Open Journal of Nephrology
Vol.05 No.04(2015), Article ID:61666,12 pages
10.4236/ojneph.2015.54016
Evaluation of Angiopoietin-2 Serum Level as a Marker of Cardiovascular Risk in Children with Chronic Kidney Disease
Manal Abdel-Salam1*, Asmaa Abd EL Wakeel1, Soheir Ibrahim1, Tagreed Abdel-Rahman2, Hend Ezzat 3, Randa Sabour4
1Department of Pediatrics, Faculty of Medicine, Al-Azhar University, Cairo, Egypt
2Department of Cardiology, Faculty of Medicine, Al-Azhar University, Cairo, Egypt
3Department of Clinical Pathology, Faculty of Medicine, Al-Azhar University, Cairo, Egypt
4Department of Radiology, Faculty of Medicine, Al-Azhar University, Cairo, Egypt

Copyright © 2015 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).


Received 6 August 2015; accepted 30 November 2015; published 3 December 2015

ABSTRACT
Background: Cardiovascular complications are a major clinical problem in uremic patients accounting for 44% of all deaths in this population. Angiopoietin cytokines are involved with controlling micro vascular permeability, vasodilatation and vasoconstriction by signaling smooth muscle cells surrounding vessels. Aim: To assess Angiopoietin-2 serum level as an early marker of cardiovascular risks in children with chronic kidney disease on regular hemodialysis and correlate with intimal medial thickness and echo data in those children. Patients and methods: The study included 40 children with CKD on regular hemodialysis (HD), and they were selected from the hemodialysis unit of Al-Zahraa Hospital, Al-Azhar University, during the period from December 2014 to April 2015. Another group of 40 apparently healthy children, matches age and sex with patients group as a controls. Angiopoietin-2 serum level, Doppler ultrasound (U/S) to assess: intima-media thickness (IMT) and the peak systolic velocity (PSV) of the main arteries including the (aorta, carotid and femoral) arteries, conventional echo and tissue Doppler imaging (TDI) of mitral and tricuspid annular velocities are obtained for both groups. Results: Children on regular HD have significantly higher (Angiopoietin-2) serum level compared to their controls, and it is (161.35 ± 38.30 ng/ml) and (9.25 ± 12.64 ng/ml) respectively (p, 0.000) and increases in the aorta, carotid and femoral (IMT) with significant increase in their mean systolic velocities in patients group compared to the controls. Significant increase in tricuspid valve late diastolic velocity (TVA vel m/s) and (E/e’ ratio) obtained by (TDI), its abnormalities threshold is detected in patients group than controls, with significant increase right ventricular systolic pulmonary pressure in patients compared to the controls. Conclusions: Higher prevalence of right ventricular dysfunction is detected by conventional and TDI echo in children on hemodialysis. Angiopoietin-2 can be used as an ideal biomarker which may progress to play an adjunctive role with echocardiography in assessing cardiovascular risk of children with CKD on regular hemodialysis.
Keywords:
Chronic Kidney Disease, Angiopoietin-2, Intima-Medial Thickness

1. Introduction
Cardiovascular diseases (CVD) were known to be the most important causes of morbidity and mortality in children with chronic kidney disease (CKD), particularly in those undergoing dialysis (DI). Although conventional cardiovascular risk factors occurred less frequently in children, those related to uremia might be present (Scavarda et al., 2014) [1] .
Manifestations of CVD in childhood CKD included arterial stiffening (Covic et al., 2012) [2] , calcification (Shroff et al., 2008) [3] , premature atherosclerosis (Dursunetal, 2009) [4] and left ventricular hypertrophy (Mitsnefes et al., 2006) [5] . Over time, CKD developing in children was associated with increased cardiovascular mortality that markedly accelerated once dialysis initiated (McDonald et al., 2004; Oh et al., 2002) [6] [7] .
Among the members of the Angiopoietin (Ang) family Tie 2 receptor and its ligands, Ang-1 and Ang-2 had attracted much attention as the factors related to angiogenesis and inflammation (Davis et al., 1996; Fam et al., 2003) [8] [9] . The Ang/Tie system tightly controlled the endothelial phenotype during angiogenesis and vascular inflammation in a unique and non-redundant fashion (Brindle et al., 2006; Fiedler and Augustin, 2006) [10] [11] .
Ang-1 was usually considered as beneficial for endothelial cell function. In contrast, Ang-2 was released from Weibel-Palade bodies by various stimuli (Fiedler et al., 2004; Kuo et al., 2008) [12] [13] and acted as an antagonist of Ang-1.
Elevated circulating Ang-2 had been reported in adults with CKD. David and colleagues (David et al., 2010) [14] found an inverse relationship between circulating Ang-2 levels and glomerular filtration rate in adults with CKD. Two other studies reported that Ang-2 levels were elevated in adults on hemodialysis (HD) or peritoneal dialysis (PD) compared with healthy controls (David et al., 2009; David et al., 2012) [15] [16] . In one of these studies Ang-2 correlated with scoring for coronary and peripheral arterial disease (David et al., 2009) [15] . In the other study, Ang-2 correlated with cholesterol, high-sensitive C-reactive protein and osteoprotegerin and was an independent predictor of mortality (David et al., 2012) [16] .
We aimed to investigate Angiopoietin-2 serum level as an early marker of cardiovascular risk in children on hemodialysis and to correlate with other studied cardiovascular risk predictors.
2. Materials and Methods
This is a case control study conducted during the period from December 2014 to April 2015, it included 40 children recruited from Alzhraa University Hospital (Cairo, Egypt), these are the patients numbers who attended at the pediatric hemodialysis unit during the period of the study, they were on regular hemodialysis more than three months at the time of the study, for 4 hours/setting, 3 times weekly, with low flux polysulphone dialyzer by 4008 Fresenius machine. They were 15 female and 25 male. The most common cause of CKD in patients group was unknown 20 cases (50%) followed by posterior urethral valve 6, (15%), their ages ranged from 3 to 16 year. A group of 40 apparently healthy children, matched age and sex with patients group served as controls. Children with congenital, rheumatic heart diseases, and primary vascular disorder were excluded from the study; they were subjected to full history taking including etiology, onset of CKD, duration of hemodialysis, and laboratory investigations. Informed consent was obtained from the participating parents in adherence with the guidelines of the ethical committee of Alzhraa hospital, AL-Azhar University, Cairo, Egypt. This study was conducted with the participation of pediatric (nephrology and hemodialysis), radiology, clinical pathology and cardiology departments of internal clinic.
2.1. Sampling
・ Two ml were drawn and placed into a vacutainer tube containing EDTA for complete blood picture (CBC) using automated cell counter model Sysmex KxN21.
・ Five ml were drawn and placed into a plain tube, centrifuged within 30 minutes of collection and serum was separated and divided into:
1) One portion for BUN, serum creatinine, ca, ph, cholesterol and triglyceride at the same day;
2) Another portion stored at −20˚C for until Angiopoietin-2 time of assay.
Assessment of Angiopoietin-2: Using enzyme-linked immunosorbent assay (ELISA).
The kit assay Human ANG-II level in the sample, use purified Human ANG-II antibody to coat microtiter plate wells, make solid-phase antibody, then add ANG-II to wells, combined ANG-II antibody which with enzyme labeled, become antibody-antigen-enzyme-antibody complex, after washing completely, add substrate, substrate become blue color at HRP enzyme-catalyzed, reaction is terminated by the addition of sulphuric acid solution and the color change is measured spectrophotometrically at wavelength of 450 nm. The concentration of Ang-2 in the samples is then determined by comparing the optic density of the samples to the standard curve (Gillard et al., 2005) [17] .
2.2. ECHO Assessment
・ Trans-thoracic echocardiography (TTE) studies
Echo Doppler was performed in cardiology department, using Vivid-7 GE system (GE Ultrasound; Horten, Norway) with tissue Doppler imaging capability. Cases were examined by multi frequency (2.5 MHz) matrix probe M3S. TTE, M-Mode, 2D, Doppler (pulsed and continuous wave), color flow mapping in the standard views from all accessible windows were obtained with ECG physiosignal displayed with all detected echo- Doppler study. All measurements were made by the same investigator over at least three cardiac cycles and the average value for each parameter was calculated. To avoid the effect of volume overload; echocardiography was done within 1-2 hours after dialysis where patients’ weight is close to their target one.
・ Two dimensional echocardiography
Routine examination was done from the parasternal, apical and subcostal views focusing on: exclusion of congenital heart disease and guidance for M-Mode and color Doppler.
M-Mode echocardiography:
They included the followings:
Left ventricular end diastolic dimension (LVEDD), Left ventricular end systolic dimension (LVESD), Inter ventricular septal diameter (IVSD), Left ventricular posterior wall diameter (LVPWD), Left atrial diameter (antero-posterior diameter), Aortic root diameter, Left ventricular ejection fraction (EF %) and Left ventricular fraction of shortening (FS).
Parameters were obtained by the guidance of two dimensional (2-D) echocardiography from the parasternal long axis view, at the level of the papillary muscle and at the level of aorta and left atrium using the leading edge technique (Sahn et al., 1978) [18] . TAPSE is easily obtainable and represents a measure of RV longitudinal function. It is measured by M-mode echocardiography with the cursor optimally aligned along the direction of the tricuspid lateral annulus in the apical four-chamber view TAPSE < 17 mm is highly suggestive of RV systolic dysfunction (Giuscaetal, 2010) [19] .
Echocardiography evaluation of LV and RV diastolic functions:
Pulsed wave Doppler signals of mitral and tricuspid inflow were estimated for:
・ Peak E and A wave velocities;
・ E deceleration time;
・ Ratio of peak mitral E-wave velocity to peak mitral A-wave velocity (E/A ratio).
The TDI function was activated to obtain color velocity displayed over the apical 4 chamber and apical two chamber views where the cine loops were digitally stored for later off line analysis.
TDI systolic, early & late diastolic velocities were obtained. TDI measures include:
・ Peak systolic velocity (Sa);
・ Early diastolic velocity (Ea);
・ Late diastolic velocity (Aa).
Then, ratio of mitral Doppler flow velocity to the early mitral annular velocity (E/e’ ratio) was calculated. Same measurements were obtained from the tricuspid valve (E/e’ ratio) was calculated abnormality threshold is >6.0, an updated from the American Society of Echocardiography and the European Association of Cardiovascular Imaging (Lang et al., 2015) [20] .
To avoid influential effects on LV and RV inflow velocities, the error of beat-to-beat variability in flow dynamics, especially on the right side, due to respiration was markedly minimized by averaging multiple consecutive beats so that to reduce error and increase reproducibility.
2.3. Doppler Ultrasound (U/S) Assessment
Intima-media thickness (IMT) and peak systolic velocity (PSV) of the main arteries including the aorta, carotid and femoral arteries. Both intima-media thickness and peak systolic velocity were measured using the Doppler ultrasound apparatus in Al-Zahraa Hospital, Al-Azhar University.
2.3.1. Intima-Media Thickness (IMT)
IMT was measured by gray scale ultrasound using 7.5 MHZ probe. The IMT was measured as the distance between the leading edge of the lumen-intima interface and the media-adventitia interface on the far wall of the artery.
MHZ probe. The IMT was measured as the distance between the leading edge of the lumen-intima interface and the media-adventitia interface on the far wall of the artery.
2.3.2. Peak Systolic Velocity (PSV)
PSV was measured by gray scale ultrasound using 7.5 MHZ probe. Doppler examination was done through B- Mode, color mode and peak systolic velocity was measured. In B-Mode, the vessel appears anechoic with hyperechoic walls (Chavhan et al., 2008) [21] .
MHZ probe. Doppler examination was done through B- Mode, color mode and peak systolic velocity was measured. In B-Mode, the vessel appears anechoic with hyperechoic walls (Chavhan et al., 2008) [21] .
2.4. Statistics Statistics
Data were collected, revised, coded and entered to the Statistical Package for Social Science (IBM SPSS) version 20. Spearman correlation coefficients were used to assess the relation between two studied parameters in the same group. Receiver operating characteristic curve was used to assess the best cut off point with sensitivity and specificity.
Limitations of the Study
Some limitations of the study include its cross-sectional design, the relatively low number of participants, and their heterogeneity due to different causes of renal failure and frequent pharmacological treatment.
3. Results
Table 1 shows comparison between dialysis children and healthy controls regarding demographic data, anthropometric measurements and blood pressure, it revealed: significant decrease in weight and height in dialysis children compared to their controls and also patients group had high blood pressure as expected.
Table 2 shows comparison between dialysis children and healthy controls regarding laboratory data, it revealed: significant increase in BUN, (creatinine, cholesterol, triglyceride, ph, alkaline phosphtase, PTH and Angiopoietin-2) serum levels in patients group than healthy controls, while there is significant decrease in RBCs counts, Hb level, serum ca and Mg levels in dialysis children than healthy controls.
Table 3 shows comparison between dialysis children and healthy controls regarding echo parameters and Doppler ultrasound assessment of IMT and peak systolic velocities of the big arteries, it revealed: significant increase in inter ventricular septum in diastole (IVSd cm) in patients on hemodialysis compared to their controls and shows significant decrease in the right ventricular diastolic functions as evidenced by significant increase TV A vel m/s and decrease in TV E/A ratio in children on dialysis versus controls, while there is no significant difference regarding left ventricular functions. Also the same table revealed significant increase in the pulmonary pressure in hemodialysis children than the control group.
Table 4 shows the ratio of tricuspid Doppler flow velocity to the early tricuspid annular velocity (E/e’ ratio) among patients group, it revealed: 32 of patients had diastolic dysfunction (TV E/e ≥ 6). Angiopoetin-2 is sig-
Table 1. Comparison between patients group and the controls regarding age, sex, anthropometric measures and blood pressure.
*Data were presented as number and percentages and compared using Chi-square test.
Table 2. Comparison between patients group and the controls regarding laboratory data.
nificantly higher in cases with diastolic dysfunction.
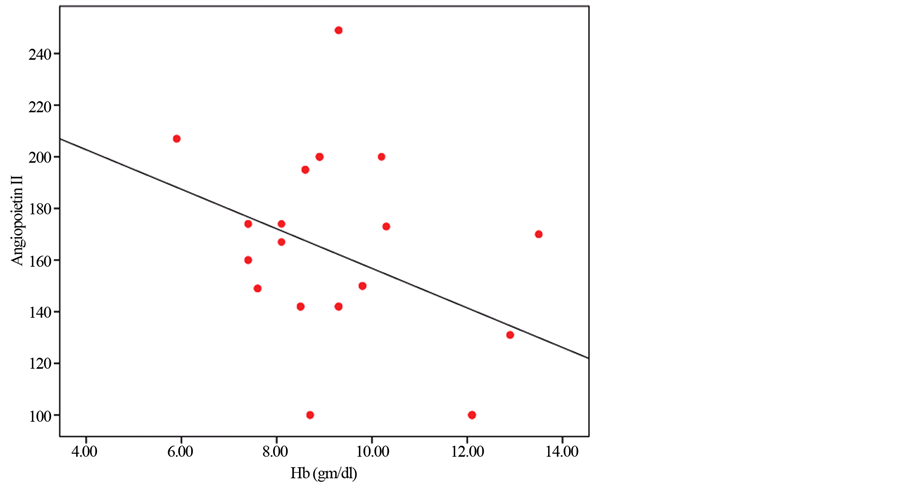
Figure 1 shows negative correlation between Angiopoietin-2 and hemoglobin level.
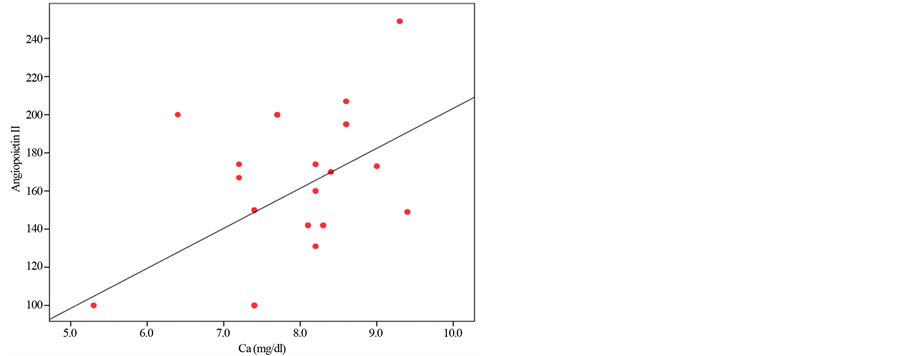
Figure 2 shows positive correlation between Angiopoietin-2 and serum ca level.
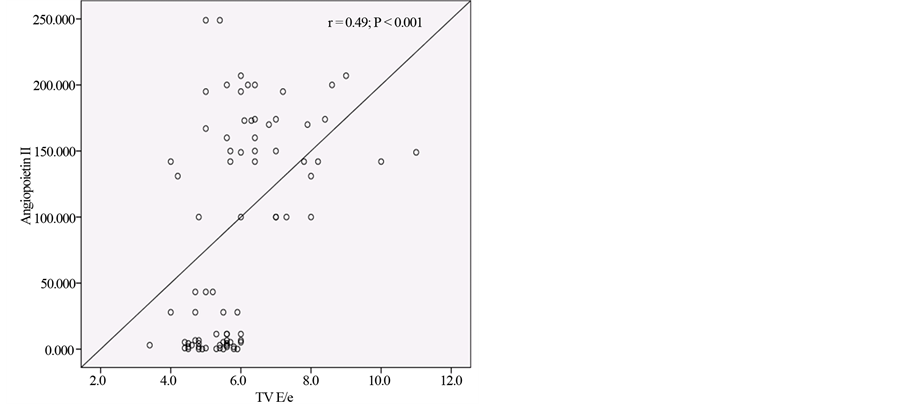
Figure 3 shows positive correlation between Angiopoietin-2 and TV E/e.
Table 5 shows correlation between Angiopoietin-2 serum level and some of the studied parameters, it revealed: significant positive correlation between Angiopoietin-2 and systolic blood pressure, serum ca and TV E/e ratio, while there is a statistical negative correlation with PTH, Hb levels and TV E vel m/s.
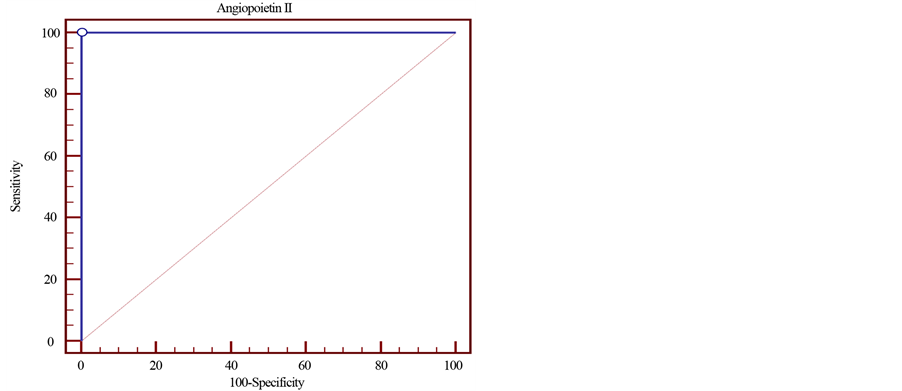
Table 6, Figure 4 show the cut of point, sensitivity and specificity of Angiopoietin-2 as an early marker for diagnosis of cardiovascular risk in children on hemodialysis; it revealed: the cut of point of Angiopoietin-2 is 43 ug/ml with 100% sensitivity and specificity in the prediction of cardiovascular risks in children on hemodialysis.
4. Discussion
Children with CKD on regular hemodialysis were assessed regarding the cardiovascular risks, it provided further evidence that children with CKD were at increased cardiovascular risk, because the majority of these children were anemic, hypertensive and dyslipidemic. In addition, children with CKD had significantly greater IMT of
Table 3. Comparison between patients group and the controls regarding the studied Doppler ultrasound and echo parameters.
Interventricular septum thickness in diastole (IVSTd); Interventricular septum thickness in systole (IVSTs); Left ventricular posterior wall thickness in diastole (LVPWTd); Left ventricular posterior wall thickness in systole (PWTs); Peak early diastolic trans-mitral flow velocity (E) MV A vel m/s; Peak late diastolic trans-mitral flow velocity (A); Tricuspid annulus plane systolic excursion (TAPSE); Peak early diastolic trans-tricuspid flow velocity (E); Peak late diastolic trans-tricuspid flow velocity (A); Early to late peak velocities (E/A ratio); Right ventricular systolic pressure (RVSP mmhg).
Table 4. Ratio of tricuspid Doppler flow velocity to the early tricuspid annular velocity (E/e’ ratio) among pa- tients group.
Figure 1. Negative correlation between Angiopoietin-2 and hemoglobin level.
Table 5. Correlation between Angiopoietin-2 and some of the studied parameters.
Table 6. The cut of point, sensitivity, specificity of Angiopiotin-2 as an early marker in detec- tion of cardiovascular risk in children on hemodialysis.
big arteries compared with healthy controls. It might also be that for abnormal calcium-phosphorus metabolism to have a detrimental effect on the vasculature, there needed to be a prolonged exposure and/or that calcium and phosphorus abnormalities were more important in the development of abnormal cIMT in children with advanced CKD (Parekh et al., 2002; Groothoff et al., 2002) [22] [23] . National Heart, Lung, and Blood Institute (NHLBI) consensus guidelines for cardiovascular health and risk reduction in children and adolescents
Figure 2. Positive correlation between Angiopoietin-2 and serum ca level.
Figure 3. Positive correlation between Angiopoietin-2 and TV E/e ratio.
Figure 4. ROC curve demonstrates Angiopoietin-2 sensitivity and specificity in predicting cardiovascular risk in children on hemodialysis.
recommended that children with CKD were considered in the highest risk of cardiovascular risk (NHLB, 2011) [24] .
Other investigators had similarly demonstrated that children with varying degrees of kidney dysfunction had higher cIMT than the general population, with dialysis patients having the most extremely elevated measurements (Litwin et al., 2005; Mitsnefes et al., 2005) [25] [26] . What made our results particularly striking was that this finding still held among young children compared with normative data of healthy controls. Our findings confirmed the previously described presence of advanced preclinical atherosclerosis in children with ESRD. The IMT values were observed in the study and control groups were highly similar to those found in previous studies (Mitsnefes et al., 2004; Jourdan et al., 2005; Poyrazoglu et al., 2006; Civilibal et al., 2007) [27] - [30] .
Echocardiographic examination was the main diagnostic tool enabling noninvasive evaluation of cardiac risks; according to our results, there was a significant increase in IVSd and EDV (end diastolic volume) in dialysis group than controls, and these findings revealed that LVH was predominant in CKD children on regular hemodialysis as LVH was defined by thickening of IVS and LVPW (Peco-Antić and Paripović, 2014) [31] . LVH was an adaptive response to chronic pressure and volume overload allowing maintenance of systolic function.
LVH was the most relevant cardiac abnormality in children with CKD stage 5 caused by pressure and volume overload which led to overgrowth of cardiomyocytes with thickening of both interventricular septum (IVS) and left ventricular posterior wall (LVPW) (Peco-Antić and Paripović, 2014) [31] . In an agreement with our study, Drozdzz et al. (2014) [32] and Adiele et al. (2014) [33] reported a significant increase in IVSd in dialysis patients than the healthy control.
We did not detect any abnormalities of the left ventricle functions between patients and healthy controls, while we detected right ventricular diastolic dysfunction as we found significant increase in TV A vel m/s and decrease in TV E/A ratio, also we detected increase in TV E/e ratio (TDI, assessment) in children on hemodialysis than healthy controls. Diastolic dysfunction appeared to occur earlier than systolic dysfunction. Rudhani et al. (2010) [34] reported on a case control study reduced right ventricular diastolic function, which might be explained by the presence of left ventricular overload as well as increased pressure on the right heart and intravascular volume. Also, in a study of ESRD patients by Virtanen et al (1998) [20] - [35] , mean E/A was 1.5 ± 0.5. London et al. (1993) [36] reported a significant reduction in E/A ratio in haemodialysis patients as compared to controls. We recorded a significant increase in the RVSP in children on hemodialysis than the control group. Risk factors for PH in CKD patients were AVF endothelial dysfunction which was a systemic pathological state characterized by an imbalance between vasodilating and vasoconstricting substances produced by the endothelium was a major determinant of PH, and episodes of nocturnal hypoxia were frequent in both predialysis and dialysis CKD patients, exposure to dialysis membranes and systemic diseases associated with CKD (Bolignano et al., 2013) [37] .
There are accumulating evidences that the increase in cardiovascular disease burden is present in patients with chronic kidney disease, particularly those on hemodialysis. We will not go deeper in the study of other risk factors related to the CVS in this population because the most has been previously studied in depth and we aimed to assess Ang-2 as a new marker of risk before the development of cardiac symptoms. Unfortunately, no enough data are available regarding this issue in pediatrics.
This study provides evidence that compared to healthy children, serum Ang-2 levels were markedly elevated in children on dialysis and this is in agreement with Rukshana et al. (2013) [38] , with significant positive correlation and systolic blood pressure, serum ca levels and negative correlation with PTH. There is no significant correlation between serum Ang-2 with Doppler ultrasound and Echo parameters of both left and right ventricle except significant positive correlation with TV E vel m/s and TV E/e ratio which is detected by TDI, is an index of right ventricular relaxation. These findings may indicate that circulating Ang-2 is a marker for the early cardiovascular changes particularly those with diastolic dysfunctions which occurring early in children with CKD on dialysis. There is no a lot of the study are available concerned with this issue. There are several potential mechanisms for the increase in circulating Ang-2 in patients with CKD. The increase in Ang-2 may be a direct consequence of elevated blood pressure. Korff and colleagues [39] demonstrated that hypertension in mice led to release of stored Ang-2 from Weibel-Palade bodies. There is also evidence that mediators of vascular tone such as Angiotensin-2 can directly alter Ang-2 expression Otani et al. (2001) [40] . A lack of endothelial nitric oxide may also predispose to a release of Weibel-Palade bodies that would theoretically increase Ang-2 levels (Nakayama et al., 2010) [41] . Our study demonstrated that Ang-2 is highly sensitive and specific marker for early diagnosis of cardiovascular risks in children on hemodialysis with 100% sensitivity and specificity. In conclusion, patients on hemodialysis have higher prevalence of right ventricular diastolic dysfunction that appears to occur earlier than systolic dysfunction. Angiotensin-2 can be an ideal biomarker may progress to play an adjunctive role with echocardiography in assessing cardiovascular risk of CKD subjects.
Cite this paper
ManalAbdel-Salam,Asmaa AbdEL Wakeel,SoheirIbrahim,TagreedAbdel-Rahman,HendEzzat,RandaSabour, (2015) Evaluation of Angiopoietin-2 Serum Level as a Marker of Cardiovascular Risk in Children with Chronic Kidney Disease. Open Journal of Nephrology,05,105-116. doi: 10.4236/ojneph.2015.54016
References
- 1. Scavarda, V.T., Pinheiro, A.C., Costa, S.D., Andrade, Z.M., Carvalhaes, J.T.A., Campos, O., Carvalho, A.C. and Moises, V.A. (2014) Children with Chronic Renal Disease Undergoing Dialysis or Conservative Treatment—Differences in Structural and Functional Echocardiographic Parameters. Echocardiography. 31, 1131-1137.
http://dx.doi.org/10.1111/echo.12525 - 2. Covic, A., Mardare, N., Gusbeth-Tatomir, P., Brumaru, O., Gavrilovici, C., et al. (2006) Increased Arterial Stiffness in Children on Haemodialysis. Nephrology Dialysis Transplantation, 21, 729-735.
http://dx.doi.org/10.1093/ndt/gfi196 - 3. Shroff, R.C., McNair, R., Figg, N., Skepper, J.N., Schurgers, L., et al. (2008) Dialysis Accelerates Medial Vascular Calcification in Part by Triggering Smooth Muscle Cell Apoptosis. Circulation, 118, 1748-1757.
http://dx.doi.org/10.1161/CIRCULATIONAHA.108.783738 - 4. Dursun, I., Poyrazoglu, H.M., Gunduz, Z., Ulger, H., Yykylmaz, A., et al. (2009) The Relationship between Endothelial Microparticles and Arterial Stiffness and Atherosclerosis in Children with Chronic Kidney Disease. Nephrology Dialysis Transplantation, 24, 2511-2518.
http://dx.doi.org/10.1093/ndt/gfp066 - 5. Mitsnefes, M.M., Barletta, G.M., Dresner, I.G., Chand, D.H., Geary, D., et al. (2006) Severe Cardiac Hypertrophy and Long-Term Dialysis: The Midwest Pediatric Nephrology Consortium Study. Pediatric Nephrology, 21, 1167-1170.
http://dx.doi.org/10.1007/s00467-006-0180-9 - 6. McDonald, S.P. and Craig, J.C., Australian and New Zealand Paediatric Nephrology Association (2004) Long-Term Survival of Children with End-Stage Renal Disease. New England Journal of Medicine, 350, 2654-2662.
http://dx.doi.org/10.1056/NEJMoa031643 - 7. Oh, J., Wunsch, R., Turzer, M., Bahner, M., Raggi, P., et al. (2002) Advanced Coronary and Carotid Arteriopathy in Young Adults with Childhood-Onset Chronic Renal Failure. Circulation, 106, 100-105.
http://dx.doi.org/10.1161/01.CIR.0000020222.63035.C0 - 8. Davis, S., Aldrich, T.H., Jones, P.F., et al. (1996) Isolation of Angiopoietin-1, a Ligand for the Tie 2 Receptor, by Secretion-Trap Expression Cloning. Cell, 87, 1161-1169.
http://dx.doi.org/10.1016/S0092-8674(00)81812-7 - 9. Fam, N.P., Verma, S., Kutryk, M., et al. (2003) Clinician Guide to Angiogenesis. Circulation, 108, 2613-2618.
http://dx.doi.org/10.1161/01.CIR.0000102939.04279.75 - 10. Brindle, N.P., Saharinen, P. and Alitalo, K. (2006) Signaling and Functions of Angiopoietin-1 in Vascular Protection. Circulation Research, 98, 1014-1023.
http://dx.doi.org/10.1161/01.RES.0000218275.54089.12 - 11. Fiedler, U. and Augustin, H.G. (2006) Angiopoietins: A Link between Angiogenesis and Inflammation. Trends in Immunology, 27, 552-558.
http://dx.doi.org/10.1016/j.it.2006.10.004 - 12. Fiedler, U., Scharpfenecker, M., Koidl, S., Hegen, A., Grunow, V., et al. (2004) The Tie-2 Ligand Angiopoietin-2 Is Stored and Rapidly Released upon Stimulation from Endothelial Cell Weibel-Palade Bodies. Blood, 103, 4150-4156.
http://dx.doi.org/10.1182/blood-2003-10-3685 - 13. Kuo, M.C., Patschan, D., Patschan, S., Cohen-Gould, L., Park, H.C., et al. (2008) Ischemia-Induced Exocytosis of Weibel-Palade Bodies Mobilizes Stem Cells. Journal of the American Society of Nephrology, 19, 2321-2330.
http://dx.doi.org/10.1681/ASN.2007111200 - 14. David, S., Kumpers, P., Lukasz, A., Fliser, D., Martens-Lobenhoffer, J., et al. (2010) Circulating Angiopoietin-2 Levels Increase with Progress of Chronic Kidney Disease. Nephrology Dialysis Transplantation, 25, 2571-2576.
http://dx.doi.org/10.1093/ndt/gfq060 - 15. David, S., Kümpers, P., Hellpap, J., Horn, R., Leitolf, H., et al. (2009) Angiopoietin-2 and Cardiovascular Disease in Dialysis and Kidney Transplantation. American Journal of Kidney Diseases, 53, 770-778.
http://dx.doi.org/10.1053/j.ajkd.2008.11.030 - 16. David, S., John, S.G., Jefferies, H.J., Sigrist, M.K., Kümpers, P., et al. (2012) Angiopoietin-2 Levels Predict Mortality in CKD Patients. Nephrology Dialysis Transplantation, 27, 1867-1872.
http://dx.doi.org/10.1093/ndt/gfr551 - 17. Gillard, B.K., Chen, Y.S., Gaubatz, J.W., et al. (2005) Plasma Factors Required for Human Apolipoprotein A-II Dimerization. Biochemistry, 44, 471-479.
http://dx.doi.org/10.1021/bi048591j - 18. Sahn, D.J., DeMaria, A., Kisslo, J., et al. (1978) Recommendations regarding Quantitation in M-Mode Echocardiography: Results of a Survey of Echocardiographic Measurements. Circulation, 58, 1072-1083.
http://dx.doi.org/10.1161/01.CIR.58.6.1072 - 19. Giusca, S., Dambrauskaite, V., Scheurwegs, C., D’Hooge, J., Claus, P., Herbots, L., et al. (2010) Deformation Imaging Describes Right Ventricular Function Better than Longitudinal Displacement of the Tricuspid Ring. Heart, 96, 281-288.
http://dx.doi.org/10.1136/hrt.2009.171728 - 20. Lang, R.M., Badano, L.P., Mor-Avi, V., Afilalo, J., Armstrong, A., Ernande, L., Flachskampf, F.A., Foster, E., Goldstein, S.A., Kuznetsova, T., Lancellotti, P., Muraru, D., Picard, M.H., Rietzschel, E.R., Rudski, L., Spencer, K.T., Tsang, W. and Voigt, J.U. (2015) Recommendations for Cardiac Chamber Quantification by Echocardiography in Adults: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Journal of the American Society of Echocardiography, 28, 1-39.
http://dx.doi.org/10.1016/j.echo.2014.10.003 - 21. Chavhan, G.B., Parra, D.A., Andrea, M. and Oscar, M.N. (2008) Normal Doppler Spectral Waveforms of Major Pediatric Vessels: Specific Patterns. Radiographics, 28, 691-706.
http://dx.doi.org/10.1148/rg.283075095 - 22. Parekh, R.S., Carroll, C.E., Wolfe, R.A. and Port, F.K. (2002) Cardiovascular Mortality in Children and Young Adults with End-Stage Kidney Disease. Journal of Pediatrics, 141, 191-197.
http://dx.doi.org/10.1067/mpd.2002.125910 - 23. Groothoff, J.W., Gruppen, M.P., Offringa, M., Hutten, J., Lilien, M.R., Van De Kar, N.J., Wolff, E.D., Davin, J.C. and Heymans, H.S. (2002) Mortality and Causes of Death of End-Stage Renal Disease in Children: A Dutch Cohort Study. Kidney International, 61, 621-629.
http://dx.doi.org/10.1046/j.1523-1755.2002.00156.x - 24. Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents National Heart, Lung, and Blood Institute (2011) Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents: Summary Report. Pediatrics, 128, S213-S256.
- 25. Litwin, M., Wühl, E., Jourdan, C., Trelewicz, J., Niemirska, A., Fahr, K., Jobs, K., Grenda, R., Wawer, Z.T., Rajszys, P., Tröger, J., Mehls, O. and Schaefer, F. (2005) Altered Morphologic Properties of Large Arteries in Children with Chronic Renal Failure and after Renal Transplantation. Journal of the American Society of Nephrology, 16, 1494-1500.
http://dx.doi.org/10.1681/ASN.2004110932 - 26. Mitsnefes, M.M., Kimball, T.R., Kartal, J., Witt, S.A., Glascock, B.J., Khoury, P.R. and Daniels, S.R. (2005) Cardiac and Vascular Adaptation in Pediatric Patients. Journal of the American Society of Nephrology, 16, 2796-2803.
http://dx.doi.org/10.1681/ASN.2005030291 - 27. Mitsnefes, M.M., Kimball, T.R., Witt, S.A., Glascock, B.J., Khoury, P.R. and Daniels, S.R. (2004) Abnormal Carotid Artery Structure and Function in Children and Adolescents with Successful Renal Transplantation. Circulation, 110, 97-101.
- 28. Jourdan, C., Wuhl, E., Litwin, M., Fahr, K., Trelewicz, J., Jobs, K., Schenk, J.P., Grenda, R., Mehls, O., Troger, J. and Schaefer, F. (2005) Normative Values for Intima-Media Thickness and Distensibility of Large Arteries in Healthy Adolescents. Journal of Hypertension, 23, 1707-1715.
http://dx.doi.org/10.1097/01.hjh.0000178834.26353.d5 - 29. Poyrazoglu, H.M., Düsünsel, R., Yikilmaz, A., Narin, N., Anarat, R., Gündüz, Z., Coskun, A., Baykan, A. and Öztürk, A. (2007) Carotid Artery Thickness in Children and Young Adults with End Stage Renal Disease. Paediatric Nephrology, 22, 109-116.
http://dx.doi.org/10.1007/s00467-006-0268-2 - 30. Civilibal, M., Caliskan, S., Oflaz, H., Sever, L., Candan, C., Canpolat, N., Kasapcopur, O., Bugra, Z. and Arisoy, N. (2007) Traditional and “New” Cardiovascular Risk Markers and Factors in Pediatric Dialysis Patients. Paediatric Nephrology, 22, 1021-1029.
http://dx.doi.org/10.1007/s00467-007-0451-0 - 31. Peco-Antic, A. and Paripovic, D. (2014) Renal Hypertension and Cardiovascular Disorder in Children with Chronic Kidney Disease Faculty of Medicine, University of Belgrade, Belgrade, Serbia. Srpski arhiv za celokupno lekarstvo, 142, 113-117.
http://dx.doi.org/10.2298/SARH1402113P - 32. Drozdzz, D., Kordon, Z., Kwinta, P., Drozdz, M., Miklaszewska, M., Zachwieja, K., Rudzinski, A. and Pietrzyk, J.A. (2014) Left Ventricular Hypertrophy at Different Stages of Chronic Kidney Disease in Children and Adolescents. Experimental & Clinical Cardiology, 20, 4111-4116.
- 33. Adiele, D.K., Okafor, H.U., Ojinnaka, N.C., Onwubere, B.J.C., Odetunde, O.I. and Uwaezuoke, S.N. (2014) Echocardiographic Findings in Children with Chronic Kidney Disease as Seen in the Resource-Limited Setting. Journal of Nephrology & Therapeutics, 4, 158.
http://dx.doi.org/10.4172/2161-0959.1000158 - 34. Rudhani, I.D., Bajraktari, G., Kryziu, E., Zylfiu, B., Sadiku, S., Elezi, Y., Rexhepaj, N., Vitia, A., Emini, M., Abazi, M., Berbatovci-Ukimeraj, M., Kryeziu, K., Hsanagjekaj, V., Korca, H. and Ukimeri, A. (2010) Left and Right Ventricular Diastolic Function in Hemodialysis Patients. Saudi Journal of Kidney Diseases and Transplantation, 21, 1053-1057.
- 35. Virtanen, V.K., Saha, H.S.T., Groundstroem, K.W.E., et al. (1998) Calcium Infusion and Left Ventricular Diastolic Function in Patients with Chronic Renal Failure. Nephrology Dialysis Transplantation, 13, 384-388.
http://dx.doi.org/10.1093/oxfordjournals.ndt.a027834 - 36. London, G.M., Marchais, S.J., Guerin, A.P., et al. (1993) Cardiac Hypertrophy and Arterial Alteration in End-Stage Renal Disease: Hemodynamic Factors. Kidney International, 41, S42-S49.
- 37. Bolignano, D., Rastelli, S., Agarwal, R., Fliser, D., Massy, Z., Ortiz, A., Wiecek, A., et al. (2013) Pulmonary Hypertension in CKD. American Journal of Kidney Diseases, 61, 612-622.
http://dx.doi.org/10.1053/j.ajkd.2012.07.029 - 38. Shroff, R.C., Price, K.L., Kolatsi-Joannou, M., Todd, A.F., Wells, D., Deanfield, J., Johnson, R.J., Rees, L., Woolf, A.S. and Long, D.A. (2013) Circulating Angiopoietin-2 Is a Marker for Early Cardiovascular Disease in Children on Chronic Dialysis. PLoS ONE, 8, e56273.
www.plosone.org
http://dx.doi.org/10.1371/journal.pone.0056273 - 39. Korff, T., Ernst, E., Nobiling, R., Feldner, A., Reiss, Y., et al. (2012) Angiopoietin-1 Mediates Inhibition of Hypertension-Induced Release of Angiopoietin-2 from Endothelial Cells. Cardiovascular Research, 94, 510-518.
http://dx.doi.org/10.1093/cvr/cvs124 - 40. Otani, A., Takagi, H., Oh, H., Koyama, S. and Honda, Y. (2001) Angiotensin II Induces Expression of the Tie2 Receptor Ligand, Angiopoietin-2, in Bovine Retinal Endothelial Cells. Diabetes, 50, 867-875.
http://dx.doi.org/10.2337/diabetes.50.4.867 - 41. Nakayama, T., Sato, W., Yoshimura, A., Zhang, L., Kosugi, T., et al. (2010) Endothelial von Willebrand Factor Release Due to eNOS Deficiency Predisposes to Thrombotic Microangiopathy in Mouse Aging Kidney. American Journal of Pathology, 176, 2198-2208.
http://dx.doi.org/10.2353/ajpath.2010.090316
NOTES
*Corresponding author.