International Journal of Clinical Medicine
Vol.4 No.6(2013), Article ID:33139,4 pages DOI:10.4236/ijcm.2013.46050
Primary Small Bowel Tumour Presenting as Bowel Obstruction in a Patient with a Virgin Abdomen
![]()
1Department of Surgery, Pamela Youde Nethersole Eastern Hospital, Hong Kong, China; 2Faculty of Medicine, Imperial College of Science, Technology and Medicine, South Kensington Campus, London, UK.
Email: xilin.wu@nhs.net
Copyright © 2013 Xilin Wu et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received March 26th, 2013; revised May 3rd, 2013; accepted May 20th, 2013
Keywords: Small Bowel Tumour; Bowel Obstruction; Virgin Abdomen
ABSTRACT
Primary malignancies of the small bowel are rare and usually present with vague, non-specific symptoms. This leads to diagnostic difficulties for both physician and radiologists alike. We present a case of a 54-year-old lady with a virgin abdomen who initially presented to hospital with vague abdominal symptoms suggestive of gastroenteritis. She responded partially to conservative treatment but re-presented to hospital 3 weeks later with obstructive symptoms. Contrast CT was inconclusive with regards to diagnosis. A diagnostic laparoscopy was performed, revealing an infiltrative growth. Consequently, she underwent segmental laparoscopic-assisted small bowel resection and recovered well postoperatively. After further histological and endoscopic investigations, a final diagnosis of primary small bowel adenocarcinoma was given. As the prognosis of small bowel malignancy is stage-dependent, this case demonstrates a high index of suspicion is necessary to reach early diagnosis, especially for symptoms non-responsive to conventional treatment. Diagnostic laparoscopy should be considered early as a definitive diagnostic tool.
1. Introduction
Cancer of the small bowel is a rare malignancy with an incidence of 1.9 per 100,000 persons per year. This is significantly lower than the incidence of colorectal cancers at 47.9 per 100,000 persons per year [1]. Whilst there are vast amounts of literature on colorectal cancers, few studies exist looking into small bowel malignancies. The lack of awareness, together with its non-specific presentation, cumulates in relative difficulties in diagnosing small bowel tumours. We report a case of a patient suffering from small bowel obstruction who, after a string of investigations, was finally given this elusive diagnosis.
2. Case Presentation
A 54-year-old lady was referred to our hospital with unresolved small bowel obstruction. Three weeks before, she initially presented to a private hospital with epigastric pain and persistent vomiting. Aside from peptic ulcer disease, she has no other significant past medical history and has never had abdominal surgery. She denied any other gastrointestinal symptoms such as recent change in bowel habit or weight loss. She was managed conservatively with an initial diagnosis of gastroenteritis, with little progress. Abdominal computed tomography (CT) showed non-specific small bowel thickening in the left lower abdomen. A small bowel enema was also performed, revealing possibly some skip lesions in the rest of small bowel. Unfortunately at this point the patient self-discharged against medical advice, prior to a full assessment of these lesions.
The patient returned to the private hospital within 3 weeks, now with obvious symptoms of small bowel obstruction. In particular she had developed abdominal distension, although there was no tenderness on palpation. She also reported lack of bowel movement for the past few days. An abdominal X-ray showed dilated loops of small bowel. Subsequently a nasogastric tube was inserted, draining 2 litres of bile-stained fluid in the first day. She was kept nil by mouth, fluid resuscitated and started on total parenteral nutrition. Repeated abdominal contrast CT was performed, revealing concentric mural thickening at the proximal ileum. This was associated with minor streakiness over the mesenteric fat and prominent lymph nodes. The differential diagnoses at this juncture were abdominal tuberculosis, inflammatory bowel disease and lymphoma of the small intestine.
As her symptoms were not resolving, she was referred to our hospital for further management. Diagnostic laparoscopy was decided in this patient with proximal small bowel obstruction, which revealed dilated small bowel loops down to the proximal ileum with a circumferential growth over a short segment of the ileum, while the small bowel distal to the lesion was collapsed. Laparoscopic-assisted segmental small bowel resection with primary anastomosis was performed (Figures 1 and 2). The port site for the laparoscope was extended slightly for the retrieval of the dilated segment of small bowel, which was then decompressed and resected. The primary anastomosis was performed extra-corporeally. This saved the patient from having a large midline laparotomy wound with better postoperative outcomes in terms of less pain, fewer wound complications, faster recovery, shorter hospital stay, as well as better cosmesis. Furthermore, the laparoscopic approach is associated with producing less post-operative intra-abdominal adhesions compared to open surgery. The patient made an uneventful recovery post-operatively.

Figure 1. Luminal view of the resected bowel showing the stenotic infiltrative growth across the entire wall of small intestines.

Figure 2. Resected section of small bowel showing stricture at the site of infiltrative growth.
Subsequent histological examination showed the stricture segment to be infiltrated by adenocarcinoma (Figure 3), invading to the subserosal soft tissue. Since primary small bowel adenocarcinoma is uncommon, further immunostaining was done for characterisation of the tumour (Figure 4). This suggested it was gastrointestinal in origin. Finally, endoscopy and colonoscopy were done 3 weeks post-discharge. As no lesions were identified, a diagnosis of primary adenocarcinoma of the small bowel (pT3N0) was made.
3. Discussion
Primary small bowel malignancies are rare, accounting for only 2% of all gastrointestinal malignancies [2]. Previously, adenocarcinoma was considered the most common histological subtype of primary small bowel malignancy. However a recent study suggests it is now being surpassed by carcinoid tumours [3].
Due to its rare nature, diagnosis of primary small bowel malignancies has proved challenging. On average there is a 6 - 8 month delay in diagnosis, attributed partly to the inaccessibility of the small bowel to endoscopic investigations [4]. Maglinte et al. also reported failure by clinicians to organise appropriate investigations and failure by radiologists to make appropriate diagnosis as contributing factors to the delay [5].
The first hurdle in diagnosis arises from the initial presentation. This is often vague and non-specific. Symptoms include abdominal pain, distension, nausea, vomiting, constipation, weight loss and gastrointestinal bleeding. Our patient presented with non-specific symptoms of insidious onset. Only when she failed to respond to treatment were further investigations carried out. Whilst the CT scan alerted us to a thickening in the bowel wall, it was unable to determine the exact nature of the lesion. In fact studies have shown CT to yield poor

Figure 3. Histological section of small bowel infiltrated with adenocarcinoma. Tissue section stained with haematoxylin and eosin; magnification ×100.
 (a)
(a) (b)
(b)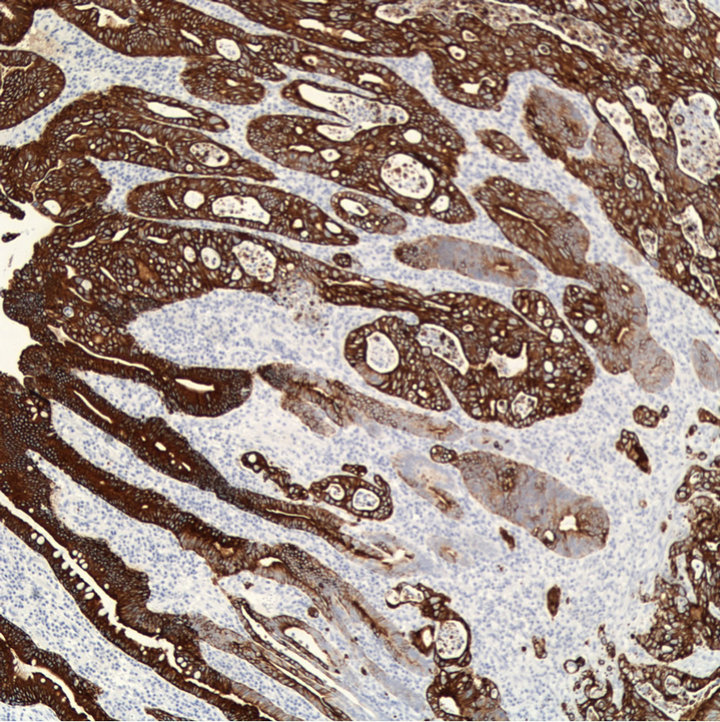 (c)
(c)
Figure 4. Immunostaining with antibodies against cytokeratin 7 (a), 19 (b), and 20 (c), indicating small bowel adencarcinoma; magnification ×100.
sensitivity for detecting small bowel pathologies. One study noted only 57% accuracy in detecting small bowel malignancies [6]. In another study on 217 patients, CT scans were used to establish diagnosis in only 14% of patients. Laparotomy was the most common method, accounting for 30% of diagnosis [7]. In this particular case since the lesion is over the proximal ileum (as shown in the pre-operative CT scan), in a virgin abdomen with minimal abdominal distension, we decided to use the minimally invasive approach of laparoscopy. The open method was used in the creation of port site for insertion of the laparoscope in order to avoid bowel injury. Since laparoscopic operative exposure and manipulation can be difficult in the presence of distended fragile bowel loops, gentle manipulation of dilated bowel with atraumatic forceps by experienced surgeons is important to avoid bowel injury. Of course, newer innovations of double balloon and video endoscopy show promise for improving diagnosis, especially when lesions cannot be identified radiologically [8]. However the need for bowel preparation and the risk of capsule retention mean they were inappropriate for our patient, given her obstructive symptoms.
Our patient’s histology results showed infiltration by adenocarcinoma cells. However it was important not to jump to the conclusion of primary small bowel adenocarcinoma. Metastases account for over 50% of all small bowel neoplasms [9], with the most common primary coming from the colon, stomach, pancreas, skin (melaenoma), breast and lungs. For our patient, immunostaining of the lesion helped direct further investigations for the primary site. Finally, the diagnosis of primary adenocarcinoma of the small bowel was only made after malignancies elsewhere in the gastrointestinal tract were ruled out with endoscopy and colonoscopy.
In conclusion, our case highlights the insidious, nonspecific nature in which primary small bowel adenocarcinomas present. This, together with its low incidence, makes diagnosis especially challenging. The two most important prognostic indicators of small bowel adenocarcinoma are resectability and staging of the cancer at diagnosis [10]. Therefore a high index of suspicion and a low threshold for investigation, especially for symptoms non-responsive to conventional treatment, are necessary to minimise delay in diagnosis and to ensure best patient outcomes. As a CT scan might not be conclusive, diagnostic laparoscopy should be considered early as a definitive diagnostic tool.
REFERENCES
- S. F. Altekruse, C. L. Kosary, M. Krapcho, N. Neyman, R. Aminou, W. Waldron, J. Ruhl, N. Howlader, Z. Tatalovich, H. Cho, A. Mariotto, M. P. Eisner, D. R. Lewis, K. Cronin, H. S. Chen, E. J. Feuer, D. G. Stinchcomb and B. K. Edwards, Eds., “SEER Cancer Statistics Review, 1975- 2007,” National Cancer Institute, Bethesda, 2010. http://seer.cancer.gov/csr/1975_2007/
- J. R. Howe, L. H. Karnell, H. R. Menck and C. ScottConner, “Adenocarcinoma of the Small Bowel Review of the National Cancer Data Base, 1985-1995,” Cancer, Vol. 86, No. 12, 1999, pp. 2693-2706. doi:10.1002/(SICI)1097-0142(19991215)86:12<2693::AID-CNCR14>3.0.CO;2-U
- K. Y. Bilimoria, D. J. Bentrem, J. D. Wayne, C. Y. Ko, C. L. Bennett and M. S. Talamonti, “Small Bowel Cancer in the United States: Changes in Epidemiology, Treatment, and Survival Over the Last 20 Years,” Annals of Surgery, Vol. 249, No. 1, 2009, pp. 63-71. doi:10.1097/SLA.0b013e31818e4641
- R. M. Zollinger Jr., “Primary Neoplasms of the Small Intestine,” American Journal of Surgery, Vol. 151, No. 6, 1986, pp. 654-658. doi:10.1016/0002-9610(86)90035-8
- D. D. T. Maglinte, K. O’Connor, J. Bessette, S. M. Chernish and F. M. Kelvin, “The Role of the Physician in the Late Diagnosis of Primary Malignant Tumors of the Small Intestine,” American Journal of Gastroenterology, Vol. 86, No. 3, 1991, pp. 304-306.
- A. J. Minardi Jr., G. B. Zibari, D. F. Aultman, R. W. McMillan and J. C. McDonald, “Small-Bowel Tumors,” Journal of the American College of Surgeons, Vol. 186, No. 6, 1998, pp. 664-668. doi:10.1016/S1072-7515(98)00092-1
- B. S. Dabaja, D. Suki, B. Pro, M. Bonnen and J. Ajani, “Adenocarcinoma of the Small Bowel: Presentation, Prognostic Factors and Outcome of 217 Patients,” Cancer, Vol. 101, No. 3, 2004, pp. 518-526. doi:10.1002/cncr.20404
- N. M. Lee and G. M. Eisen, “10 Years of Capsule Endoscopy: An Update,” Expert Review of Gastroenterology and Hepatology, Vol. 4, No. 4, 2010, pp. 503-512. doi:10.1586/egh.10.44
- R. M. Gore, U. K. Mehta, J. W. Berlin, V. Rao and G. M. Newmark, “Diagnosis and Staging of Small Bowel Tumours,” Cancer Imaging, Vol. 6, No. 1, 2006, pp. 209- 212. doi:10.1102/1470-7330.2006.0031
- R. R. Hutchins, A. Bani Hani, P. Kojodjojo, R. Ho and S. J. Snooks, “Adenocarcinoma of the Small Bowel,” ANZ Journal of Surgery, Vol. 71, No. 7, 2001, pp. 428-437. doi:10.1046/j.1440-1622.2001.02149.x

