Journal of Geoscience and Environment Protection
Vol.04 No.13(2016), Article ID:73148,14 pages
10.4236/gep.2016.413002
Seasonal Contrast of Some Anthropic Pollution Parameters in Water Retention in Mountainous Area under Sahelian Climate: Elementary Dynamics and Space Distribution (Territory of the Mounts Mandara, Cameroon)
Sara Lewa1*, Auguste Ombolo2, Benoit Loura3
1Department of Earth Sciences, Maroua University, Maroua, Cameroon
2Department of Hydraulic and Water Management, Maroua University, Maroua, Cameroon
3Department of Textile and Leather Engineering, Maroua University, Maroua, Cameroon

Copyright © 2016 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/



Received: October 18, 2016; Accepted: December 26, 2016; Published: December 29, 2016
ABSTRACT
In order to understand the origin, the extent and the space distribution of the seasonal anthropic pollution parameters in the water retention located in mountainous area thickly populated and which water resources is intended for the production of drinking water for serving a population of approximately 800,000 inhabitants, two sampling campaigns were carried out for the two seasons of the year known as the dry and rainy season. The samples were then conveyed and analyzed in laboratory. The parameters retained for this study were primarily the physicochemical parameters (pH, conductivity and temperature), measured in situ and second the major anthropic origins anions of (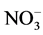 ,
, 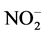 ,
, 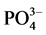 and
and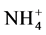 ). The results of the study show that overall the anthropic pollution parameters undergo an important seasonal variation of their concentration between the dry and rainy periods although they remain high in any season compared to the quality standards of natural water of the European Union. Their space distribution within retention remains overall random and is not function of the seasons. However, surrounding areas of the retention and the discharge system of the seasonal rivers (Mayos) which feed it present in rainy season space distributions of concentrations in anions of anthropic origin enumerated above highest.
). The results of the study show that overall the anthropic pollution parameters undergo an important seasonal variation of their concentration between the dry and rainy periods although they remain high in any season compared to the quality standards of natural water of the European Union. Their space distribution within retention remains overall random and is not function of the seasons. However, surrounding areas of the retention and the discharge system of the seasonal rivers (Mayos) which feed it present in rainy season space distributions of concentrations in anions of anthropic origin enumerated above highest.
Keywords:
Water Retention, Anthropic Pollution, Seasonal Variation, Space Distribution, Quality

1. Introduction
In a world context marked by increasing shortages and a rise of a demand for good water quality, many countries already took measures to protect their water resources. But, in spite of this progress, WHO and UNICEF underline that the world remains very far from achieving the Goals of the Millennium in the matter of development cleansing.
In addition, in spite of this drinking water shortage, agriculture responsible for 70% of all the world consumption of fresh water and groundwater and the demographic explosion vector of the fast increase in water requirements, involve a very great pressure on surface water and constitute the most important factors of the increasing of anthropic pollution parameters such as nitrates, phosphates and ammonium ions in these water [1] .
The present study aims to determine the origin, the extent and the seasonal space distribution of the concentrations of anthropic chemical anions in a water retention located in mountainous area under Sahelian climate with densities of inhabitants approaching 200 inhabitants/km2. This retention except its first vocation which is the production of drinking water to serve a population of more than 800,000 inhabitants, is also the building drain of two seasonal waterways (Mayos) draining a catchment area of a surface of approximately 70 km2 [2] . Mainly, in this work it will be all about determining the nature of this anthropic pollution through the analysis of some anthropic water pollution parameters, the considering of their seasonal fluctuation, their space distribution and their incidence on the irreversible degradation in the long term of the quality of the water retention resources.
2. Methods
2.1. Natural Setting
The study was conducted at the dam located at approximately 15 km from the town of Mokolo in far North Cameroon (Figure 1). Topography is characteristic of the mountainous area of the Mandara mounts with altitudes straying between 900 and 1500 m [3] . The climate is the Sahelian type with a precipitation ranging between 850 to 1100 mm of rain distributed a year over approximately 5 months [4] . The average temperature is of 26.0˚C and the relative humidity of the air from approximately 48% on annual average [5] . The seasonal drainage of the laying-up basin of a surface of approximately 70 km2 is ensured by two seasonal streams, the Madabrom and Mododrof [6] .
Figure 1. Map of the field study area.
The geological formations met date from the Panafricanorogenesis (500 ± 100 billion years) where the metamorphic series were granitites and rejuvenated. The major part of the base consists of metamorphic rocks (gneiss, schists) to which are added plutonic solid masses of rocks (granitoid, syenites…) [7] .
The dam retention consists of an earth embankment reinforced on both sides by a riprap slope. The dam presents a length of approximately 980 m for a height of 20 m. It presents in his center a tower of hydrant for the station of treatment located downstream at approximately 500 m. His maximum capacity is of approximately 3 million m3 on rising period.
The anthropic activities within the catchment area are primarily agricultural (food crops and of revenue). We note also some pastoral activities such as the breeding of the cattle, goats and the swine. The manufacturing and artisanal activities are non-existent or at least still at the embryonic stage [8] [9] [10] [11] .
2.2. Sampling and Measured Parameters
The various investigation campaigns on the ground were held over two periods known as the period of dry season and the period of rainy season of the year 2012-2013. The period of high dry season is between the end of March and the beginning of April and corresponds to the period of low water level while the period of the rains is located between the end of August and the beginning of September corresponding to the period of high water level in the retention. During these two periods 30 water samples were taken including 15 in period of dry season and 15 in period of rainy season. The points of sampling were selected as to cover the totality of the tank and taking in consideration the environmental specificity such as: the presence of the market gardening; the zone of drenching of the pets and the discharge system of the waterway feeding the retention.
The physicochemical parameters such as temperature, pH and conductivity were measured in the field using a Hach brand pH/conductivity meter. The concentrations of the major anthropic ions ( ,
,  ,
,  and
and ) were obtained according to the standard methods of water analysis [12] by the using of JENWAY 6320D Spectrophotometer.
) were obtained according to the standard methods of water analysis [12] by the using of JENWAY 6320D Spectrophotometer.
3. Results and Discussion
The results of physicochemical parameters that were measured in situ and the chemical analysis of anthropic pollution parameters of dry and rainy season samples are presented in Table 1.
Table 1. The results and the concentration of anthropic pollution elements in the water retention.
ND: not detected.
3.1. Statistical Characteristics of Anthropic Pollution Parameters
The chemical anthropic pollution parameters (Figure 2) represented by nitrates, nitrites, phosphates and the ammonium ion present respective percentages of 23.66%, 0.23%, 1.65% and 0.39% of the total sum of the anions in low water level against 37.91%, 0.99%, 12.74% and 1.67% of this sum in period of flooding. These slight increases of the anthropic elements of such as nitrates and phosphates respectively from approximately 14.25% and 11.09% in rainy period are in relation of the intense farming in the hydrological area catchment. In addition, the other parameters such as chlorides and sulfates although are slightly more abundant in period of rising than in period of low water level which they remain overall constant. This constancy can signify a weak impact of the seasons (dry season and rainy season) on their origin. Nevertheless in the absence of the data on the chemical signal of rainwater in this zone, the surface origin of these elements can be considered [13] .
The analysis of principal components of water of the retention reveals that the two principal factorial axes F1 and F2 (Figure 3) gather only approximately 40.98% of the variables in period of low waters and 45.55% in period of flood. This is revealing of a dispersion of the sources of origin of the chemical water composition.
In period of low water level, the F1 axis with 23.91% of the variables gathers the Temperature, the pH, Potassium, nitrates, bicarbonates and the ion ammonium. This regrouping highlights association of the ions corresponding to the anthropic pole of the water mineralization (presence of  and
and ) and translated in this fact the importance of domestic and agricultural pollution.
) and translated in this fact the importance of domestic and agricultural pollution.
In rainy period, the F1 axis which gathers respectively the temperature, conductivity, the chlorides and the bicarbonates shows the natural source of the mineralization of the water of the retention. However the F2 axis which regroups the pH, magnesium, potassium, phosphates and nitrates expresses the influence of the anthropic activities in the acquisition of its mineralization.

Figure 2. Statistical percentages of anthropic ions in dry season (a) and in rainy season (b).


Figure 3. Principal component axes of the dam water in dry season (a) and in rainy season (b).
3.2. Organic Pollution Indices
The analysis of the organic pollution indices (OPI) of the water of the retention in dry season and rainy season (Table 2) reveals that they oscillate around the
Table 2. Organic pollution indices of water retention.
OPI: organic pollution indices.
respective averages of 1.95 and 1.04. This indicates a very strong organic pollution of the whole water samples of the retention in all two seasons. However, we notice that the samples of water which presents moderate organic pollution (approximately 13.33% of the total samples of the dry season) and strong organic pollution (approximately 26.67% of the total samples of the dry season) evolve to a level of very strong organic pollution in rainy season. This transition testifies the recrudescence of anthropic pollution during the rainy season when the agro-pastoral activities and erosion (streaming, through fall…) are at their maximum [14] .
3.3. Spatial Distribution of Pollution Parameters
The ion ammonium (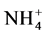 ) is produced by the reaction of ammonification which corresponds to the degradation of the nitrogenized organic material in to ammonium, which can take place in the aerobic or anaerobic zone and can appear in a broad range of environmental conditions [15] . Roughly, it is observed that the space distribution of the concentrations of the ion ammonium in the retention (Figure 4(c)) does not obey to a specific measure. However, it is noticed that the strong contents are observed in rainfed period and the low contents in low water level period which corresponds to the dry season. In addition, it is observed that the strong ammonium concentrations are located on the dead arms and in the center of the retention while the weak concentrations are close to the dam. Nevertheless, an aggregate point of strong concentrations in ammonium ion is located in the center of the retention without a particular explanation. These situations which do not have a precise explanation can be explained by weak stirring movements of the water of the retention. However, the strong concentrations in the peripheral zones in rainy seasons can be attributed to storm water runoff.
) is produced by the reaction of ammonification which corresponds to the degradation of the nitrogenized organic material in to ammonium, which can take place in the aerobic or anaerobic zone and can appear in a broad range of environmental conditions [15] . Roughly, it is observed that the space distribution of the concentrations of the ion ammonium in the retention (Figure 4(c)) does not obey to a specific measure. However, it is noticed that the strong contents are observed in rainfed period and the low contents in low water level period which corresponds to the dry season. In addition, it is observed that the strong ammonium concentrations are located on the dead arms and in the center of the retention while the weak concentrations are close to the dam. Nevertheless, an aggregate point of strong concentrations in ammonium ion is located in the center of the retention without a particular explanation. These situations which do not have a precise explanation can be explained by weak stirring movements of the water of the retention. However, the strong concentrations in the peripheral zones in rainy seasons can be attributed to storm water runoff.
Nitrites (



Figure 4. Spatial distribution of the concentration of pollution parameters in the water retention. (a) Nitrates (



The nitrates are the result of the transformation of the ammonium ions in nitrate through the process of nitrification [17] . The space distribution of nitrates within the water retaining for the two seasonal periods (Figure 4(a)) demonstrates the randomness of the spatial distribution of concentrations obtained according to the intake points and their variations according to the seasons. Overall, they are very high regardless the considered season. Nevertheless, they are higher in rainy season than in dry season. The high levels contents of the rainy season probably result from the contributions of elements by surface runoff of rain water. Indeed, the use of agricultural fertilizers during the rainy season contributes through the leaching land to the enrichment of water of the retention by nitrates. On the other hand, the high levels contents of nitrates in dry season cannot be explained by the contributions of surface waters runoff in absence of rain, but probably, the mineralization of the intrinsic organic matter of the retention. Bock et al. [17] affirm that some parts of nitrates of the retention are consumed by aquatic plants for their growth; all this corroborates the low contents of nitrates obtained at certain points of sampling to the periphery of the retention where aquatic flora abounds.
The lithosphere is the ultimate source of all the phosphorus of the biosphere. As 


3.4. Seasonal Variation of Pollution Parameters
The observation of Table 3 and Table 4 shows that the nitrates present
Table 3. Concentrations of pollution parameters in the dry season.
Table 4. Concentrations pollution parameters in the rainy season.
sensibly equal average concentrations in dry and rainy seasons of about 155.95 and 411.30 mg/l respectively; the nitrites and the ion ammonium present respectively in dry season the average contents of 1.52 and 2.57 mg/l while in rainy season these concentrations are 10.76 and 18.11 mg/l respectively. With regard to phosphates, although not being a dominating major element in water, its values remain important for the perception of anthropic pollution. It is noticed however that its value, very low in dry season (about 10.88 mg/l), becomes 138.20 mg/l in rainy season. This augmentation is enormous because it highlights the major role of the rain waters runoff on its growth within the retention. If the strong concentrations of nitrates and phosphates observed during the rainy season can be put on the uncontrolled use of fertilizers upstream of the water retention by agricultural activities [21] , the ammonium ion on the other hand highlights the extent of the animal and human rejections.
The study of 


3.5. Quality of the Water Resources
The observation of Table 5 which recapitulates the state of seasonal quality of the water in the retention shows that the nitrates, the nitrites, the phosphates and the ammonium ion often largely exceed the limiting thresholds fixed by the European standard quality of natural waters. Thereby, the 


Table 5. Seasonal quality of the water in the retention.


Figure 5. Seasonal variation of 
Lastly, the ammonium ion with a concentration in dry season of 2.57 mg/l, reaches a concentration in rainy season of 18.11 mg/l which seven times the concentration observed in dry season. Considering that the origin of the majority of these anions is exclusively external because they come for their majorities from the anthropic actions, their strong concentrations observed in rainy season depend on the badly controlled agropastoral activities and the phenomenon of grounds scrubbing by rain waters.
In the light of this report, it should be noted that a good management of the water retention resources must be considered and this one will be possible only if the public authorities establish strict and effective regulation of all anthropic activities near the dam. Durand and Petit [23] prescribed to ensure a good protection of the water resources a delimitation of three perimeters of protection to apprehend the position risks coming from the human activities. It will be about of envisaging:
-A perimeter of immediate protection with objective of avoiding any direct introduction of polluting substances into the water retention and preventing the degradation of the infrastructure;
-A perimeter of close protection where activities or installations which can harm directly or indirectly taken water quality can be prohibited or regulated;
-And finally a perimeter of distant protection to regulate activities or installations which can degrade the quality of the water resources.
4. Conclusions
The analysis of principal component of the water of the retention reveals that in low water period the F1 axis gathers the pH, the nitrates and the ammonium ion and in that fact translated the impact of domestic and agricultural pollution constraints. The F2 axis which gathers chlorides, sulfates and bicarbonates highlights the natural source of the mineralization of the water of the retention. In period of high water level, the two axes F1F2 gather the parameters of water natural mineralization while only the F3 axis materializes anthropic pollution.
Anthropic pollution in the study area has primarily agropastoral and domestic origin. Indeed, if the variations of NO3-/K+ ratio lay emphasis on the impact of the use of fertilizers or the mineralization of the organic matter on the concentrations of nitrates in water, the organic pollution indices (OPI) put more emphasis on the degree of this organic pollution.
The space distribution of some major anthropic elements such as nitrates (



Considering the increasing of the anthropic pollution elements, the effective management of this water resource according to environmental and socioeconomic issues, will only be possible with the establishment of a strict and effective regulation of anthropic activities by public authorities. Furthermore, delimitation of security perimeter upstream and around the retention will be at longer essential to complete de protection of the water resource.
Cite this paper
Lewa, S., Ombolo, A. and Loura, B. (2016) Seasonal Contrast of Some Anthropic Pollution Parameters in Water Retention in Mountainous Area under Sahelian Climate: Elementary Dynamics and Space Distribution (Territory of the Mounts Mandara, Cameroon). Journal of Geoscience and Environment Protection, 4, 27-40 http://dx.doi.org/10.4236/gep.2016.413002
References
- 1. Cooper, C.M. (1993) Biological Effects of Agriculturally Derived Surface Water Pollutants on Aquatic Systems. Journal of Environmental Quality, 22, 402-408.
https://doi.org/10.2134/jeq1993.00472425002200030003x - 2. Callède, J. and Delfieu, G. (1967) Hydrology of the North-Cameroon Mayos: The Mayo Tsanaga Basin—Campaign of the Year 1966. Yaounde, Orstom, 46.
- 3. Morin, S. (1979) Oro-Hydrography, Geomorphology. In: Atlas of the United Republic of Cameroon, Ed Young Africa, 13-17.
- 4. Olivry, J.-C. (1986) Rivers of Cameroon. Mesres-Orstom. Paris, Orstom, Coll. Hydrological Monographs No. 9, 733.
- 5. L’Hote, Y. and Mahé, G. (1996) West and Central Africa; Annual Precipitations Averages (Period of 1951-1989). Scale Map 1/6,000,000, 90 × 60 cm. Paris, Orstom.
- 6. Le Gourières, D. (1962) Study of the Mayo Mokolo Catchment Area. Yaounde, Orstom-Ircam, Graphs, 23.
- 7. Dumont, J.C. and Peronne, Y. (1966) Directions for Use on the Maroua Sheet. Geological Map of Recognition at the Level of 1/500,000 D.M.G.C., Yaounde.
- 8. Seignobos, C. (1982) Fat, Parks and Agrarian Civilizations (Chad and North-Cameroon). Books of Overseas, 139, 129-269.
- 9. Pillet, P. (1987) Vegetable Crops in the Northern Provinces of Cameroon. Missions of Identification. Mesres/IRA, Station of Foumbot, Program Market Gardenings, 19.
- 10. Holtzman, J. (1981) Breeding and Marketing of Cattle in the Mandara Mountains. Michigan State University, East Lansing.
- 11. Boulet, J. (1975) Magoumaz, the Study of Mountain Terroir in Mafa Country. Orstom, Yaounde, 147.
- 12. Rodier, J., Legube, B., Merlet, N. and Coll (2009) The Analysis of the Water. 9th Edition, Dunod, Paris, 1203.
- 13. Hauhouot, C. (2004) Anthropogenic Pressures on the Natural Environments of South-East Ivory Coast. Geo-Eco-Trop, 28, 69-82.
- 14. Bahroun, S. (2007) Impact of Urban and Industrial Wastewaters on Natural Water in the Area of El Tarf. Memory of Magister, University of Annaba, 160.
- 15. Ladd, J.N. and Jackson, R.B. (1982) Biochemistry of Ammonification. In: Stevenson, F.H., Ed., Nitrogen in Agricultural Soils, American Society of Agronomy, Madison, 173-228.
- 16. Prosser, J.I. (1989) Autotrophic Nitrification in Bacteria. Advances in Microbial Physiology, 30, 125-181.
https://doi.org/10.1016/S0065-2911(08)60112-5 - 17. Bock, E., Koops, H.P., Ahlers, B. and Harms, H. (1992) Oxidation of Inorganic Nitrogen Compounds as Energy Source. In: Balows, A., Trüper, H.G., Dworkin, M., Harder, W. and Schleifer, K.H., Eds., The Prokaryotes: A Handbook on Biology of Bacteria: Ecophysiology, Isolation, Identification, Applications, Springer, New York, 414-430.
- 18. Reeburgh, W.S. (1997) Figures Summarizing the Global Cycles of Biogeochemically Important Elements. Bulletin of the Ecological Society of America, 78, 260-267.
- 19. Carpenter, E.J., Montoya, J., Burns, J., Mulholland, M., Subramaniam, A. and Capone, D.G. (1999) Extensive Bloom of a N2-Fixing Diatom/Cyanobacterial Association in the Tropical Atlantic Ocean. Marine Ecology Progress Series, 185, 272-283.
https://doi.org/10.3354/meps185273 - 20. Bennet, E.M., Carpentier, S.R. and Caracao, N.F. (2001) Human Impact on Erodible Phosphorus and Eutrophication: A Global Perspective. Bioscience, 51, 227-234.
https://doi.org/10.1641/0006-3568(2001)051[0227:HIOEPA]2.0.CO;2 - 21. Déradji, F., Bousnoubra, H., Kherici, N., Roméo, M. and Caruba, R. (2007) Impact of Organdnic Pollution on the Quality of Surface Waters in the Algerian North-East. Sécheresse, 18, 27.
- 22. Djabri, L. (1996) Mechanism of Pollution and Vulnerability of the Water of Seybouse: Geological, Industrial, Agricultural and Urban Origins. State Thesis of the University of Annaba, Annaba, 176.
- 23. Durand, F. and Petit, V. (1997) Planning Guide of Catchments Intended for Drinking Water Supply and the Perimeters of Immediate Protection. BRGM Report R 39473, 38 p, 1 Year.





