International Journal of Clinical Medicine
Vol.4 No.7(2013), Article ID:33913,6 pages DOI:10.4236/ijcm.2013.47055
Comparison of the Outcome of Treatment of Chronic Osteomyelitis by Surgical Debridement with and without Local Antibiotic Delivery System: Experience from a Nigerian Teaching Hospital
![]()
1Department of Orthopaedics and Traumatology, University of Calabar Teaching Hospital, Calabar, Nigeria; 2Department of Community Medicine, University of Calabar Teaching Hospital, Calabar, Nigeria; 3Department of Anaesthesiology, University of Calabar Teaching Hospital, Calabar, Nigeria.
Email: *iaikpeme@yahoo.com
Copyright © 2013 Ikpeme A. Ikpeme et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received April 25th, 2013; revised May 20th, 2013; accepted May 30th, 2013
Keywords: Chronic osteomyelitis; debridement/sequestrectomy; local antibiotic systems; outcomes
ABSTRACT
Background: Chronic osteomyelitis is still common in the developing world and presents a continuing therapeutic challenge. Antibiotics cannot penetrate the dense fibrotic scar tissue that surrounds infected and avascular bone which perpetuates the infection. Surgical debridement/sequestrectomy is the cornerstone to treatment and aims to create a viable, vascularized base which promotes healing. Surgical debridement necessarily creates a dead space which must be dealt with to prevent re-infection. Local antibiotic delivery systems serve the dual purpose of obliterating dead space and creating a sterile local environment with high bactericidal concentrations. Aim: To determine the outcomes in patients with chronic osteomyelitis who received debridement/sequestrectomy alone, and those who received the procedure combined with a local antibiotic delivery system in the University of Calabar Teaching Hospital. Patients and Methods: A prospective descriptive analysis of patients managed surgically for chronic osteomyelitis from July 2007 to December, 2012. Patients’ biodata, aetiology, organisms, treatment options and outcomes were analysed. Results: Forty-four patients presented with the condition and accepted surgery. Male:Female ratio was 2.1:1, and mean age was 27.27 ± 17.48 years. The tibia was the most commonly affected bone (45.5%), Staphylococcus aureus was the commonest organism (56.8% of sinus cultures; 73% of marrow/sequestral cultures) and previous acute haematogenous osteomyelitis was the commonest mechanism. The use of a local antibiotic delivery system improved cure rates from 57.7% to 77.8%). Conclusion: Multiple surgical interventions increase the socioeconomic costs of treating this condition and have a direct impact on the economies of individuals especially in the developing world. Surgical interventions should aim at achieving maximum impact with minimum repetition of the processes. Adequate surgical debridement combined with a local antibiotic delivery system offer improved chances of obtaining cure in this therapeutically challenging disease.
1. Introduction
Chronic osteomyelitis is often characterized by variable periods of quiescence followed by flare phenomena. This may continue throughout the life of the individual with varying consequences ranging from “the minor nuisance of a persistent discharging sinus to pathologic fracture of the infected bone”, and in a very small percentage of individuals malignant transformation at the site of pathology [1,2]. The disease continues to present a therapeutic challenge in Orthopaedics [1], and different treatment modalities have been described [3-7]. The principles of treatment of chronic osteomyelitis consist of eradicating diseased (infected) bone and avascular soft tissue followed by obliteration of dead space, restoration of blood supply, stabilization, adequate soft tissue coverage and reconstruction as maybe necessary [8]. Eradication of diseased bone may involve resection of bone segments. The goals of treatment include local control of infection and provision of good quality vascularized soft tissue near the affected bone [3,8].
Debridement and sequestrectomy with soft tissue cover used alone or in combination with local antibiotic delivery systems is the mainstay of surgical intervention with the aim of clearing infected foci and dealing with the resultant dead space [2,3]. Systemic antibiotics are used as adjunctive therapy [2,9,10]. Immediate soft tissue closure after sequestrectomy used as a lone treatment option is probably the most commonly used procedure [3,7]. Although high success rates have been reported [3], it may not be possible to avoid pocketing pus with the risk of recurrence of infection [7]. Antibiotic therapy alone or combined with repeated incision and drainage of the involved bone and sequestrum has yielded poor results in some reports [1,3]. Ineffective antibiotic concentrations at the site of infection are as a result of ischaemia within the sequestrum and surrounding infected area. Aggressive surgical management combined with antibiotics, especially in methods that ensure a high local concentration at the affected site have been advocated [1,3]. Aggressive surgery aims to remove all infected material and scar tissue and restore a viable, vascularized base. This often results in the creation of a dead space which may become reinfected.
Besides the use of debridement and sequestrectomy as a sole management option, the procedure may be combined with other procedures like muscle flap interposition, bonegrafting, primary or secondary skingrafting, antibiotic impregnated beads or the Lautenbach technique [1,9,10]. The aims of these additional procedures include the management of dead space and sterilization of the cavity until healthy, clean granulation tissue create a well vascularized base. Each technique has it’s drawbacks. Polymethylmethacrylate is non-biodegradable and requires a subsequent surgical procedure to remove the beads. This is to prevent the beads serving as nidus for reinfection. Biodegradable beads using calcium sulfate or hydroxyapatite obviate the need for a subsequent procedure. The Lautenbach technique and the use of mechanical pumps do not immediately obliterate the dead space that follows aggressive debridement [11,12]. Pump failure may also lead to leakage and reinfection with hydrophilic organisms [13].
Despite literature in support of multi-modality management for chronic osteomyelitis involving local antibiotic delivery system [14-16], the disease is often managed by debridement and sequestrectomy combined with soft tissue cover and without a local antibiotic delivery system in our setting. There is support for this modality of treatment in the literature [3,17-19]. However, there is experimental evidence that the results obtained by offering patients debridement and sequestrectomy alone are inferior to the use of a local antibiotic delivery system in this condition [20]. In a society where the decision to accept surgical intervention is often difficult, it is important that the surgical options offered patients who accept intervention be selected and targeted to achieve maximum therapeutic impact in a condition that is often difficult to cure. This study will document the outcomes in patients with osteomyelitis who were offered surgical debridement/soft tissue cover alone and those in whom debridement and sequestrectomy was augmented with a local antibiotic delivery system in our hospital.
2. Aim
To determine the outcomes of using debridement and sequestrectomy alone and debridement/sequestrectomy augmented with a local antibiotic delivery system in the management of chronic osteomyelitis.
3. Patients and Methods
All patients who presented at the University of Calabar Teaching Hospital, Calabar with chronic osteomyelitis and were treated by surgery between July 2007 and December 2012 were prospectively entered into the study. Consent for sequestrectomy/debridement used alone or used in combination with a local antibiotic delivery system was obtained from the patients before treatment was instituted. All patients received standard post-operative antibiotics which were culture based for at least 6 weeks.
Information obtained included patients biodata, duration of symptoms, aetiology, organism(s) cultured in the sinus tract and sequestrum/marrow currettings as well as the affected bones. The treatment modality and outcome of treatment were also documented. We defined treatment failure as the recurrence of a discharging sinus within one year of surgery. Statistical analysis was performed using IBM SPSS Statistic Version 20 (North Castle, New York, USA). Data was summarized using frequencies, percentages, means and standard deviations. Results were presented in frequency tables. Tests of association were carried out using the Likelihood ration (X2) and Fisher’s exact test. The p-value was set at 0.05.
4. Results
There were 44 patients with chronic osteomyelitis who underwent surgery during the period under review. There were 30 males (68.2%) and 14 females (31.8%) giving a Male:Female ratio of 2.1:1. The mean age of patients was 27.27 ± 17.48 years and the mean duration of symptoms was 29.76 ± 30.06 months. Previous acute haematogenous osteomyelitis was the commonest aetiopathologic mechanism accounting for 18 (40.9%) of cases followed by previous open fracture with 16 cases (36.4%) and previous open reduction and internal fixation (10 cases; 22.7%), (Table 1).
Table 2 shows that the tibia was the commonest location accounting for a total of 20 cases (45.5%), followed by the femur in 13 patients (29.4%). There were 4 patients with humeral infections (9%). Staphylococcus aureus was the commonest causative organism occurring as a monomicrobial culture in 21 sinus tract cultures (56.8%) and 19 sequestral or marrow curetting cultures (73%). A polymicrobial flora was cultured in 14 subjects (37.8%) from the sinus tract. In these 14 patients with a mixed sinus tract microbiological culture, Staphylococcus aureus occurred in 7 cases (50%); (Table 3). In 28 individuals, Staphylococcus aureus was cultured in the sinus tract, and in 16 (57%) of these, the marrow/sequestral
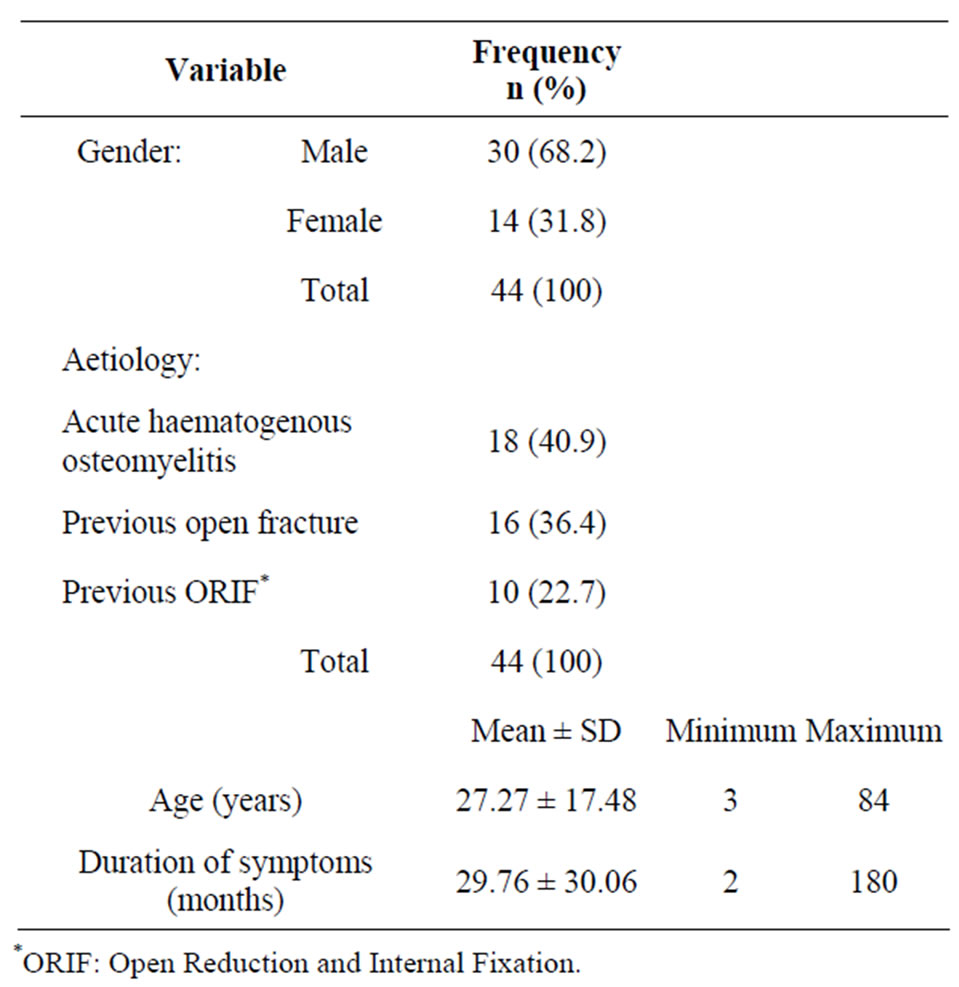
Table 1. Clinical and aetiologic variables.

Table 2. Anatomic location of chronic osteomyelitis.
cultures were positive for Staphylococcus aureus.
Table 4 shows that debridement and sequestrectomy was used as the sole treatment modality in 26 patients (59.1%) while the procedure was combined with a local antibiotic delivery system in 18 patients (40.9%). Treatment outcomes showed that overall, 29 patients (65.9%) were cured while recurrence occurred in 15 (34.1%). Among those who received debridement and sequestrectomy alone, a cure was achieved in 15 patients (57.7%) while recurrence occurred in 11 patients (42.3%). In those patients in whom debridement and sequestrectomy was combined with a local antibiotic delivery system, cure was achieved in 14 patients (77.8%) while recurrence occurred in 4 patients (22.2%).
5. Discussion
Despite advances in surgical techniques and antibiotic therapy, chronic osteomyelitis continues to challenge the orthopaedic surgeon and different treatment approaches have been reported with varying results [10,21,22]. The aim of treatment is the creation of a viable, vascularized base which promotes nutrition, oxygenation and healing. Surgical intervention is often indicated in chronic osteomyelitis inorder to clear the sequestrum, excessive fibrous tissue formation and biofilm which reduce cortical blood supply and impair antibiotic penetration into areas of necrotic bone [1]. Systemic antibiotic therapy as sole treatment option is often unsafe and ineffective in chronic osteomyelitis because the concentration of antibiotics required to penetrate the biofilm and kill the enclosed bacteria is 10 - 100 times the standard bactericidal concentration [15], with the increased potential for systemic toxicity to the patient. Systemic antibiotics are therefore used as adjuncts to surgical debridement in this condition [2].
The mean age of patients in this series was 27.27 ±

Table 3. Comparison of sinus tract and sequestral/marrow cultures.
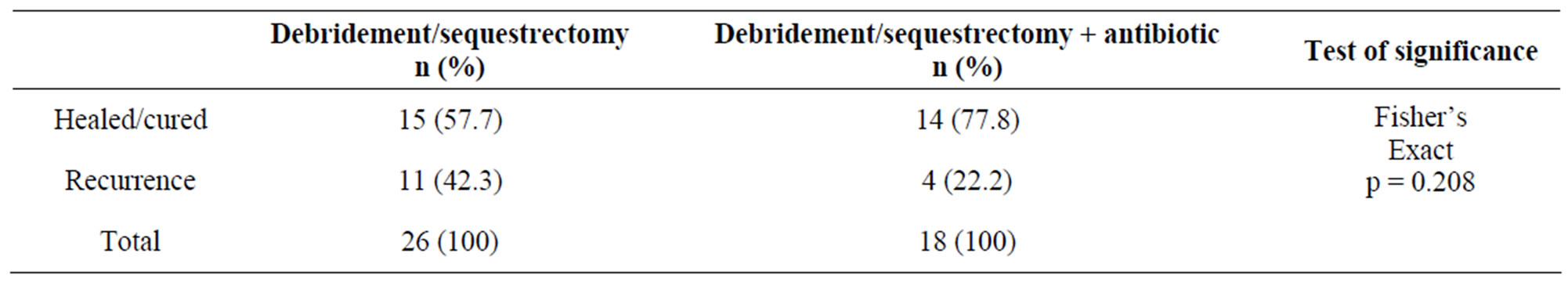
Table 4. Treatment options and outcomes.
17.48 years, comparing well with another Nigerian study with a mean of 21.9 years but lower than the age in studies from East Africa and Nepal [23-25]. The challenge posed by supportive diagnostic services in the developing world has been highlighted in this study. There were 37 sinus tract cultures (84%) but only 26 sequestral/marrow curretting cultures (59%) in our study. Sequestral/marrow curretting cultures are more significant in determining adjunctive antibiotic therapy in chronic osteomyelitis [26,27]. Swab cultures were done in only half of the patients in an East African study [24].
Staphylococcus aureus is often identified as the dominant organism causing chronic osteomyelitis [27-29], while the tibia is the most commonly involved bone [23, 24,28]. The tibia was the involved bone in twenty patients (45.5%) in this series. The subcutaneous location of the tibia makes it prone to open fractures which was second only to previous acute haematogenous osteomyelitis as the most frequent aetiopathogenetic mechanism in this series, accounting for 36.4% of cases. Our results agree with other published data which document previous acute haematogenous osteomyelitis (40.9% in this study) and previous trauma as the commonest causes of chronic osteomyelitis [23-25].
“Local antibiotics have been used to prevent or treat skeletal infection since the 1970s with significant results” [15]. A local antibiotic delivery system ensures a constantly high antibiotic concentration in the local haematoma/seroma than with systemic antibiotic therapy, and without the risk of serious systemic absorption and the associated complications [15,30,31]. The outcomes following a local antibiotic delivery system in experimental studies are superior to sequestrectomy and debridement alone and has been supported by our results in this clinical investigation. In this study, a cure was achieved in a total of 29 patients (65.9%). When sequestrectomy/debridement was used as a mono-treatment option, 15 out of 26 patients (57.7%) obtained a cure. When surgical debridement and sequestrectomy was combined with a local antibiotic delivery system, the cure rate increased to 77.8% (14 out of 18 patients). When both treatment options are compared, there was a 35% improvement in the cure rates when a local antibiotic delivery system was used in conjunction with debridement and sequestrectomy in this series. Our results differ from another Nigerian study in which 103 out of 107 (96.3%) patients were “adjudged cured” using saucerization, sequestrectomy and curettage alone [23].
Chronic osteomyelitis is a significant cause of morbidity in orthopaedics [23,29-32]. The presence of infected bone devoid of blood supply (sequestrum) ensures that cure of the condition with “antibiotic therapy alone is rarely, if ever, possible” [32]. The cornerstone for treatment is adequate surgical debridement with removal of all infected bone. This procedure necessarily creates a potential dead space which must be dealt with appropriately to reduce the chances of re-infection. Local antibiotic delivery systems do not only provide the opportunity to deal with the dead space, they offer the chance for sterilization of the local environment and therefore promote healing. Antibiotic impregnated polymethylmethacrylate (PMMA) and the low pressure antibiotic irrigation and clearance system (Lautenbach technique) are two commonly used local antibiotic delivery systems [3, 9,12,13]. The use of non-biodegradable PMMA bone cement raises two issues—the need for a second surgery to remove the beads and the risk of retained beads which can then serve as a nidus for re-infection. The Lautenbach procedure presents the challenges of wound leakage and re-colonization by hospital-acquired hydrophilic organisms.
However, the development and use of biodegradable antibiotic impregnated systems obviate the need for a second surgery while retaining the advantages of local antibiotic delivery [11,15]. In low income economies with poverty and low healthcare investments, treatment options for difficult conditions must aim at achieving maximum impact using minimum repetition in the intervention processes. The overall socioeconomic cost following recurrence would make a local antibiotic delivery system a viable option in all patients undergoing surgical therapy for chronic osteomyelitis. Our study shows that almost 78% of patients were cured when a local antibiotic delivery system was used while only 58% of patients obtained cure without a local antibiotic delivery system.
This study evaluated outcomes in this disease with and without the use of a local antibiotic delivery system. We used non-degradable polymethylmetacrylate (PPMA) impregnated antibiotics in 8 individuals and the antibiotic irrigation and drainage method in 10 individuals. Among the 4 recurrences in the local antibiotic group, 1 occurred in the PMMA group while 3 occurred in the antibiotic irrigation and drainage group. Although antibiotic impregnated bone cement potentially appears to provide superior results in our study, other literature document near equal outcomes [13].
7. Conclusion
Chronic osteomyelitis remains a major challenge in Orthopaedics with the risk of significant morbidity. The expensive treatment of this condition especially in the setting of multiple surgical interventions adds to the socioeconomic challenges of individuals in low income countries where the disease is common. Adequate surgical debridement, combined with a local antibiotic delivery system offer improved chances of obtaining cure in this otherwise therapeutically challenging disease, and should be seriously considered by clinicians working in these settings. The availability of bio-degradable antibiotic vehicles combined with adequate surgical debridement hold a huge promise for consistently improved outcomes for the treatment of this condition in future.
REFERENCES
- P. Wirganowicz, “Aggressive Surgical Management of Chronic Osteomyelitis,” The University of Pennsylvania Orthopaedic Journal, Vol. 12, 1999, pp. 7-12. http://upoj.org/site/files/v12/v12_05.pdf
- I. A. Ikpeme, N. E. Ngim and A. A. Ikpeme, “Diagnosis and Treatment of Pyogenic Bone Infections,” African Health Sciences, Vol. 10, No. 1, 2010, pp. 82-88.
- G. Cierny III and D. DiPasquale, “Treatment of Chronic Infection,” Journal of the American Academy of Orthopaedic Surgeons, Vol. 14, No. 10, 2006, pp. S105-S110.
- J. P. Anthony, S. J. Mathes and B. S. Alpert, “The Muscle Flap in the Treatment of Chronic Lower Extremity Osteomyelitis: Results in Patients over 5 Years after Treatment,” Plastic and Reconstructive Surgery, Vol. 88, No. 2, 1991, pp. 311-318. doi:10.1097/00006534-199108000-00023
- R. H. Fitzerald Jr., P. E. Ruttle, P. G. Arnold, J. P. Kelly and G. B. Irons, “Local Muscle Flaps in the Treatment of Chronic Osteomyelitis,” The Journal of Bone & Joint Surgery (American), Vol. 67, No. 2, 1986, pp. 175-185.
- A. Ateschrang, B. G. Ochs, M. Lüdemann, K. Weise and D. Albrecht, “Fibula and Tibia Fusion with Cancellous Allograft Vitalized with Autologous Bone Marrow: First Results for Infected Tibial Non-Union,” Archives of Orthopaedic and Trauma Surgery, Vol. 129, No. 1, 2009, pp. 97-104. doi:10.1007/s00402-008-0699-2
- E. M. Evans and D. M. Davies, “The Treatment of Chronic Osteomyelitis by Saucerization and Secondary Skin Grafting,” The Journal of Bone & Joint Surgery (British), Vol. 51B, No. 3, 1969, pp. 454-457.
- L. Lazzarini, J. T. Mader and J. H. Calhoun, “Osteomyelitis in Long Bones,” Journal of Bone and Joint Surgery (American), Vol. 86, No. 10, 2004, pp. 2305-2318.
- L. Solomon, H. Srinivasan, S. Tuli and S. Govender, “Infection,” In: L. Solomon, D. Warwick and S. Nayagam, Eds., Apley’s System of Orthopaedics and Fractures, Hodder Arnold, London, 2010, pp. 29-59.
- L. O. Conterno and C. R. da Silva Filho, “Antibiotics for Treating Chronic Osteomyelitis in Adults,” Cochrane Database of Systemic Reviews, Vol. 8, No. 3, 2009, Article ID: CD004439.
- S. Gitelis and G. T. Brebach, “The Treatment of Chronic Osteomyelitis with a Biodegradable Antibiotic-Impregnated Implant,” Journal of Orthopaedic Surgery (Hong Kong), Vol. 10, No. 1, 2002, pp. 53-60.
- M. A. Hashmi, P. Norman and M. Saleh, “The Management of Chronic Osteomyelitis Using the Lautenbach Method,” Journal of Bone and Joint Surgery (British), Vol. 86, No. 2, 2004, pp. 269-275. doi:10.1302/0301-620X.86B2.14011
- M. J. Patzakis, “Management of Acute and Chronic Osteomyelitis,” In: M. W. Chapman, Ed., Chapman’s Orthopaedic Surgery, Lippincott Williams & Wilkins, 2001, pp. 3534-3559.
- B. Parsons and E. Straus, “Surgical Management of Chronic Osteomyelitis,” The American Journal of Surgery, Vol. 188, No. 1, 2004, pp. 57-66. doi:10.1016/S0002-9610(03)00292-7
- J. S. Gogia, J. P. Mechan, P. E. Di Cesare and A. A. Jamali, “Local Antibiotic Therapy in Osteomyelitis,” Seminars in Plastic Surgery, Vol. 23, No. 2, 2009, pp. 100- 107. doi:10.1055/s-0029-1214162
- M. S. Moon and J. L. Moon, “Management of Osteomyelitis Editorial,” Journal of Orthopaedic Surgery, Vol. 8, No. 2, 2000, pp. 8-10.
- R. K. Beals and R. E. Bryant, “The Treatment of Chronic Open Osteomyelitis of the Tibia in Adults,” Clinical Orthopaedics and Related Research, Vol. 433, 2005, pp. 212-217.
- M. A. McNally, J. O. Small, H. G. Tofighi and R. A. B. Mollan, “Two Stage Management of Chronic Osteomyelitis of the Long Bones. The Belfast Technique,” Journal of Bone and Joint Surgery (British), Vol. 75-B, No. 3, 1993, pp. 375-380.
- S. G. Kim and H. S. Jang, “Treatment of Chronic Osteomyelitis in Korea,” Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology and Endodontology, Vol. 92, No. 4, 2001, pp. 394-398. doi:10.1067/moe.2001.117810
- M. Kaya, G. Simsek-Kaya. N. Gürsan. E. Kirei, E. Dayi and B. Gündoğdu, “Local Treatment of Chronic Osteomyelitis with Surgical Debridement and Tigecycline— Impregnated Calcium Hydroxyapatite: An Experimental Study,” Oral Surgery, Oral Medicine, Oral Pathology and Oral Radiology, Vol. 113, No. 3, 2012, pp. 340-347. doi:10.1016/j.tripleo.2011.03.032
- V. Lok, E. Bal, A. Sebik and H. Aydinok, “Long-Term Results of Treatment Including Creation of a Gutter and Muscle Transposition for Chronic Sclerosing Osteomyelitis,” Acta Orthopaedica et Traumatologica, Vol. 43, No. 6, 2009, pp. 510-514. doi:10.3944/AOTT.2009.510
- I. M. Smith, O. M. Austine and A. G. Batchelor, “The Treatment of Chronic Osteomyelitis: A 10 Year Audit,” Journal of Plastic, Reconstructive & Aesthetic Surgery, Vol. 59, No. 1, 2006, pp. 1-15.
- S. B. Agaja and R. O. Ayorinde, “Chronic Osteomyelitis in Ilorin, Nigeria,” South African Journal of Surgery, Vol. 46, No. 4, 2008, pp. 116-118.
- W. L. Biruk and K. Wubshet, “Chronic Osteomyelitis at Tikur Anbessa Hospital, Addis Ababa University, Ethiopia,” East and Central African Journal of Surgery, Vol. 12, No. 1, 2007, pp. 33-41.
- B. K. Shrestha, T. Rajbhandary, B. Bijukachhe and A. K. Banskota, “Surgical Interventions in Chronic Osteomyelitis,” Kathmandu University Medical Journal, Vol. 3, No. 1, 2005, pp. 50-54.
- A. L. Akinyoola, O. O. Adegbehingbe and A. O. Aboderin, “Therapeutic Decision in Chronic Osteomyelitis: Sinus Track Culture versus Intraoperative Bone Culture,” Archives of Orthopaedic and Trauma Surgery, Vol. 129, No. 4, 2009, pp. 449-453. doi:10.1007/s00402-008-0621-y
- T. O. Alonge, S. O. Ogunlade and A. N. Fashina, “Microbial Isolates in Chronic Osteomyelitis—A Guide to Management,” African Journal of Medicine and Medical Sciences, Vol. 31, No. 2, 2002, pp. 167-169.
- A. A. Salman, E. Hussein, A. Yonis and R. A. Radwani, “Epidemiological and Bacteriological Study of Chronic Osteomyelitis,” Tikrit Medical Journal, Vol. 14, No. 1, 2008, pp. 59-62.
- M. A. Haitham and A. A. Mahmood, “Bacterial Etiology of Chronic Osteomyelitis Involving Anaerobes,” Annals of the College of Medicine Mosul, Vol. 36, No. 1-2, 2010, pp. 106-113.
- H. Wahlig, E. Dingeldein, R. Bergmann and K. Reuss, “The Release of Gentamicin from Polymethylmethacrylate Beads. An Experimental and Pharmacokinetic Study,” Journal of Bone and Joint Surgery (British), Vol. 60, No. 2, 1978, pp. 270-275.
- S. F. Hoff, R. H. Fitzgerald Jr. and P. J. Kelly, “The Depot Administration of Penicillin G and Gentamicin in Acrylic Bone Cement,” Journal of Bone and Joint Surgery (American), Vol. 63, No. 5, 1981, pp. 798-804.
- B. A. Cunha, “Osteomyelitis in Elderly Patients,” Clinical Infectious Diseases, Vol. 35, No. 3, 2002, pp. 287-293. doi:10.1086/341417
NOTES
*Corresponding author.

