American Journal of Analytical Chemistry
Vol. 3 No. 2 (2012) , Article ID: 17281 , 8 pages DOI:10.4236/ajac.2012.32014
Studies on Degradation of Diquat Pesticide in Aqueous Solutions Using Electrochemical Method
1Chemistry Department, Al-Azhar University, Gaza, Palestine
2Chemistry Department, College of Sciences, Al-Aqsa University, Gaza, Palestine
Email: *hazemona1@yahoo.co.uk
Received October 31, 2011; revised December 16, 2011; accepted December 29, 2011
Keywords: Carbon Paste Electrode; Herbicide; C/Pbo2; Electrode; Electrooxidation; Diquat Dibromide
ABSTRACT
The C/PbO2 electrode assisted electrochemical removal of diquat dibromide herbicides solutions has been the subject of the present investigation under several operating conditions. The optimum conditions of the treatment process are: current density of 150 mA/cm2, pH 2.2, NaCl concentration 2 g/L, temperature of 10˚C and initial diquat concentration of 50 mg/L. The time of electrolysis is 60 min for degradation rate of diquat and chemical oxygen demand (COD) removal is 210 min. The results were obtained by UV-Vis spectrophotometer and the present designed electrode was coincident.
1. Introduction
Diquat is a bipyridylium herbicide generally marketed as a dibromide salt. The physical properties of the diquat herbicide such as easiness of handling (crystalline salt), low vapor pressure (minimal fumes), high water solubility (700 g·L–1 at 20˚C), high binding potential (soil binding cause deactivation and immobilization), and fast working (once photosynthesis begins) make them suitable for agricultural uses. The double positive charge on the diquat cation causes it to be adsorbed tightly to the negatively charged clay minerals present in the soil. As a result, diquat remains in the upper layers of soil for a long time and is unlikely to leach to groundwater. Diquat is registered for a variety of application including weed control on orchard floors, preplant for weed killers for many crops preharvest desiccants crops such as potatoes, and aquatic weed control [1]. Diquat is acutely toxic when it is absorbed through the skin and the possibility for poisoning increases with repeated exposure. Cataract formation is the most significant effect of chronic exposure to diquat that is currently recognized [2]. Most instances of poisoning involve the ingestion or inhalation of concentrated commercial products. However, there is a potential for human exposure to diquat in the general population through residues on food crops or aerial drift and field runoff to aquatic systems during spraying applications. Diquat is thought by other researchers to have the potential to cause birth defects, toxic to fish, algae and other aquatic organism and also causes eye irritation. Its half-life is less than 48 hours in water [3]. The United States Environmental Protection Agency (USEPA) maximum contamination levels (MCL) for diquat in drinking water is 20 µg·L–1.
It was found that pesticides containing N atoms such as Diquat 2HBr [1,1′-ethylene-2,2′-bipyridylium ion] [4] can form chloramines when using aqueous solution of NaCl as electrolyte [5]. However, it is important to point out that presence of NaCl in solution can also confer an enhanced activity to the electrodes. In this case the Cl− species at the electrode surface act as intermediates in the electron transfer between the pesticide molecule and the electrode [6]. The anode material is another important restrictive factor which determinates reaction parameters such as current efficiency, selectivity and product composition. Several works have reported the use of Ti or Pt electrodes [7,8] with results less adequate for considering it as anodic material for removal of pesticides from aquatic media. The formation of complex mixture of oxidation byproducts in solution, no detoxification of solution, or desactivation phenomena of anodes are some of the limitations of these type of electrodes for their use in the electrochemical method of direct oxidation. Better results has been achieved by using metallic oxides such as advanced oxidation processes (AOPs) for removal of pesticides from aqueous media such as SnO2, PbO2, or RuO2 [8], dimensionally stable anodes [6], and more recently boron-doped diamond surfaces [9,10].
After a very careful review of the literature, no mention of the degradation of diquat was spotted, therefore, intended to explore the effects of electrochemical degradation of diquat. Electrochemical treatment of wastewater offers high removal efficiencies and has lower temperature requirements compared to non-electrochemical treatment. Oxygen transfer is usually favored on an anode material with high oxygen evolution overpotential. Lead dioxide, is characterized by high oxygen overpotential. Therefore, it is one of the most commonly used anodes for the electrochemical degradation of many pollutants [11,12] particularly when it is doped with metallic cations whose oxides have low oxygen evolution overpotential.
Because the removal of diquat from the environment is very necessary we thought of studying the degradation of diquat to harmless species using electrochemical method. This method could be effectively applied for highly contaminated aquatics with diquat. In this work, a suitable mass of diquat dibromide herbicide was dissolved in double distilled water to make a concentration of 50 mg·L–1. The C/PbO2 electrode assisted electrochemical removal of diquat dibromide herbicides solutions has been the subject of the present investigation under several operating conditions. The concentration of the undecomposed diquat ion was traced by a newly developed potentiometric carbon paste electrode [13]. The results were compared with those obtained using the usual UV-Vis spectroscopy-based detector and were found coincident.
2. Materials and Methods
2.1. Chemicals
Double distilled water was used throughout all experiments. diquat dibromide (DqBr2, M.wt = 344.05 g·mol−1), was obtained from Aldrich Chemical Company. Graphite powder, 2-nitrophenyl octyl ether (2-NPOE), were purchased from Aldrich and used as received. phosphotungstic acid (PTA) H3[PW12O40], and sodium tetraphenylborate (Na-TPB) Na[C24H20B], were obtained from Sigma.
Different standard solutions of diquat with concentration from 10 - 100 ppm were prepared to measure the degradation under different conditions. Standard solutions of potassium dichromate (K2Cr2O7) and sulfuric acid (H2SO4) reagent with silver sulfate (Ag2SO4) were prepared to measure the COD [14].
2.2. Apparatus and Preparation of Carbon Paste Electrode
All EMF measurements were carried out with the following assembly: Hg, Hg2Cl2(s), KCl(sat.) ||sample solution| carbon paste electrode. The potential measurements were carried out at 25˚C ± 0.1˚C with a digital pH meter with a Pocket pH/mV Meters, pH315i (WissenschaftlichTechnische Werkstatten GmbH (WTW)-Germany) under stirring conditions at room temperature (25.0˚C ± 1.0˚C). Modified carbon paste was prepared as described by our group [13].
2.3. Preparation of the Carbon/Lead Dioxide (C/PbO2 Electrode)
Pretreatment of Carbon rod (8 mm × 25 cm) was carried out following the procedure applied by Narasimham and Udupa [15]. The carbon rod was soaked in 5% NaOH solution, washed with distilled water, dried in furnace at 105˚C, for 15 min., cooked with linseed oil to reduce the porosity of rod. After the previous treatment of the electrode, it is ready to receive doped PbO2. The electrodeposition of PbO2 was performed at constant anodic current of 20 mA·cm–2 from 12% w/v Pb(NO3)2 solution containing 5% w/v CuSO4·5H2O and 3% cetyltrimethylammonium bromide (CETAB) as surfactant. The role of the surfactant is to minimize the surface tension of the solution. Electrodeposition was carried out for 60 min. at 80˚C with continuous stirring [16].
2.4. Electrolysis
Pyrex glass cell containing 50 mL diquat dibromide solution with the prepared C/PbO2 electrodes as anode (where lead oxide is the surface material) and austenitic stainless steel as cathode. DC power supply (model GP4303D, LG Precision Co. Ltd, Korea) was used. The samples were then analyzed by three methods: UV-Vis spectrophotometer at 271 nm (Shimadzu, Japan), potentiometric determination of diquat using the presented designed electrode and COD (mg O2·L–1), which was measured by a closed reflux titrimetric method [17]. The experimental conditions were varied to test the effect of the following parameters: conductive electrolyte type, NaCl concentration, temperature, current density, pH, time of electrolysis and the initial concentration of diquat.
3. Result and Discussion
3.1. Mechanism of Electrochemical Oxidation of Diquat Dibromide
The electro-degradation of diquat dibromide may be occurred in electrocatalytic oxidation [18,19] as well as the electrochlorination method [19]. In addition, the possible intermediate and the reaction products of diquat dibromide through the electrodegradation may be 1,2,3,4-tetrahydro-1-oxopyrido [1,2-a]-5-pyrazinium ion, 3,4-dihydro-2H-pyrido [1,2a] pyrazine-1,6-dione and diquat dipyridone as supposed [20].
3.2. Effect of Various Factors on the Rate of Degradation
Type of conductive electrolyte, current density, pH of simulated solution, temperature, time interval of treatment, initial concentration, and NaCl concentration were studied and optimized. The remaining concentration (mg·L−1) and COD removal (mg O2·L−1) were illustrated in Figures 1-6.
3.3. Effect of Types of Electrolytes
Electrolytes of 2 g·L−1 of the following salts: NaCl, CaCl2, KCl, Na2CO3, NaF, and Na2SO4 were studied by C/PbO2 electrode. Figure 1 shows that the best electrocatalytic degradation of diquat dibromide is found when using NaCl or KCl as electrolytes. The electrocatalytic efficiency of diquat dibromide was 99.84% using C/PbO2 electrode while that of COD removal was 100%. NaCl appears to be the most effective electrolyte from which the removal of diquat dibromide and significant depletion of COD was observed. While Na2SO4 and Na2CO3 electrolytes showed poor results. The Cl− anion enhances the degradation of pollutants. Therefore, addition of KCl or NaCl provides the effective Cl− ion. This behavior may be due to the small ion size of K+ and Na+ which increases the ion mobilities and the loss ability of Cl− ion. Na2SO4 and Na3CO3 electrolytes showed the least efficiency in the degradation of pollutant. This may be attributed to the formation of an adherent film on the anode surface which poisons the electrode surface. Also, these electrolytes do not contain chloride ion (Cl−) in their structures and they may form stable intermediate species that could not be oxidized by direct electrolysis. These observations were also confirmed in other studies [18] CaCl2 is less effective electrolyte than NaCl in sodium hypochlorite production. This may be due to the formation of less soluble calcium hypochlorite Ca(OCl)2 than NaOCl.
3.4. Effect of the NaCl Concentration
Different concentrations of NaCl were applied to investtigate their effect on the removal of diquat dibromide and the corresponding COD elimination as indicated in Figure 2. The results indicated that an increase of the electrolyte concentration up to 2 g·L–1 lead to increase in the diquat dibromide degradation rate and COD removal for C/PbO2 electrode. Further increase of the NaCl concentration has a negative reflection on the degradation rate of diquat dibromide and COD removal.
3.5. Effect of Current Density
As shown in Figure 3 diquat dibromide degradation and COD removal increase with increasing the applied current density up to 150 mA/cm2 by using C/PbO2 electrode. Further increase of the current density was followed by gradual decrease in diquat dibromide degradation and COD removal due to the increase in temperature. Above a temperature 35˚C, sodium hypochlorite tends to chemically decompose to sodium chlorate [21].
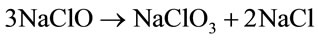
So when temperature rises higher than 35˚C, production of NaClO decreases. But at higher current densities the rate of hypochlorite decomposition increases with the increase in current density.
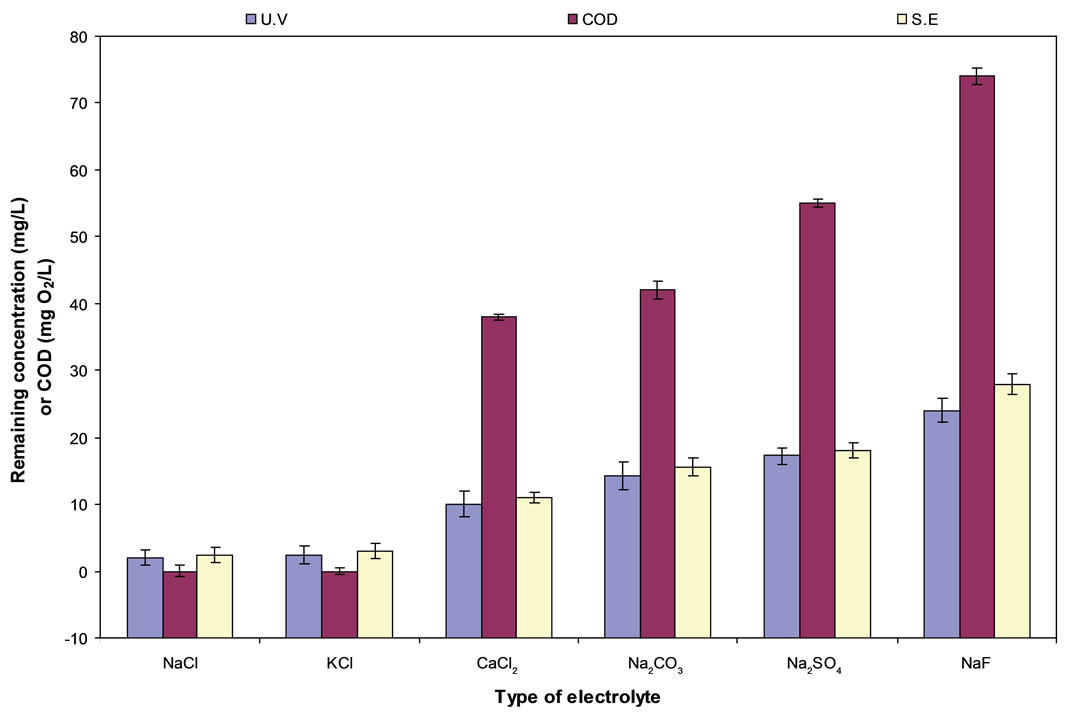
Figure 1. The effect of conductive type of electrolyte on diquate dibromide and COD removal using C/PbO2 electrode.
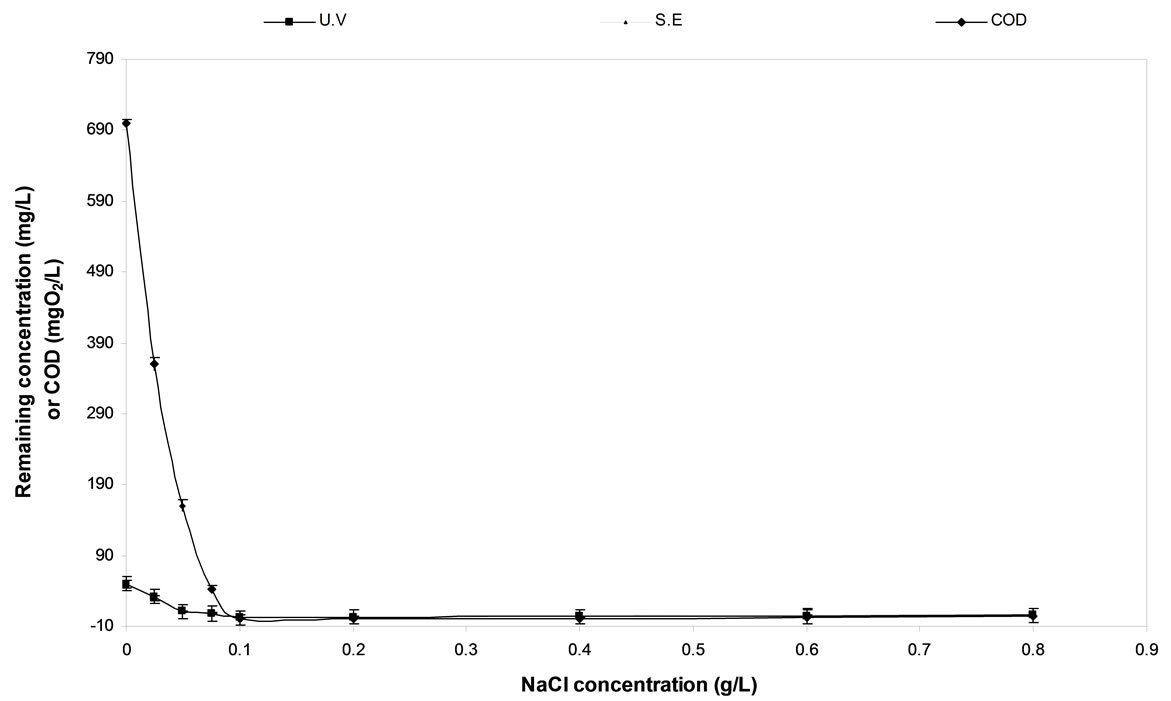
Figure 2. The Effect of NaCl concentration on diquat dibromide and COD removal using C/PbO2 electrode.
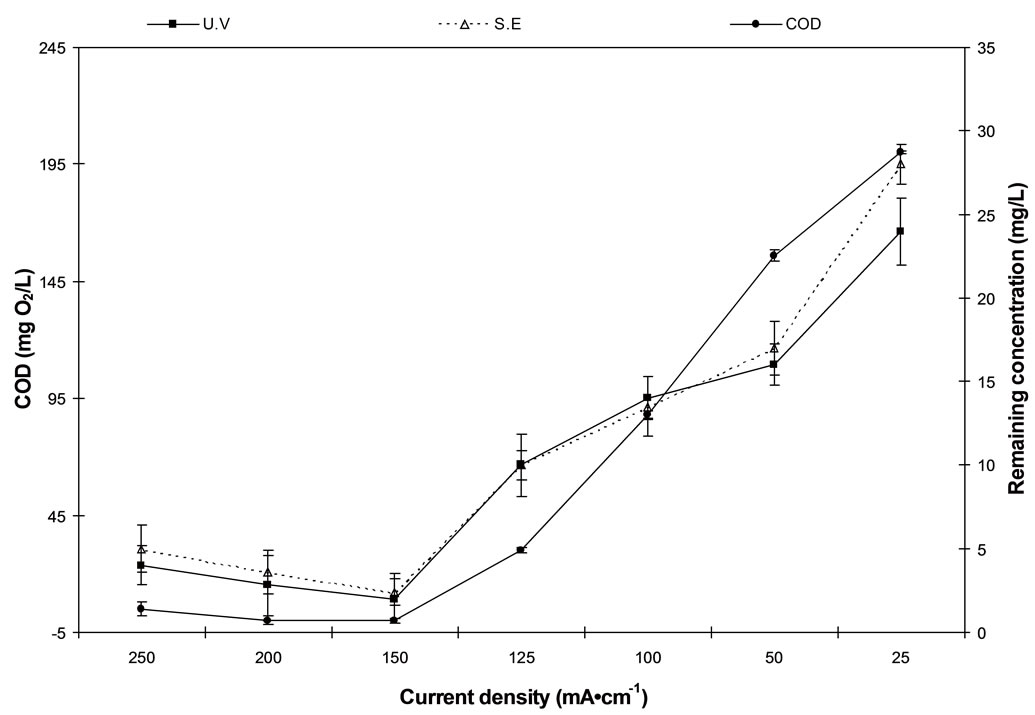
Figure 3. The effect of cuurent density on diquat dibromide and COD removal using C/PbO2 electrode.
3.6. Effect of pH Value
The pH dependence of the electrochemical degradation of diquat dibromide was illustrated in Figure 4. The pH values of the solutions were adjusted by adding drops of H2SO4 and NaOH. The reactions were carried out for 60 min. under the following experimental conditions: the initial concentration of 50 mg·L−1, a current density of 150 mA·cm−2, a temperature of 10˚C and NaCl concentration of 2 g·L−1. The distance between the two electrodes was adjusted to be 1 cm.
It was found that the maximum removal of diquat dibromide and COD using C/PbO2 electrode was achieved in acidic medium at pH 2.2. Under this condition the NaCl solution liberates Cl2 gas which is considered as the active species for the degradation of diquat dibromide.
3.7. Effect of the Electrolysis Time
Diquat dibromide was effectively removed in 60 min by electrochemical oxidation using C/PbO2 electrode. In addition, COD removal was in 210 min.
3.8. Effect of Temperature
The effect of temperature on the electrochemical degradation of diquat dibromide and COD removal was showed in Figure 5. The rate of the diquat dibromide degradation and COD removal decrease significantly with the increase of the solution temperature above 40˚C. Further decrease of the temperature below 10˚C did not bring any significant effect. Therefore, 10˚C was confirmed as optimal electrolysis temperature under the same experimental conditions mentioned previously.
The sodium hypochlorite production rate depends on temperature [22]. There was reduction in the sodium hypochlorite production rate within 23˚C and 30˚C. Over this small temperature range, the sodium hypochlorite production rate was lowered and sodium hypochlorite tends to chemically decompose to sodium chlorate at 35˚C [21]. It is clear from the above Figure 5 that the optimum temperature of sodium hypochlorite production was 10˚C for C/PbO2 electrode. At low temperature, the loss of chlorine gas decreases, so the sodium hypochlorite is increased.
3.9. Effect of Initial Diquat Dibromide Dosage
The effect of initial diquat dibromide and corresponding COD removal on the rate of degradation were studied within the range of (10 - 250 mg/L).The data shown graphically in Figure 6. The observed data reveals that the total removal of diquat dibromide and COD can be achieved from samples containing up to 50 mg/L. However, increasing of diquat dibromide concentration above this level resulted in a decrease in the rate of electrocatalytic degradation. It was repeatedly observed that the COD removal was practically complete as the value measured for the residual diquat dibromide was consistently less than 0.14%. This result can be easily seen by examining the figures.
3.10. Potentiometric Determination of Using the Presented Designed Electrode
A newly designed chemically modified carbon paste electrode (the presently designed electrode) as well as the spectrophotometric method was used in the determination of all diquat samples. Calibration curves were prepared and used to calculate the concentration of diquat in all solutions by the UV method as well as the potentiometric method. In all cases the results obtained by both methods were coincident. This can be seen clearly from Figures 1-6.
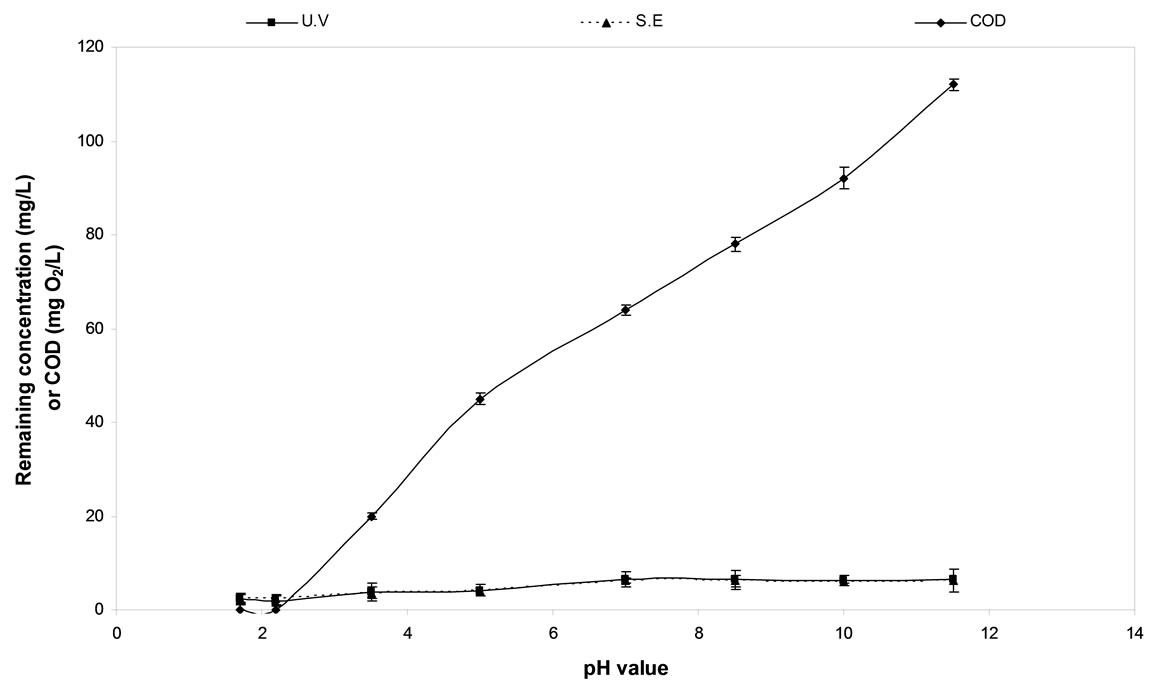
Figure 4. The effect of pH on diquate dibromide and COD removal using C/PbO2 electrode.
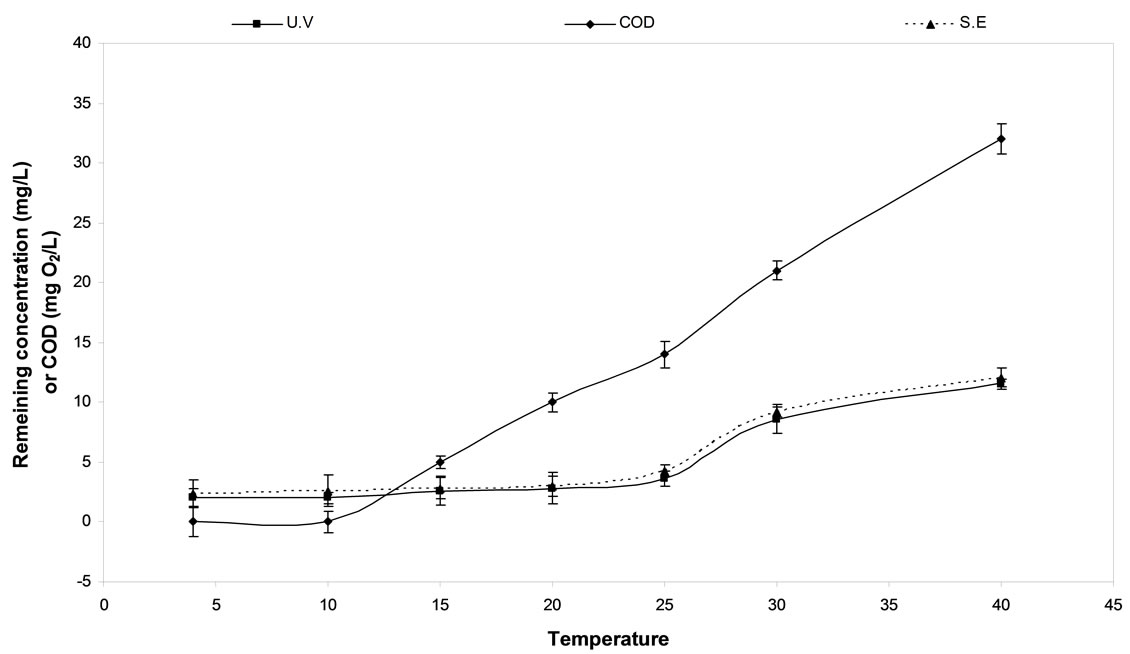
Figure 5. The effect of temperature on Diquat dibromide and COD removal using C/PbO2 electrode.
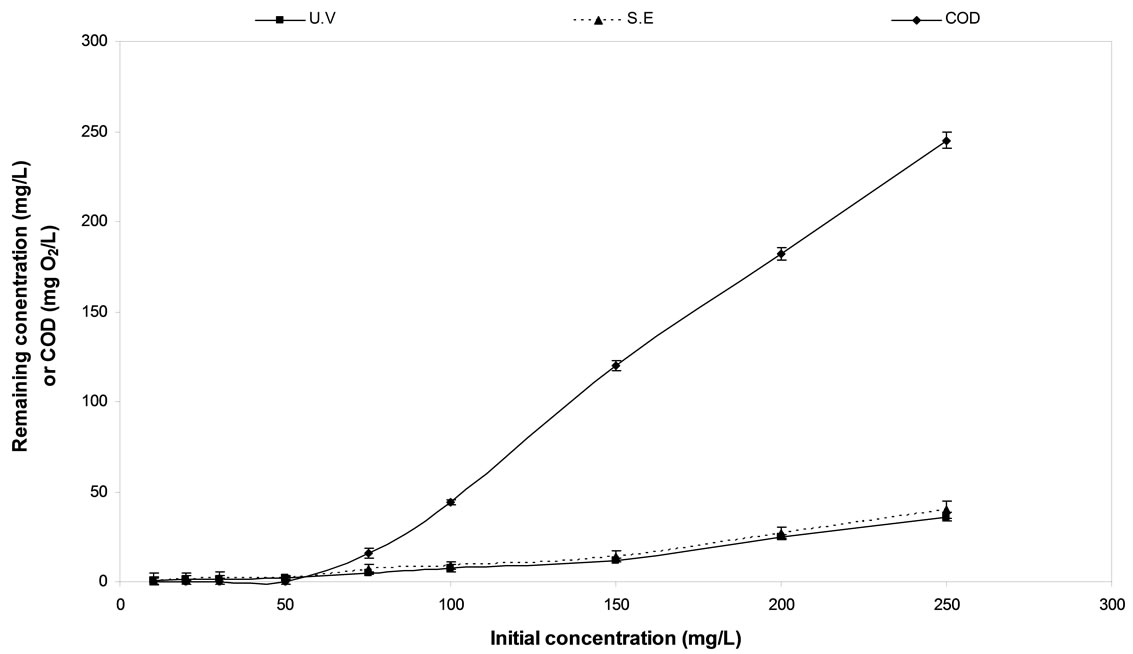
Figure 6. The effect of the initial concentration on diquat dibromide and COD removal using C/PbO2 electrode.
4. Conclusion
In this work The C/PbO2 electrode assisted electrochemical removal of diquat dibromide herbicides solutions has been the subject of the present investigation under several parameters. Those parameters include conductive electrolyte, current density, temperature, initial concentration of diquat dibromide, pH and time. The optimum condition for C/PbO2 electrode are: NaCl (2 g·L–1), temperature within (5˚C - 10˚C), degradation time of 60 min, initial concentration of 50 mg·L–1, and current density (150 mA·cm–2). The degradation of diquat dibromide was nearly completed (99.84%) using C/PbO2 electrode at pH 2.2.
REFERENCES
- T. Chichila and S. Walters, “Liquid Chromatographic Determination of Paraquat and Diquat in Crops Using a Silica Column with Aqueous Ionic Mobile Phase,” Journal—Association of Official Analytical Chemists, Vol. 74, 1991, pp. 961-967.
- N. I. Sax, “Dangerous Properties of Industrial Materials,” Sixth edition, VanNostrand Reinhold Company, New York, 1984.
- TOXNET, “National Library of Medicine’s Toxicology Data Network,” Hazardous Substances Databank, Public Health Service, National Institute of Health, U. S. Department of Health and Human Services, NLM, Bethesda, 1985.
- G. L. Berg, Ed., “Farm Chemicals Handbook,” Meister Publishing Company, Willoughby, 1986.
- F. Mogyoródy, “Electrochemical Degradation of Thiocarbamates in NaCl Solution,” Journal of Applied Electrochemistry, Vol. 36, No. 7, 2006, pp. 773-781. doi:10.1007/s10800-006-9136-9
- D. W. Miwa, G. R. P. Malpass, S. A. S. Machado and A. J. Motheo, “Electrochemical Degradation of Carbaryl on Oxide Electrodes,” Water Research, Vol. 40, No. 17, 2006, pp. 3281-3289. doi:10.1016/j.watres.2006.06.033
- A. Vlyssides, D. Arapoglou, S. Mai and E. M. Barampouti, “Electrochemical Oxidation of Two Organophosphoric Obsolete Pesticide Stocks,” International Journal of Environment and Pollution, Vol. 23, No. 3, 2005, pp. 289-299.
- C. Pulgarin and J. Kiwi, “Overview on Photocatalytic and Electrocatalytic Pretreatment of Industrial Non-Biodegradable Pollutants and Pesticides,” Chimie, Vol. 50, 1996, pp. 50-55.
- J. Gao, G. Zhao, W. Shi and D. Li, “Microwave Activated Electrochemical Degradation of 2,4-Dichlorophenoxyacetic Acid at Boron-Doped Diamond Electrode,” Chemosphere, Vol. 75, No. 4, 2009, pp. 519-525. doi:10.1016/j.chemosphere.2008.12.018
- C. Flox, P. L. Cabot, F. Centelas, J. A. Garrido, R. M. Rodríguez, C. Arias and E. Brillas, “Electrochemical Combustion of Herbicide Mecoprop in Aqueous Medium Using a Flow Reactor with a Boron-Doped Diamond Anode,” Chemosphere, Vol. 64, No. 6, 2006, pp. 892-902. doi:10.1016/j.chemosphere.2006.01.050
- M. Polcaro, S. Palmas, F. Renoldi and M. Mascia, “On the Performance of Ti/SnO2 and Ti/PbO2 Anodesin Electrochemical Degradation of 2-Chlorophenol for Wastewater Treatment,” Journal of Applied Electrochemistry, Vol. 29, No. 2, 1999, pp. 147-151. doi:10.1023/A:1003411906212
- C. A. Martınez-Huitle, M. A. Quiroz, C. Comninellis, S. Ferro and A. D. Battisti, “Electrochemical Incineration of Chloranilic Acid Using Ti/IrO2, Pb/PbO2 and Si/BDD electrodes,” Electrochimica Acta, Vol. 50, No. 4, 2004, pp. 949-956. doi:10.1016/j.electacta.2004.07.035
- M. H. Abu Shawish N. Abu Ghalwa, M. Hamada and H. Basheer, “Modified Carbon Paste Electrode for Potentiometric Determination of Diquat Dibromide Pesticide in Water and Urine samples,” Materials Science and Engineering C, 2011, in Press.
- L. G. Turner and R. E. Carawan, “Using COD to Measure Lost Product,” North Carolina Cooperative Extension Service, No. CD-38, North Carolina State University, 1996.
- K. C. Narasimham and H. V. K. Udupa, “Preparation and Applications of Graphite Substrate Lead Dioxide (GSLD) Anode,” Journal of the Electrochemical Society, Vol. 123, No. 9, 1976, pp. 1294-1298. doi:10.1149/1.2133063
- M. H. Mashhadizadeh, M. Talakesh, M. Peste, A. Momeni, H. Hamidian and M. Mazlum, “A Novel Modified Carbon Paste Electrode for potentiometric Determination of Mercury(II) Ion,” Electroanalysis, Vol. 18, No. 22, 2006 pp. 2174-2179. doi:10.1002/elan.200603643
- PHA, AWWA and WEF, “Standard Methods for the Examination of Water and Wastewater,” 18th Edition, American Public Health Association, Washington DC, 1992.
- H. S. Awad and N. Abo Galwa, “Electrochemical Degradation of Acid Blue and Basic Brown dyes on Pb/PbO2 Electrode in the Presence of Different Conductive Electrolyte and Effect of Various Operating Factors,” Chemosphere, Vol. 61, No. 9, 2005, pp. 1327-1335. doi:10.1016/j.chemosphere.2005.03.054
- M. Panizza, C. Bocca and G. Cerisola, “Electrochemical Treatment of Waste Water Containing Poliaromatic Organic Pollutants,” Water Research, Vol. 34, No. 9, 2000, pp. 2601-2605. doi:10.1016/S0043-1354(00)00145-7
- H. Florencio, M. Pires, E. Castro, L. A. Nunes, R. M. Borges and F. M. Costa, “Photodegradation of Diquat and Paraquat in Aqueous Solutions by Titanium Dioxide: Evolution of Degradation Reactions and Characterization of Intermediate,” Chemosphere, Vol. 55, No. 3, 2004, pp. 345-355. doi:10.1016/j.chemosphere.2003.11.013
- K. Asokan and K. Subramanian, “Design of a Tank Electrolyser for in-Situ Generation of NaClO,” Proceedings of the World Congress on Engineering and Computer Science, Vol. 1, 2009, pp. 139-142.
- A. Kraft, M. Stadelmann, M. Blaschke, D. Kreysig, B. Sandt, F. Schröder and J. Rennau, “Electrochemical Water Disinfection Part 1: Hypochlorite Production from Very Dilute Chloride Solutions,” Journal of Applied Electrochemistry, Vol. 29, 1999, pp. 861-868.
NOTES
*Corresponding author.

