Open Journal of Nephrology
Vol.4 No.1(2014), Article ID:43548,5 pages DOI:10.4236/ojneph.2014.41002
Effects of Darbepoetin-α on Oxidative Stress Marker in Patients with Chronic Renal Failure
Norio Nakamura1*, Michiko Shimada2, Ikuyo Narita2, Yuko Shimaya2, Takeshi Fujita2, Reiichi Murakami2, Hiroshi Osawa3, Hideaki Yamabe2, Ken Okumura2
1Community Medicine, Hirosaki University Graduate School of Medicine, Japan
2Department of Cardiology, Respiratory Medicine and Nephrology, Hirosaki University Graduate School of Medicine, Hirosaki, Japan
3Department of General Medicine, Hirosaki University Hospital, Hirosaki, Japan
Email: *nnakamur@r2.dion.ne.jp
Copyright © 2014 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/


Received 27 December 2013; revised 26 January 2014; accepted 25 February 2014
ABSTRACT
Objective: Long-acting darbepoetin-α (DA) has recently been used to treat renal anemia in patients with chronic renal failure. It is considered clinically useful because its duration of action is longer than that of conventional epoetin-α. In this study, we investigated changes in the levels of the oxidative stress marker malondialdehyde-modified low density lipoprotein (MDA-LDL), renal anemia, and renal function when patients were treated for chronic renal failure switched from epoetin-α to DA. Materials and Methods: The subjects included nine patients with chronic renal failure and renal anemia who were treated with epoetin-α on an outpatient basis at our department. Blood was sampled prior to the switch and at 3, 6, and 12 months after the switch. We then investigated changes in MDA-LDL, hemoglobin (Hb), and creatinine (Cr) levels. Results: There were no significant changes in MDA-LDL and Hb levels after switching to DA. A significant increase was observed in Cr levels after 12 months compared with those prior to switching. Conclusion: Once-amonth administration of DA did not result in an increase in oxidative stress, and therefore, DA is considered capable of controlling renal anemia.
Keywords:Chronic Renal Failure; Darbepoetin-α; Epoetin-α; Indoxyl Sulfate; MDA-LDL; Renal Anemia

1. Introduction
Reports have stated that when treating renal anemia in patients with chronic renal failure, palliative care should be initiated for progression of renal failure in addition to treating anemia [1] . Furthermore, such treatment is reported to be extremely important for the prevention of cardiovascular disease [2] . From these perspectives, the importance of treating renal anemia in patients with chronic renal failure continues to increase.
Epoetin-α was released in the market in 1990 as a drug to treat renal anemia, but from 2005 onwards, darbepoetin-α (DA) began to be used in clinical practice. DA is a formulation with a modified epoetin-α molecular structure; therefore, the duration of its hematopoietic effects is longer than that of conventional epoetin-α, thereby allowing a prolonged administration interval [3] . In clinical practice, excellent results have been reported in patients undergoing dialysis and those who are in the conservative stage of renal failure [3] .
Renal failure and uremia are known to cause oxidative stress [4] . This is believed to damage the kidneys and other organs [5] . Currently, although a few reports have investigated changes in the levels of oxidative stress markers due to DA administration, results have been inconsistent [6] [7] .
For this reason, in this study, we investigated the changes in the levels of the oxidative stress marker malondialdehyde-modified low density lipoprotein (MDA-LDL), hemoglobin (Hb), and creatinine (Cr) inconservative-stage patients with chronic renal failure who were switched from epoetin-α to DA treatment.
2. Materials and Methods
The study population comprised nine patients with conservative-stage renal failure who were treated as outpatients at the Nephrology Department of the Hirosaki University Hospital. The patients’ diseases were wellcontrolled by once-a-month administration of epoetin-α. After providing written consent, the subjects were enrolled into this trial.
Patients were switched to DA at an epoetin-α:DA ratio of 200:1. Laboratory examinations were conducted at the time of switching and at 3, 6, and 12 months after the switch. Changes in Hb, Cr, and MDA-LDL and indoxyl sulfate levels were measured. Serum MDA-LDL and serum indoxyl sulfate levels were measured by SRL Inc. (Tokyo, Japan).
According to the Japan Society for Dialysis Therapy for renal anemia treatment guidelines, patients with renal anemia should be administered iron supplements when the transferrin saturation ratio (TSAT) is ≤20% and/or when the serum ferritin level is ≤100 ng/mL, with the target Hb level of 11 g/dL.
Results were represented as mean ± SD. Scheffe’s method was used for statistical analysis, and p < 0.05 was considered statistically significant.
3. Results
Patients’ background is shown in Table 1. The underlying disease was determined by renal biopsy in one patient (focal segmental glomerulonephritis), five patients were suspected to have chronic glomerulonephritis, one patient had polycystic kidney disease, one patient was suspected to have benign nephrosclerosis, and one patient was suspected to have diabetic nephropathy. Epoetin-α was administered at the dose of 6000 units/month in one subject with comparatively well-preserved renal function, whereas all other patients were administered 12,000 units/month of epoetin-α.
Changes in serum MDA-LDL concentrations are shown in Figure 1. Increase in serum MDA-LDL was only observed in one patient at 6 months after administration, but there were no significant changes observed in any of the patients during the course of the investigation.
Changes in Hb levels are shown in Figure 2. No statistically significant changes were observed during the course of the investigation. After 12 months of administration, Hb level in one patient decreased to almost 6 g/dL, but this patient experienced abnormal vaginal bleeding due to a uterine tumor, which was thought to be the cause of the drop in Hb level.
Changes in serum Cr levels are shown in Figure 3. A tendency toward a gradual increase in Cr levels was observed in all subjects, and a significant difference was observed when the levels prior to administration were compared with those at 12 months after administration (p < 0.05).
Changes in serum in doxyl sulfate levels are shown in Figure 4. There was a wide variation in changes, but no significant changes were observed during the investigation period.
Table 1. Patients’ background.
FSGS: Focal segmental glomerulonephritis, PKD: polycystic kidney disease.
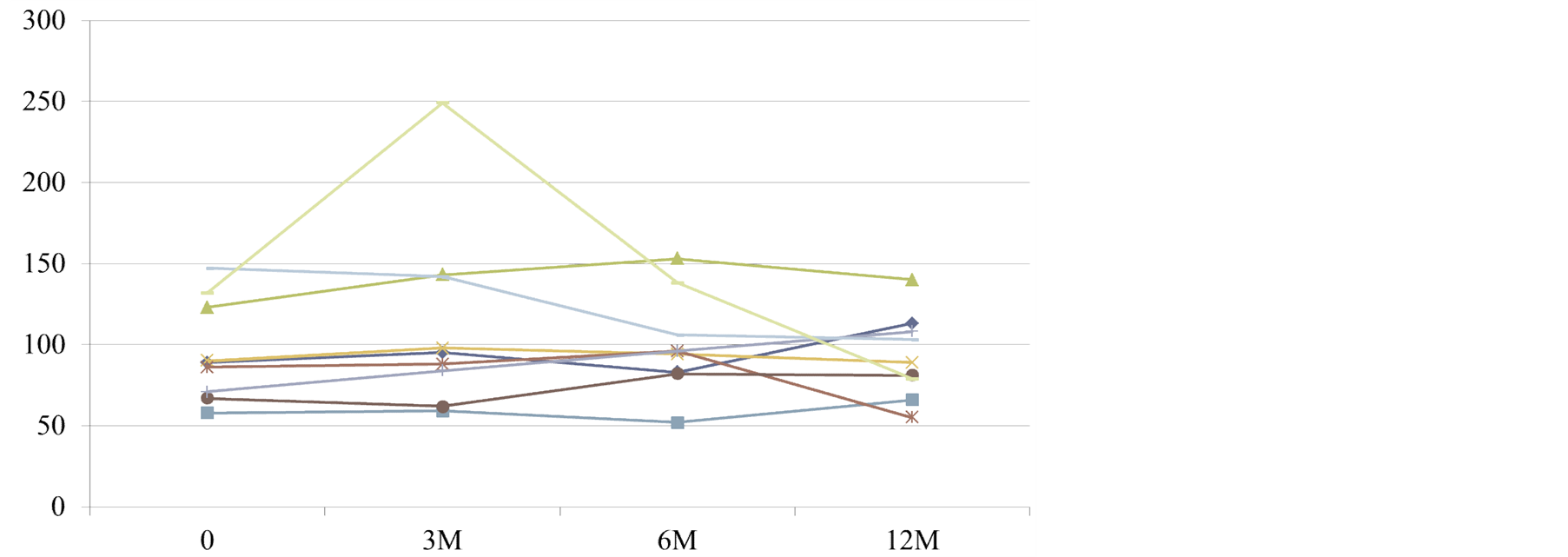
Figure 1. Changes in serum MDA-LDL levels (mg/dL) in patients with chronic renal failure.
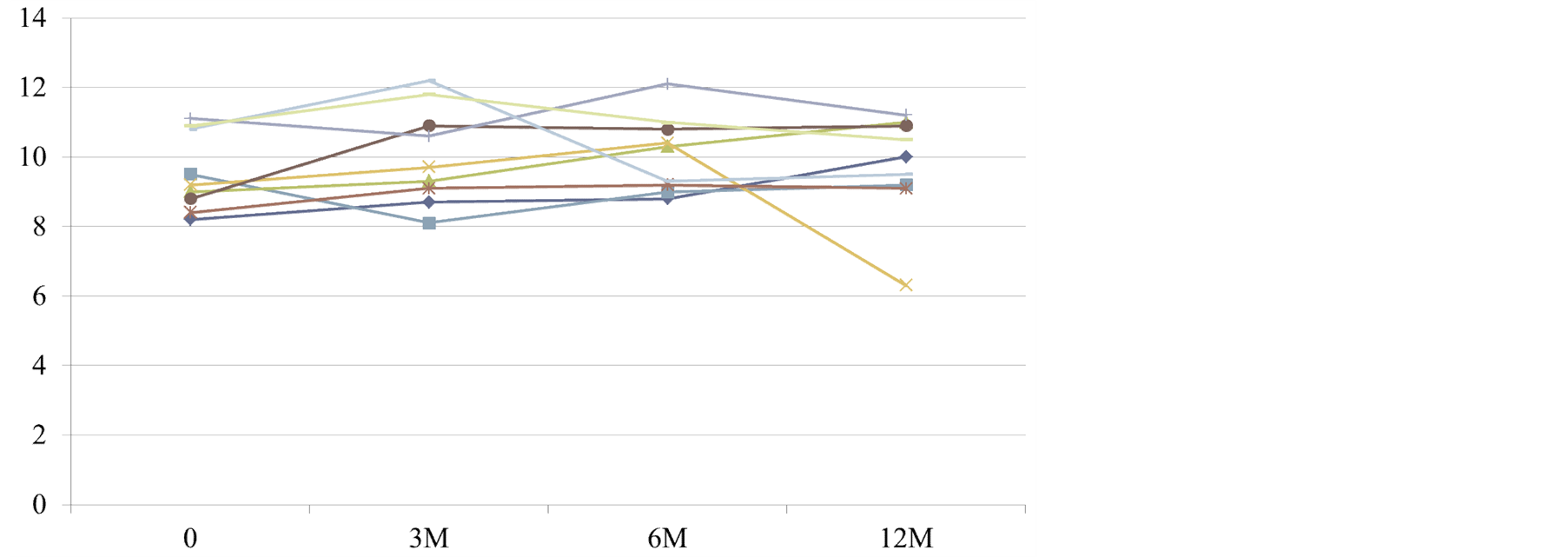
Figure 2. Changes in hemoglobin levels (g/dL) in patients with chronic renal failure.
Furthermore, no significant changes were observed in either systolic or diastolic blood pressure after the switch to DA (data not shown).
4. Discussion
In this study, we investigated serum MDA-LDL levels, Hb levels, and renal function in patients with conservative-stage renal failure who were switched from epoetin-α to DA treatment.
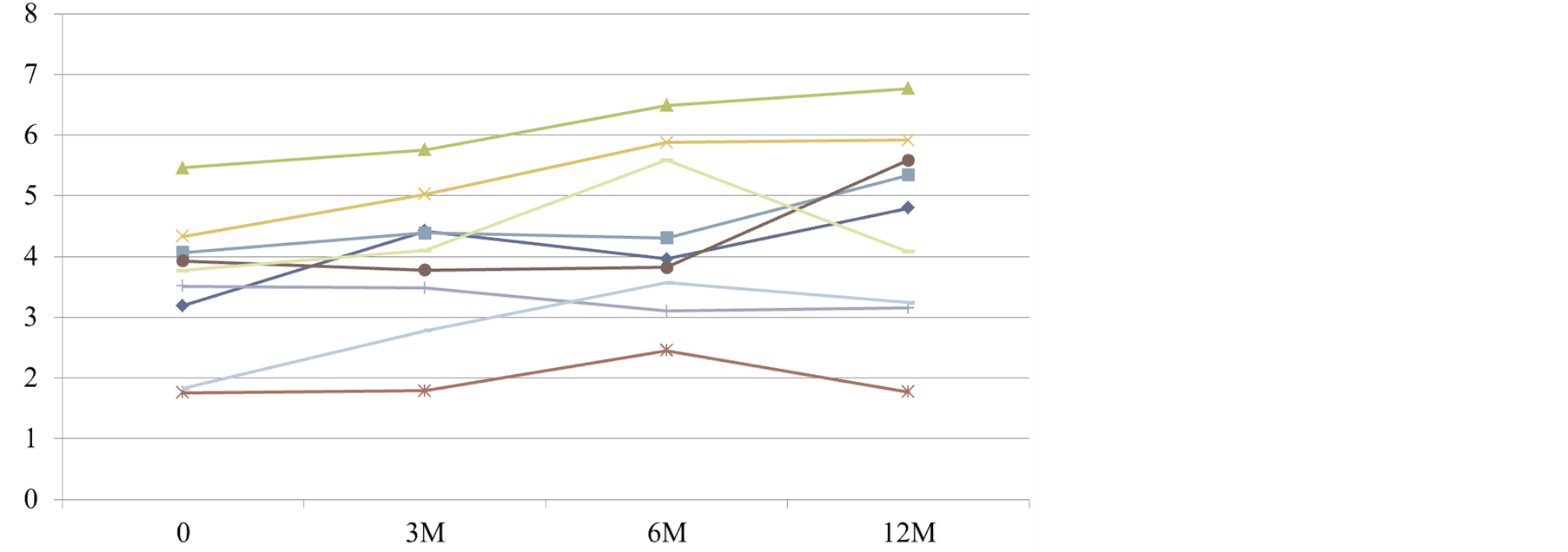
Figure 3. Changes in serum creatinine levels (mg/dL) in patients with chronic renal failure.
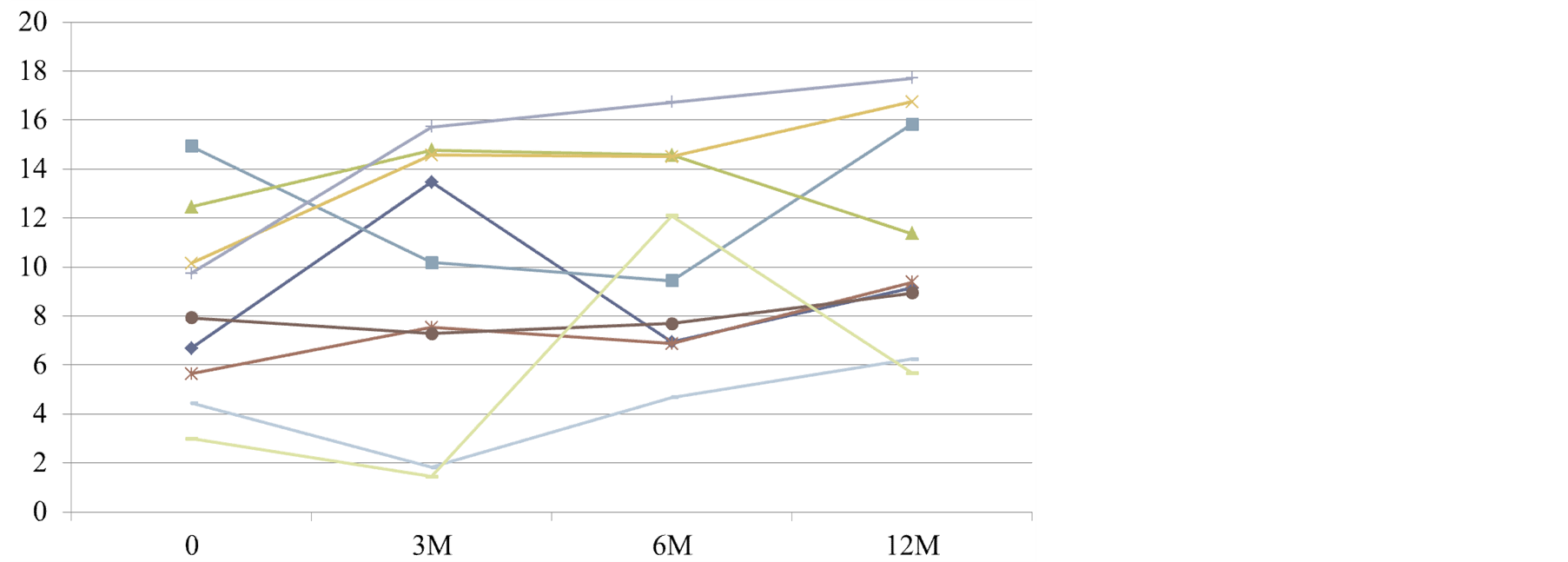
Figure 4. Changes in serum indoxyl sulfate levels (mg/mL) in patients with chronic renal failure.
During this study, when we investigated changes in the levels of MDA-LDL, an oxidative stress marker, no significant changes were observed after switching from epoetin-α to DA. MDA-LDL has recently received attention as a marker of oxidative stress and has also been suggested as being possibly related to cardiovascular events [8] . Although no obvious changes were observed in this study, serum Cr levels gradually increased. Practically, this increase may suppress the progressive oxidative stress associated with worsening renal failure.
Furthermore, Hb levels remained stable after the switch form epoetin-α to DA. After 12 months of administration, Hb level decreased in one patient, but the patient presented with abnormal vaginal bleeding due to a uterine tumor, which was thought to be the cause of the drop in Hb level. The subject received a transfusion, experienced no further problems, and was considered well-controlled.
In all cases, there was a tendency toward a gradual increase in serum Cr levels, and a significant difference was observed at 12 months after administration when compared with the level measured prior to administration. Some patients with advanced renal failure were included among the patients enrolled in this study, and thus, these subjects were believed to reflect the natural course of renal failure progression.
Moreover, there were no significant differences in the indoxyl sulfate levels. However, indoxyl sulfate is considered to promote renal failure and may act as an inhibitor of DA.
To date, few reports regarding DA and oxidative stress have been published; some of these reports have stated that oxidative stress is improved by DA administration [6] , whereas other reports have stated that no significant changes are observed [7] ; thus, no consensus has been reached yet. It is possible that these results were related to differences in the control groups and administration periods. In addition, in vitro reports have also been published, and according to Yang et al., DA suppresses TNF-α-mediated production of endothelin-1 and acts to suppress atherosclerosis [9] in human aortic epithelial cells. These effects are stronger with DA than with epoetin-α. The underlying reason proposed by Yang et al. is that DA molecular structure contains more sialic acid than epoetin-α molecular structure. This theory, however, requires further investigations.
5. Conclusion
In this study, we investigated changes in MDA-LDL and indoxyl sulfate levels, anemia, and renal function in patients with conservative-stage renal failure who were switched from epoetin-α to DA. In our study, no increase in MDA-LDL or indoxyl sulfate levels was observed in response to the once-a-month administration of DA; therefore, DA could be used to control anemia. Thus, DA is believed to be a useful treatment for renal anemia in patients with conservative-stage renal failure.
References
- Kuriyama, S., Tomonari, H., Yoshida, H., Hashimoto, T., Kawaguchi, Y. and Sakai, O. (1997) Reversal of Anemia by Erythropoietin Therapy Retards the Progression of Chronic Renal Failure, Especially in Nondiabetic Patients. Nephron, 77, 176-185. http://dx.doi.org/10.1159/000190270
- Hayashi, T., Suzuki, A., Shoji, T., Togawa, M., Okada, N., Tsubakihara, Y., Imai, E. and Hori, M. (2000) Cardiovascular Effect of Normalizing the Hematocrit Level during Erythropoietin Therapy in Predialysis Patients with Chronic Renal Failure. American Journal of Kidney Diseases, 35, 250-256. http://dx.doi.org/10.1016/S0272-6386(00)70334-9
- Kiss, Z., Elliott, S., Jedynasty, K., Tesar, V. and Szegedi, J. (2010) Discovery and Basic Pharmacology of Erythropoiesis-Stimulating Agents (ESAs), Including the Hyperglycosylated ESA, Darbepoetin Alfa: An Update of the Rationale and Clinical Impact. European Journal of Clinical Pharmacology, 66, 331-340. http://dx.doi.org/10.1007/s00228-009-0780-y
- Libetta, C., Sepe, V., Esposito, P., Galli, F. and Al Canton, D.A. (2011) Oxidative Stress and Inflammation: Implications in Uremia and Hemodialysis. Clinical Biochemistry, 44, 1189-1198.
- Sung, C.C., Hsu, Y.C., Chen, C.C., Lin, Y.F. and Wu, C.C. (2013) Oxidative Stress and Nucleic Acid Oxidation in Patients with Chronic Kidney Disease. Oxidative Medicine and Cellular Longevity, 2013, Article ID: 301982.
- Parissis, J.T., Kourea, K., Andreadou, I., Ikonomidis, I., Markantonis, S., Ioannidis, K., Paraskevaidis, I., Iliodromitis, E., Filippatos, G. and Kremastinos, D.T. (2009) Effects of darbepoetin alfa on Plasma Mediators of Oxidative and Nitrosative Stress in Anemic Patients with Chronic Heart Failure Secondary to Ischemic or Idiopathic Dilated Cardiomyopathy. American Journal of Cardiology, 103, 1134-1138. http://dx.doi.org/10.1016/j.amjcard.2008.12.041
- Malgorzewics, S., Lichodziejewska-Niemierko, M., Lizakowski, S., Liberek, T., Lysiak-Szydiowska, W. and Rutkowski, B. (2010) Oxidative Stress, Inflammation and Nutritional Status during Darbepoetin α-Treatment in Peritoneal Dialysis Patients. Clinical Nephrology, 73, 210-215.
- Lopes-Virella, W.F., Hunt, K.J., Baker, N.L., Virella, G., Moritz, T. and VADT Investigators (2012) The levels of MDA-LDL in circulating immune complexes predict myocardial infarction in the VADT study. Atherosclerosis, 224, 526-531. http://dx.doi.org/10.1016/j.atherosclerosis.2012.08.006
NOTES

*Corresponding author.


