Journal of Geoscience and Environment Protection
Vol.03 No.07(2015), Article ID:59420,8 pages
10.4236/gep.2015.37002
Assessment of Heavy Metals Pollution in Soils and Vegetation around Selected Industries in Lagos State, Nigeria
Adeola Alex Adesuyi1*, Kelechi Longinus Njoku2, Modupe Olatunde Akinola2
1Environmental Impact Assessment, Environment Department, Shell Petroleum Development Company, Port Harcourt, Nigeria
2Environmental Biology Unit, Cell Biology and Genetics Department, University of Lagos, Lagos, Nigeria
Email: *biologistalex@gmail.com, kecynjoku@yahoo.com, mayomi12@yahoo.com
Copyright © 2015 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 8 July 2015; accepted 4 September 2015; published 7 September 2015
ABSTRACT
In this study, eleven soil samples and twenty-twoplants samples were collected in the vicinity of eleven industries and a thermal station was analyzed for zinc, copper, iron, lead and cadmium. Soil sample from Egbin thermal station had the highest concentration of Zn (141.06 mg/kg) and Cu (131.70 mg/kg). Soil from international textile had the highest level of Fe and the soil from Nichemtex had the highest concentration Pb and Cd was the highest in soil from Guinness (28.91 mg/kg, 59.80 mg/kg and 1.72 mg/kg respectively). The highest concentrations of the heavy metals were observed from different plants species. Analyses of variance (p < 0.05) showed that heavy metal variation in plant and soil samples were not significant (p > 0.5). There were positive correlations between the heavy metals in the soils and the plant samples indicated that the plants obtained the heavy metals from the soil. Plants having BCF values less than one had limited ability to accumulate, translocate and phytoextract heavy metals. These plants in this study with higher Bioconcentration Factor value especially those greater than one (Croton lobatus, Borreria sp., Cyathula prostrata, Lantana camara, Ficus sp., Mimosa pudica, Eclipta prostrata, Commelina sp. etc.) were suggested for further research and assessment on their bioaccumulation abilities and phytoremediation potential.
Keywords:
Heavy Metals, Bioaccumulation, Industries, Phytoremediation

1. Introduction
Fifty percent of Nigeria’s industrial activities including 300 industries in 12 industrial estates are located in the Lagos area [1] . The high urbanization and industrial growth rate in Lagos had made it one of the most densely populated regions on the earth with a population of about 9.3 millions according to 2006 Census [2] . Thus, the continuous increase in population and industrial growth in Lagos persistently cause large volume of wastes to be generated industrially and domestically [1] . Industries have largely been responsible for discharging untreated effluents containing trace metals such as zinc (Zn), copper (Cu), manganese (Mn), cadmium (Cd), mercury (Hg), nickel (Ni), lead (Pb), Iron (Fe) and Chromium (Cr) improperly into our environments [3] -[5] . Soils and plants in nearby zone of industrial areas display increased concentration of heavy metals, serving in many cases as sinks of pollution loads [6] . Plants around industries take up large quantities of pollutants which they translocate into vegetative and generative organs at various rates [7] [8] . However, pollution in developing nations is correlated with the degree of industrialisation and the intensity of chemical usage [9] -[11] .
The concentrations of heavy metals in animals have harmful effects especially when consum above the bio-recommended limits from plants [12] . Although individual metals exhibit specific signs of their toxicity, the followings have been reported as general signs associated with cadmium, lead, iron, zinc, and copper poisoning: gastrointestinal (GI) disorders, diarrhoea, stomatitis, tremor, hemoglobinuria causing a rust-red colour to stool, ataxia, paralysis, vomiting and convulsion, depression, and pneumonia when volatile vapours and fumes are inhaled [13] . The nature of effects can be toxic (acute, chronic or sub-chronic), neurotoxic, carcinogenic, muta- genic or teratogenic [12] .
The overall objective of this study is to investigate the influence of industrial activities and associated processes with heavy metal contamination of soils and vegetation in Lagos city, and comparing heavy metal contents in soils and that of the plants (Bioaccumulation). Information being obtained can assist in identifying and subjecting bioaccumulators for remediation of polluted soils.
2. Materials and Methods
2.1. Study Area
Study areas included three local government areas in Lagos, south western Nigeria (Figure 1). It is Nigeria’s largest city, chief port, and principal economic, cultural and industrial center.
Figure 1. Map of Lagos (in Nigeria) showing sampling locations.
2.2. Description of Sampling
All the sites are located within Lagos metropolis in Lagos state is shown in Figure 1. The different sampling sites, the locations and products of industries are shown in Table 1.
2.3. Sample Collections
Samples of soils and plants were collected in the month of June, July and August 2012. Twenty-two (22) plant samples and eleven (11) soil samples were obtained from Ikeja, Ikorodu and Alimosho Local government areas (Figure 1). The soil samples were collected at depth ranging between 5 - 25 cm using a steel soil auger and kept in a tagged polythene bags. The plants were carefully uprooted and also labelled in designated polythene bags [14] .
2.4. Plant Samples Digestion and Analyses
Each plant sample was separated into leaf, stem and root. Each part was cut into pieces and air-dried; each plant part was further dried in an oven at 80˚C, milled with an electric blender into powdery consistency. Each milled plant part samples was mixed properly by rumbling and sieved through (1 mm) mesh filler [14] .
A gram of each milled homogenized sample was weighed with a digital weighing balance in to a conical flask. 5 ml of 60% hydrochloric acid (HCl) and 10 ml of 70% nitric acid (HNO3) were added into the weighed samples. The sample mixture was digested with a moderate heat (50˚C) with a hot-plate until white fumes evolved, makingit brownish solution. The heat was intensified further for few minutes to expel off most of the hydrochloric acid (HCl). 50 ml of distilled water was dispensed into the solution and heated for a few minutes, allowed to cool before it was filtered through whatman’s No 1 paper (11 µm) into a dispensed transparent plastic container that has been cleaned with detergent and treated successively with HCl and rinsed with deionized water. The filtered sample was left to stand for few minutes for the aspiration of the element according [15] . The digested samples were analyzed for zinc (Zn), lead (Pb), iron (Fe), copper (Cu) and cadmium (Cd) concentrations using atomic absorption spectrophotometer (AAS) [15] .
2.5. Soil Samples Preparation and Analyses
The soil samples were air-dried and sieved to <0.25 mm, then stored in desiccators prior to analysis of heavy metals. 0.5 g of the dry sample was weighed and digested with a mixture of nitric acid (HNO3) and perchloric acid (HClO4) as was described by [16] . 2 g sample was transferred to a Teflon beaker and 25 ml of distilled water was added. 2 ml of concentrated HNO3 was added the contents of the beaker and this was allowed to evaporate to dryness. Subsequently, three drops of concentrated sulfuric acid (H2SO4) and 10 ml of hydrogen fluoride (HF) were added. The sample was then placed on a sand bath while the temperature slowly increased to 200˚C and was allowed to evaporate to dryness. This was followed by the addition of 15 ml of concentrated
Table 1. The different sampling sites, localized industries, their products with the gps coordinates.
HNO3, 2 ml of H2SO4, and 5 ml of HClO4. Heating continued until strong fumes of SO3 were produced. The Teflon container was cooled and the solution transferred quantitatively to a 50 ml volumetric flask by adding distilled water [16] . The concentrations of Zn, Pb, Fe, Cu and Cd, were determined by atomic absorption spectrophotometer [16] .
2.6. Bioconcentration Factor (BCF)
Bioconcentration factor was calculated from the following equation as described by [17] .
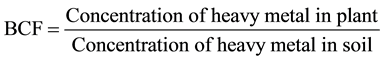
2.7. Statistical Analyses
The descriptive statistical parameters were calculated with Graph Pad Prism software package (version 6.0), the correlations of heavy metals in soils and plants were analyzed using Pearson correlation analysis. One-way analysis of variance (ANOVA) was performed to test the significance of differences in total metal concentrations in plant and soil samples. The Tukey-Kramer Multiple Comparisons Test was used to compare all heavy metal concentration levels in soils and plant.
3. Results
3.1. Heavy Metal Concentration in Soil Samples
The metal concentration level in soil samples is shown in Table 2. The soil sample from the vicinity of Egbin power thermal station has the highest concentration of zinc (141.06 mg/kg) while the least was in the soil sample from Clay industry (6.23 mg/kg). There was no significant difference (p > 0.05) in the concentration of zinc in all soil samples and they were all below the standard threshold value (EU/UK/USEPA threshold). The concentration of copper in the soil samples varied from 0.93 mg/kg in the vicinity of Chikki/Holdent Industries to 131.70 kg/mg in the soil from Egbin power thermal station. There was no significant difference (p > 0.05) in copper concentration in all soil samples. Concentration of iron in soil samples was the highest in soils at International Textile Limited (28.91 mg/kg) and least in Nichemtex industries (1.201 mg/kg). There was significant difference (p < 0.05) among iron concentration from all the different soil samples. Lead concentration in sampled soils range from 1.12 mg/kg in the vicinity of Clay industries to 59.80 kg/mg in Nichemtex industries. There was no significant difference (p > 0.05) in lead concentration in all soil samples analysed. The concentration of
Table 2. Heavy metal concentration in soil samples (mg/kg).
cadmium in soil sampled vary from zero (not detected) in some locations to 1.72 mg/kg in the vicinity of Guinness in Ikeja. There was significant difference (p < 0.05) in the concentration of cadmium in the soil samples. However, cadmium concentrations were all below the threshold values (EU/UK/USEPA threshold) for the heavy metal in soil. Analyses of variance show variation among heavy metals in soil samples to be significant (p < 0.5).
3.2. Heavy Metal Concentration in Plants
The metal concentration level of heavy metals in plant samples is shown in Table 3. The highest concentration of copper was recorded in Borreria sp. (151.50 mg/kg) with the least in Amaranthus spinosus (0.1 kg/mg). There was significant difference (p < 0.05) in copper concentration across all the plant samples. Zinc concentration level in plant samples range from 2.20 mg/kg to 19.18 mg/kg with Croton lobatus having the highest concentration while Cyathula prostrata had the least. However, there was no significant difference (p > 0.05) in zinc concentration level in 75% of all sampled plants. Iron concentrations range from 0.41 mg/kg in Ludwigra sp. to 38.01 mg/kg in Ficus sp. and there was significant difference (p < 0.05) in all plant samples. The highest concentration of lead was recorded in Corchorus oliterus (3.19 mg/kg) and the least in Cyathula prostrata (0.59 mg/kg) and there was no statistical difference (p > 0.5) across all plants. Concentrations of cadmium in plant was generally low varying from zero in so many plants to 0.02 mg/kg and were quite below the threshold values (EU/UK/ USEPA threshold) for heavy metals in plants.
Table 3. Heavy metal concentration in plant samples (mg/kg).
ND-Not Detected.
3.3. The Bioconcentration Factor (BCF)
The bioconcentration factor is shown in Table 4. BCF value is highest in copper than in any other heavy metals. For copper the BCF values ranged from 0.012 to 2.099, zinc from 0.021 to 0.500, iron from 0.080 to 1.574, in lead from 0.029 to 0.538 and cadmium from 0.00 to 0.080. Croton lobatus had the highest bioaccumulation for Zn and Cu with BCF value of 0.500 and 2.099 respectively, Cyathula prostrata has the highest bioaccumulation for Fe and Pb with BCF value of 1.932 and 0.527 respectively, while Corchorus oliterus has the highest BCF value of 0.080 for Cd.
3.4. Correlation between Metals in Soil and Plant Samples
Correlation between metals in plant and soil samples is shown in the Table 5. Thecorrelation between soil and plant for all the metals are all positive. These relations were significant for copper, iron, lead and cadmium (r = 0.718, r = 0.644, r = 0.705 and r = 0.010 respectively) but not significant for zinc (0.030). The correlation pattern indicates that the heavy-metal concentrations of Cu, Fe, and Cd in plants are associated with the concentration in
Table 4. Bioconcentration factor of Zn, Cu, Fe, Pb and Cd.
Table 5. Computed pearson correlation between heavy metals in plant and soil samples.
the soils but Zn might be influenced by other unknown sources.
4. Discussion
High values of copper observed generally in this study are consistent with values reported by [18] [19] . They identified plants to greatly uptake Cu, Cd and Zn. Cu exhibited considerable variations from location to location with the highest value observed at Egbin thermal station although still within the range of International threshold values for copper concentration in soils. Comparison of the Cu concentration in all sampled plants showed that it is higher in plants located in the vicinity of the thermal station. Melting, grinding or the cutting of copper, and emission from electricity cables, may produce fumes and dust, and exposure or inhalation of these fumes may produce potential health hazards [20] . Fumes of copper may cause metal fumes fever with flu-like symptoms and hair and skin discoloration in man although dermatitis has not been reported, it can also cause irritation of the upper respiratory tract, a metallic taste in the mouth and nausea [20] . [21] [22] explained that there are different tolerance ranges for plants, but a critical toxic level of Cu is in the range of 20 - 30 mg/kg for most plants. The risk of Cu toxicity in vegetation species is clearly evident by the fact that higher concentrations have been reported in this current study.
Cadmium is a toxic element and can cause serious health problems. The most common sources for cadmium in soil and plants are phosphate fertilizers, non-ferrous smelters, lead and zinc mines, sewage sludge application and combustion of fossil fuels [23] [24] . International threshold values for heavy metals concentration in soils for cadmium in soil are between 1.40 - 3 mg/kg [25] . At this level, in most cases, it is taken up metabolically and is easily transported to the other parts of the plant [26] . In this present study, Cd concentrations in all soil and plant samplesinvestigated in this presentstudy are low as also observed by [20] .
Zinc is essential element for plant growth, as it serves an important role in plant structure and function [27] , it is a natural constituent of soils in terrestrial ecosystem and it is taken up actively by roots [28] . In this study, there is slight pollution of Zn in the textile, paint and the thermal station with the highest concentration observed in soil of Egbin thermal station. The high concentrations of the soil could have negative effects on soil microbial population and activities [29] , provoking a low organic matter mineralization during the plant growth. These results are line with the findings of [30] which observed the soils of Industrial area to be slightly polluted with Zn.
Iron is another essential element for plant and animalgrowth. Its deficiency can cause various types of diseases; however, its high concentration also affects plant growth. Iron concentration levels in soil samples collected from all locations showed no significantdifferences with the heavy metals in plants of same sites. However, the observed concentrations of Fe in plants are slightly higher than those in soils and none is beyond the regulated standards.
Lead is regarded as highly hazardous for plants, animals and particularly for microorganisms. The main sources of lead pollution in agriculture and plants are lead mines, combustion of leaded hydrocarbon, sewage sludge applications and farmyard manure, industrial processes etc. [20] . In this study the concentration Pb in 25% of the plants were higher than the maximum acceptable range of 0.3 - 2.0 mg/kg for plants. The highest concentrations of Pb in soils were observed in Sunflag group (spintex) and Nichemtex. The concentration of Pb in soil is of a significant interest as it is far higher than what [30] recorded in a similar study in the vicinity of Sunflag group (spintex). These high concentrations of Pb in soils indicate high amount of Pb in textile effluents. This result is in line with the findings of [30] -[32] .
Plant species, physiological stage, uptake capability as well as growth rate are major determinants of metal transfer from soil to the crop [6] . The ability of plants to tolerate and accumulate these metals may provide the bases for their phytoremediation usefulness. Bioconcentration factor (BCF) can be used to assess a plant’s potential for phytoremediation purposes. The highest BCF value for Zn and Cu was observed in Croton lobatus, for Fe it was observed in Cyathula prostrata, for Pb it was observed in Borreria sp. while Corchorus oliterus had the highest BCF for Cd.
In an attempt to understand the relationship between the concentrations of corresponding metals evaluated from the soil and plant samples, the Pearson Correlation Co-efficient, r, was used. There are positive correlation between soil and plants for all the metals in all the investigated areas. These relations were significant (p < 0.05) for Cu, Fe, Pb and Cd but not significant for Zn under the current study. The results of positive correlation between soil and plants have been supported by earlier research findings by [33] [34] . These positive relations indicate that plants take in nutritional elements from the soil through their roots. However, the negative correlation relation indicated by Zn, give a strong suspicion to the fact that some elements might be assimilated through other organs of the plants other than their roots. Such elemental intake may be directly through atmospheric deposition.
5. Conclusion
The findings in this study show that plants in the vicinity of industries have high risk of having heavy metal concentrations beyond the permissible limit if safety and environmental law are not enforced. This study confirms a relationship between the concentration of heavy metals in the plants and soils. Plants having BCF values less than one had limited ability to accumulate, translocate and phytoextract heavy metals (Fitz and Wenzel, 2002). Therefore, we recommend plants in this study with higher BCF value especially those greater than one (such as Croton lobatus, Borreria sp., Cyathula prostrata, Lantana camara, Ficus sp., Mimosa pudica, Eclipta prostrata, Commelina sp. etc.) be subjected for further research and assessment on their phytoremediation abilities.
Acknowledgements
The authors wish to thank Oluwafunmilayo Famakinwa-Adesuyi, Chinedu Anikpo, Adetokunbo Soyoye and Taibat Olajumoke Odukoya for their assistance during the field data gathering exercises and special thanks to the EIA Team, Environmental Dept. of Shell Petroleum Development Company in Nigeria, Port Harcourt, especially Prof Gabriel Umoh, Prof. Valerie Nnodu, Ajibade Temitope, Ngwoke Moses and Ojesanmi Adesola for their intellectual and invaluable contributions.
Cite this paper
Adeola AlexAdesuyi,Kelechi LonginusNjoku,Modupe OlatundeAkinola, (2015) Assessment of Heavy Metals Pollution in Soils and Vegetation around Selected Industries in Lagos State, Nigeria. Journal of Geoscience and Environment Protection,03,11-19. doi: 10.4236/gep.2015.37002
References
- 1. Oresanya, O. (2000) Methods of Landfill and Landfill Equipments. A Technical Paper on Workshop on the Role of Sanitary Landfilling in Integrated Solid Waste Management, 60 p.
- 2. Odukoya, A.M., Abimbola, A.F. and Lawal, O. (2011) Potential Soil Contamination with Toxic Metals in the Vicinity of Active and Abandoned Dumpsite. Agriculture and Biology Journal of North America, 2, 785-790.
http://dx.doi.org/10.5251/abjna.2011.2.5.785.790 - 3. Ibok, U.J., Udosen, E.D. and Udoidiong, O.M. (1989) Heavy Metals in Fishes from Stream in Ikotekpene Area of Nigeria. Nigeria Journal of Technology and Research, 1, 61-68.
- 4. Chen, Y. and Chen, M. (2001) Heavy Metal Concentrations in Nine Species of Fishes Caught in Coastal Waters off Ann Ping South West Taiwan. Journal of Food and Drug Analysis, 9, 107-114.
- 5. Tiwari, A.K., De Maio, M., Singh, P.K. and Mahato, M.K. (2015) Evaluation of Surface Water Quality by Using GIS and a Heavy Metal Pollution Index (HPI) Model in a Coal Mining Area, India. Bulletin of Environmental Contamination and Toxicology.
http://dx.doi.org/10.1007/s00128-015-1558-9 - 6. Cui, Y.J., Zhu, Y.G., Zhai, R.H., Chen, D.Y., Huang, Y.Z., Qui, Y. and Ling, J.Z. (2004) Transfer of Metals from Soil to Vegetables in an Area near a Smelter in Nanning, China. Environmental International, 30, 784-790.
http://dx.doi.org/10.1016/j.envint.2004.01.003 - 7. Kovács, M., Turcsányi, G., Szoke, P., Penksza, K., Kaszab, L. and Koltay, A. (1993) Heavy Metal Content in Cereals in Industrial Regions. Acta Agrobotanica Hungaricea, 42, 171-183.
- 8. Tack, F.M.G., Van Ranst, E., Lievens, C. and Vandenberghe, R.E. (2006) Soil Solution Cd, Cu and Zn Concentrations as Affected by Short-Time Drying or Wetting: The Role of Hydrous Oxides of Fe and Mn. Geoderma, 137, 83-87.
http://dx.doi.org/10.1016/j.geoderma.2006.07.003 - 9. Filazi, A., Baskaya, R., Kum, C. and Hismiogullari, S.E. (2003) Metal Concentrations in Tissues of the Black Sea Fish Mugil auratus from Sinopicliman, Turkey. Human Experiment and. Toxicology, 22, 85-87.
http://dx.doi.org/10.1191/0960327103ht323oa - 10. Akinola, M.O., Njoku, K.L. and Ekeifo, B.E. (2008) Determination of Lead, Cadmium and Chromium in the Tissue of an Economically Important Plant Grown around a Textile Industry at Ibeshe, Ikorodu Area of Lagos. Advances in Environmental Biology Journal, 2, 25-30.
- 11. Chandra, S., Singh, P.K., Tiwari, A.K., Panigrahy, B. and Kumar, A. (2014) Evaluation of Hydrogeological Factor and Their Relationship with Seasonal Water Table Fluctuation in Dhanbad District, Jharkhand, India. ISH Journal of Hydraulic Engneering, 21, 193-206.
http://dx.doi.org/10.1080/09715010.2014.1002542 - 12. Duruibe, J.O., Ogwuegbu, M.O.C. and Egwurugwu, J.N. (2007) Heavy Metal Pollution and Human Biotoxic Effects. International Journal of Physical Science, 2, 112-118.
- 13. McCluggage, D. (1991) Heavy Metal Poisoning, NCS Magazine. The Bird Hospital, Columbus.
- 14. Akinola, M.O., Njoku, K.L. and Ifitezue, N.V. (2011) Assessment of Heavy Metals (Lead and Cadmium) Concentration in Paspalum orbiculare near Municipal Refuse Dumpsites in Lagos State, Nigeria. Journal of Ecology and the Natural Environment, 3, 509-514.
- 15. Gray, D.C. (1980) Analytical Chemistry. 3rd Edition, John Wiley and Sons Inc., New York.
- 16. Hseu, Z.Y., Chen, Z.S., Tsai, C.C., Tsui, C.C., Cheng, S.F., Liu, C.L. and Lin, H.T. (2002) Digestion Methods for Total Heavy Metals in Sediments and Soils. Water, Air and Soil Pollution, 141, 189-205.
http://dx.doi.org/10.1023/A:1021302405128 - 17. Ghosh, M. and Singh, S.P. (2005) A Comparative Study of Cadmium Phytoextraction by Accumulator and Weed Species. Environmental Pollution, 133, 365-371.
http://dx.doi.org/10.1016/j.envpol.2004.05.015 - 18. Romer, L.H. and Keller, H. (2001) Exudation of Organic Acids by Spinach and the Mobilization of Cu, Zn, and Cd in Soil. In: Horst, W.J., Ed., Plant Nutrition: Food Security and Sustainability of Agroecosystems, Kluwer Academic, Amsterdam, 556-557.
- 19. Mattina, M.I., Lannucci-Berger, W., Musante, C. and White, J.C. (2003) Concurrent Plant Uptake of Heavy Metals and Persistent Organic Pollutants from Soil. Environmental Pollution, 124, 375-378.
http://dx.doi.org/10.1016/S0269-7491(03)00060-5 - 20. Khan, M.A., Ahmad, I. and Rahman, I. (2007) Effect of Environmental Pollution on Heavy Metals Content of Withaniasomnifera. Journal of the Chinese Chemical Society, 54, 339-343.
http://dx.doi.org/10.1002/jccs.200700049 - 21. Robson, A.D. and Reuter, D.J. (1981) Diagnosis of Copper Deficiency and Toxicity. In: Loneragen, J.F., Robson, A.D. and Graham, R.D., Eds., Copper in Soils and Plants, Academic Press, London, 287-312.
- 22. Kabata-Pendias, A. and Mukherjee, A.B. (2007) Trace Elements from Soil to Human. Springer-Verlag, Berlin, 23.
http://dx.doi.org/10.1007/978-3-540-32714-1 - 23. Davies, B.E. (1990) Cadmium. In: Alloway, B.J., Ed., Heavy Metals in Soil, Glasgow, Blackie, 321.
- 24. McBride, M.B. (1995) Toxic Metal Accumulation from Agricultural Use of Sludge: Are USEPA Regulations Protective? Journal of Environmental Quality, 24, 5-18.
http://dx.doi.org/10.2134/jeq1995.00472425002400010002x - 25. Canadian Council of Ministers for the Environment (CCME) (2001) Canadian Water Quality Guidelines for the Protection of Aquatic Life: Summary Table. CCME Water Quality Index 1.0. User’s Manual, Winnipeg, 5.
- 26. Pendias, A.K. and Pendias, H. (1992) Trace Elements in Soils and Plants. 2nd Edition, CRC Press, Boca Raton.
- 27. Erum, S. and Ahmad, S.S. (2010) Integrated Assessment of Heavy Metals (Fe, Zn, Pb, Ni, Cd and Cr) Pollution along Motorway M-2, Pakistan. Soil and Environment, 29, 110-116.
- 28. Aubert, H. and Pinta, M. (1997) Trace Element in Soils. Elsevier, Amsterdam, 395-398.
- 29. Dai, J., Becquer, T., Rouiller, J.H., Reversat, G., Bernhard-Reversat, F. and Lavelle, P. (2004) Influence of Heavy Metals on C and N Mineralisation and Microbial Biomass in Zn-, Pb-, Cu-, and Cd-Contaminated Soils. Applied Soil Ecology, 25, 99-109.
http://dx.doi.org/10.1016/j.apsoil.2003.09.003 - 30. Njoku, K.L., Akinola, M.O. and Zighadina, T.M. (2013) A Study on the Spatial Distribution of Heavy Metal in Industrial Area of Ikorodu, Lagos State, Nigeria. Journal of Research in Environmental Science and Toxicology, 2, 64-70.
- 31. Van Assche, F. and Clijsters, H. (1990) Effects of Metals on Enzyme Activity in Plants. Plant, Cell and Environment, 13, 195-206.
http://dx.doi.org/10.1111/j.1365-3040.1990.tb01304.x - 32. Jensen, A. and Bro-Rasmussen, F. (1992) Environmental Cadmium in Europe. Reviews of Environmental Contamination and Toxicology, 125, 101-181.
http://dx.doi.org/10.1007/978-1-4612-2890-5_3 - 33. Fatoki, O.S. (2003) Lead, Cadmium and Zinc Accumulation along Some Selected Major Roads of Eastern Cape. International Journal of Environmental Studies, 60, 199-204.
http://dx.doi.org/10.1080/00207230304734 - 34. Fatoki, O.S. (2003) Lead, Cadmium and Zinc Accumulation along Some Selected Major Roads of Eastern Cape. International Journal of Environmental Studies, 60, 199-204.
http://dx.doi.org/10.1080/00207230304734. - 35. Addo, M.A., Darko, E.O., Gordon, C., Nyarko, B.J.B., Gbadago, J.K., Nyarko, E., Affum, H.A. and Botwe, B.O. (2012) Evaluation of Heavy Metals Contamination of Soil and Vegetation in the Vicinity of Cement Factory in the Volta Region, Ghana. International Journal of Science and Technology, 2, 40-50.
NOTES

*Corresponding author.



