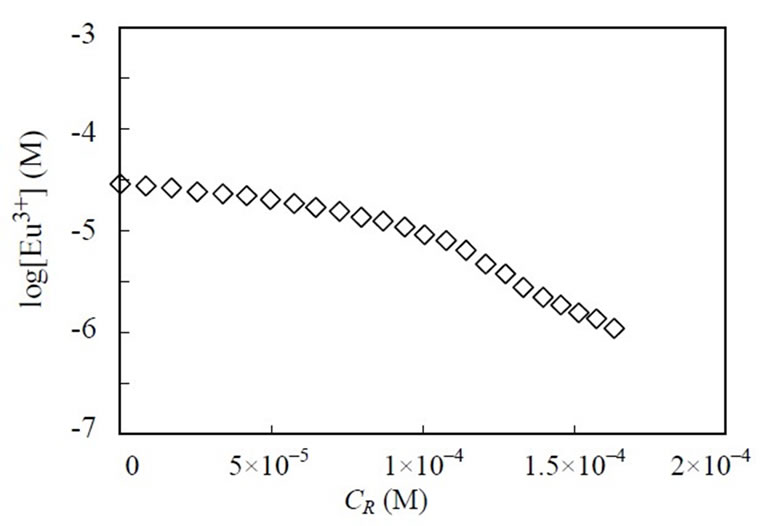
Figure 5. Titration plots of logarithm of free europium concentration as function of CR at constant pHc of 4.87 and I = 0.01 (NaCl). The [Eu]tot is 3 × 10–5 M, the titrant is 2 × 10–3 M of ALHA.
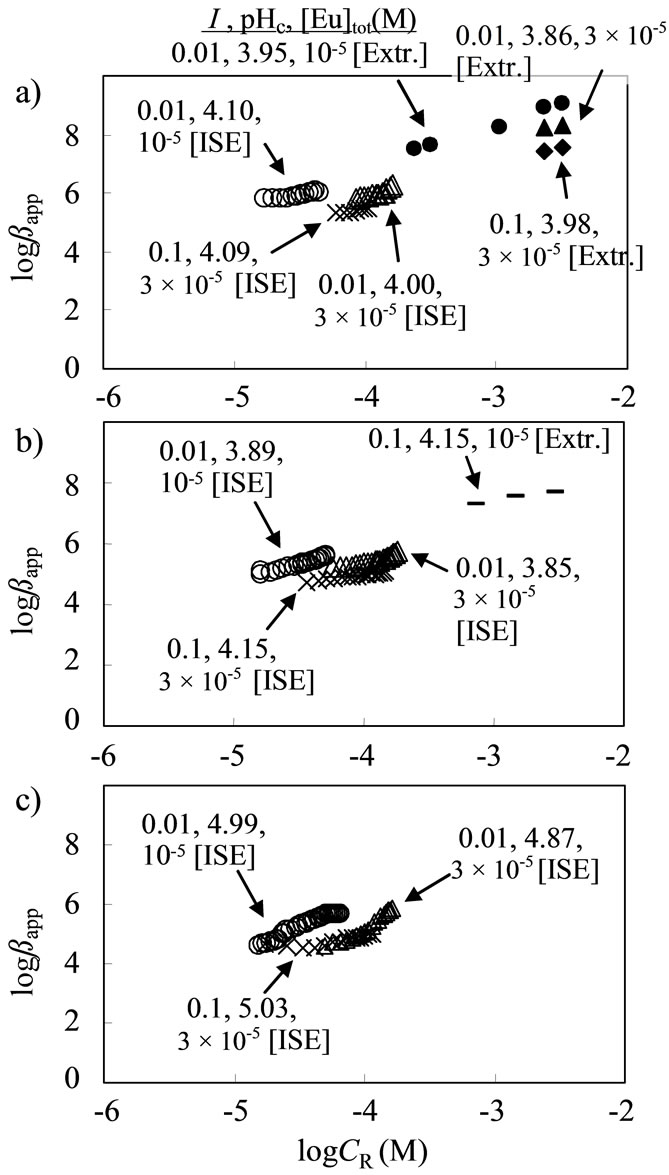
Figure 6. Dependence of logCR, I, [Eu]tot, and analytical method on logβapp: (a) SHA; (b, c) ALHA.
ALHA compared to SFA might be related to the logK/(1–m) value in Table 1, which provided a measure of the strength of proton binding as the acidity. The high value is affected by strong intramolecular hydrogen
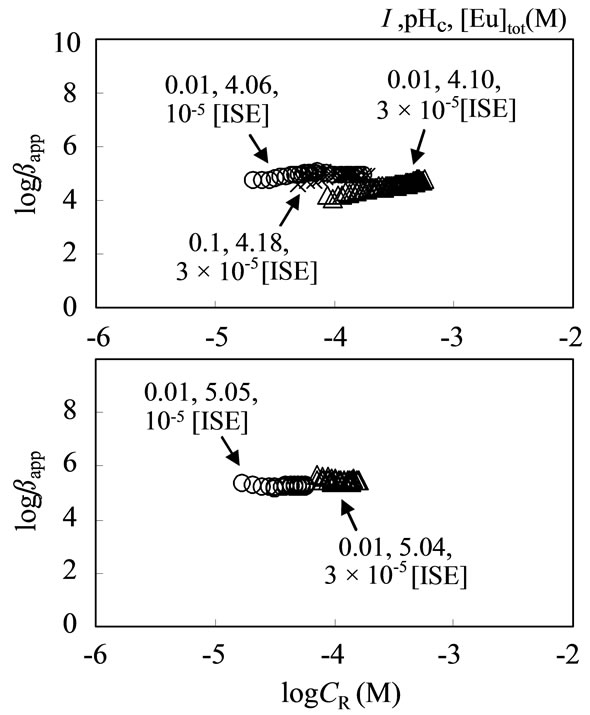
Figure 7. Dependence of logCR, [Eu]tot and I on logβapp for SFA.

Figure 8. Intercomparison of logβapp for humic substances at pHc ~ 4, I = 0.01 (NaCl), and [Eu]tot = 10–5 M (a) and 3 × 10–5 M (b). Data were obtained by the titration with ISE.
bonds among the neighboring multi-functional groups. This fact might introduce a strong chelate complexation, resulting in the higher βapp values. However, it is difficult to correlate the ratio of the number of carboxylic and phenolic functional groups,  , to the reason for the different βapp values. The other reason would be the different molecular weights and sizes of the humic substances from the electrostatic point of view. In general, the larger molecules of HA has a greater tendency to aggregate than FA. If the polyvalent metal ion Eu3+ was incorporated by the aggregation, the βapp might increase.
, to the reason for the different βapp values. The other reason would be the different molecular weights and sizes of the humic substances from the electrostatic point of view. In general, the larger molecules of HA has a greater tendency to aggregate than FA. If the polyvalent metal ion Eu3+ was incorporated by the aggregation, the βapp might increase.
5. Conclusion
The apparent formation constants of europium ions with humic acid and fulvic acid could be determined by potentiometric titration using an ion selective electrode (ISE) under several solution conditions. The electrode exhibited a stable and quick Nernstian response during the period of measurement. Unlike the batch-type solvent extraction method, the present titration can acquire multiple data points at once without troublesome experimental preparations.
REFERENCES
- J. I. Kim and T. Sekine, “Complexation of Neptunium(V) with Humic-Acid,” Radiochimica Acta, Vol. 55, No. 4, 1991, pp. 187-192.
- V. Moulin, J. Tits and G. Ouzounian, “Actinide Speciation in the Presence of Humic Substances in Natural-Water Conditions,” Radiochimica Acta, Vol. 58, 1992, pp. 179-190.
- Y. Takahashi, Y. Minai, S. Ambe, Y. Makide, F. Ambe and T. Tominaga, “Simultaneous Determination of Stability Constants of Humate Complexes with Various Metal Ions Using Multitracer Technique,” Science of the Total Environment, Vol. 198, No. 1, 1997, pp. 61-71. doi:10.1016/S0048-9697(97)05442-9
- D. Wenming, Z. Hongxia, H. Meide and T. Zuyi, “Use of the Ion Exchange Method for the Determination of Stability Constants of Trivalent Metal Complexes with Humic and Fulvic Acids—Part I: Eu3+ and Am3+ Complexes in Weakly Acidic Conditions,” Applied Radiation and Isotopes, Vol. 56, No. 6, 2002, pp. 959-965. doi:10.1016/S0969-8043(01)00055-0
- M. Schnitzer and S. I. M. Skinner, “Organo-Metallic Interactions in Soils: 7. Stability Constants of Pb++-, Ni++-, Mn++-, Co++-, Ca++-, and Mg++-Fulvic Acid Complexes,” Soil Science, Vol. 103, No. 4, 1967, pp. 247-252. doi:10.1097/00010694-196704000-00004
- M. Schnitzer and S. I. M. Skinner, “Organo-Metallic Interactions in Soils: 5. Stability Constants of Cu++-, Fe++-, and Zn++-Fulvic Acid Complexes,” Soil Science, Vol. 102, No. 6, 1966, pp. 361-365. doi:10.1097/00010694-196612000-00002
- M. Caceci, “The Interaction of Humic-Acid with Europium(III) Complexation Strength as a Function of Load and pH,” Radiochimica Acta, Vol. 39, 1985, pp. 51-56.
- J. H. Ephraim, “Heterogeneity as a Concept in the Interpretation of Metal-Ion Binding by Humic Substances—The Binding of Zinc by an Aquatic Fulvic Acid,” Analytica Chimica Acta, Vol. 267, No. 1, 1992, pp. 39-45. doi:10.1016/0003-2670(92)85004-P
- M. A. Glaus, W. Hummel and L. R. Van Loon, “Equilibrium Dialysis-Ligand Exchange: Adaptation of the Method for Determination of Conditional Stability Constants of Radionuclide-Fulvic Acid Complexes,” Analytica Chimica Acta, Vol. 303, No. 2-3, 1995, pp. 321-331. doi:10.1016/0003-2670(94)00534-S
- A. Kirishima, T. Ohnishi, N. Sato and O. Tochiyama, “Simplified Modeling of the Complexation of Humic Substance for Equilibrium Calculations,” Journal of Nuclear Science and Technology, Vol. 47, No. 11, 2010, pp. 1044-1054. doi:10.1080/18811248.2010.9711669
- J. G. Hering and F. M. M. Morel, “Humic Acid Complexation Calcium and Copper,” Environmental Science and Technology, Vol. 22, No. 10, 1988, pp. 1234-1237. doi:10.1021/es00175a018
- J. P. Pinheiro, A. M. Mota and M. F. Benedetti, “Lead and Calcium Binding to Fulvic Acids: Salt Effect and Competition,” Environmental Science and Technology, Vol. 33, No. 19, 1999, pp. 3398-3404. doi:10.1021/es990210f
- T. Ito, C. Goto and K. Noguchi, “Lanthanoid Ion-Selective Solvent Polymeric Membrane Electrode Based on 1-Phenyl-3-methyl-4-octadecanoyl-5-pyrazolone,” Analytica Chimica Acta, Vol. 443, No. 1, 2001, pp. 41-51. doi:10.1016/S0003-2670(01)01192-8
- M. R. Ganjali, N. Davarkhah, H. Ganjali, B. Larijani, P. Norouzi and M. Hossieni, “A Novel Europium(III) Sensor Based on 4E-4-(2-phenylviazenyl)-2-((E)-(2-aminoethylimino)methyl)phenol,” International Journal of Electrochemical Science, Vol. 4, No. 6, 2009, pp. 762-771.
- J. B. Harrell, A. D. Jones and G. R. Choppin, “A Liquid Ion-Exchange Membrane Electrode for Polyvalent Cations,” Analytical Chemistry, Vol. 41, No. 11, 1969, pp. 1459-1462. doi:10.1021/ac60280a026
- D. A. Chowdhury, T. Ogata, S. Kamata and K. Ohashi, “Samarium(III)-Selective Electrode Using Neutral Bis (thiaalkylxanthato)alkanes,” Analytical Chemistry, Vol. 68, No. 2, 1996, pp. 366-370. doi:10.1021/ac950814b
- Y. Kitatsuji, H. Aoyagi, Z. Yoshida and S. Kihara, “Plutonium(III)-Ion Selective Electrode of Liquid Membrane Type Using Multidentate Phosphine Oxide Ionophore,” Analytica Chimica Acta, Vol. 387, No. 2, 1999, pp. 181- 187. doi:10.1016/S0003-2670(99)00121-X
- T. Sasaki, H. Yoshida, Y. Kitatsuji, I. Takagi and H. Moriyama, “Formation Constants of Eu(III)-Carboxylates Determined by Ion Selective Liquid Membrane Electrode,” Chemistry Letters, Vol. 40, 2011, pp. 870-871. doi:10.1246/cl.2011.870
- J. I. Kim, G. Buckau, G. H. Li, H. Duschner and N. Psarros, “Characterization of Humic and Fulvic Acids from Gorleben Groundwater,” Fresenius Journal of Analytical Chemistry, Vol. 338, No. 3, 1990, pp. 245-252. doi:10.1007/BF00323017
- A. Kirishima, T. Ohnishi, N. Sato and O. Tochiyama, “Determination of the Phenolic-Group Capacities of Humic Substances by Non-Aqueous Titration Technique,” Talanta, Vol. 79, No. 2, 2009, pp. 446-453. doi:10.1016/j.talanta.2009.04.008
- T. Sasaki, S. Aoyama, H. Yoshida, Y. Kulyako, M. Samsonov, T. Kobayashi, I. Takagi, B. Miyasoedov and H. Moriyama, “Apparent Formation Constants of Pu(IV) and Th(IV) with Humic Acids Determined by Solvent Extraction Method,” Radiochimica Acta, 2012, in press.
- “International Humic Substances Society,” 2012. http://www.humicsubstances.org
- G. Gran, “Determination of the Equivalence Point in Potentiometric Titrations. Part II,” Analyst, Vol. 77, No. 920, 1952, pp. 661-671. doi:10.1039/an9527700661
- K. Fujiwara, H. Yamana, T. Fujii, K. Kawamoto, T. Sasaki and H. Moriyama, “Solubility of Uranium(IV) Hydrous Oxide in High pH Solution under Reducing Condition,” Radiochimica Acta, Vol. 93, No. 6, 2005, pp. 347- 350. doi:10.1524/ract.93.6.347.65643
- T. Sasaki, S. Kubo, T. Kubota, I. Takagi and H. Moriyama, “Complex Formation of Lanthanides(III) and Actinides(III) with Dicarboxylates Containing Soft Donor Groups,” Journal of Alloys and Compounds, Vol. 408- 412, 2006, pp. 1283-1286. doi:10.1016/j.jallcom.2005.04.130
- C. F. Baes and R. E. Mesmer, “The Hydrolysis of Cations,” John Wiley & Sons, New York, 1976.
- J. Du, N. Sato and O. Tochiyama, “Potentiometric Study on the Proton Binding of Humic Substances,” Journal of Nuclear and Radiochemical Sciences, Vol. 6, No. 1, 2005, pp. 25-29.
- O. Tochiyama, Y. Nibbori, K. Tanaka, H. Yoshino, T. Kubota, A. Kirishima and B. Setiawan, “Modeling of the Complex Formation of Metal Ions with Humic Acids,” Radiochimica Acta, Vol. 92, No. 9-11, 2004, pp. 559-565. doi:10.1524/ract.92.9.559.54996
- K. Müller and T. Sasaki, “Complex Formation of Np(V) with Fulvic Acid at Tracer Metal Concentration,” Radiochimica Acta, 2012, in press.
- T. Sasaki, T. Kobayashi, I. Takagi and H. Moriyama, “Discrete Fragment Model for Complex Formation of Europium(III) with Humic Acid,” Journal of Nuclear Science and Technology, Vol. 45, No. 8, 2008, pp. 718- 724. doi:10.3327/jnst.45.718
- W. Hummel, M. A. Glaus and L. R. Van Loon, “Trace Metal-Humate Interactions. II. The ‘Conservative Roof’ Model and Its Application,” Applied Geochemistry, Vol. 15, 2000, pp. 975-1001. doi:10.1016/S0883-2927(99)00100-6
NOTES
*Corresponding author.

