Open Journal of Rheumatology and Autoimmune Diseases
Vol.3 No.4(2013), Article ID:39699,7 pages DOI:10.4236/ojra.2013.34029
Diagnostic Value of Antibodies against a Modified Citrullinated Vimentin in Rheumatoid Arthritis
![]()
1Department of Clinical Pathology, Faculty of Medicine, Sohag University, Sohag, Egypt; 2Department of Rheumatology and Rehabilitation, Faculty of Medicine, Sohag University, Sohag, Egypt.
Email: rmhamdy@yahoo.com
Copyright © 2013 SaharAbou El-Fetou, Hanan S. Abozaid. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received April 25th, 2013; revised May 25th, 2013; accepted June 2nd, 2013
Keywords: Anti-Cyclic Citrullinated Peptide (Anti-CCP2); Anti-Citrullinated Vimentin Antibody (Anti-CMV) Rheumatoid Arthritis (RA)
ABSTRACT
Aim of Work: To investigate the value of the detection of antibodies against modified citrullinated vimentin antibodies (anti-MCV) in comparison with anti-CCP2- for the diagnosis of rheumatoid arthritis (RA). Patients and Methods: The study Included Forty patients with Rheumatoid arthritis (RA). They under went assessment by the disease activity score (DAS-28), visual analogue scale (VAS) and health assessment questionnaire (HAQ). Thirty healthy subjects matched for age and sex served as a control group. Blood samples were obtained from patients and controls for erythrocyte sedimentation rate (ESR), C reactive protein (CRP), rheumatoid factor (RF). Anti-CCP2 and anti-MCV were determined using ELISA technique. Results: Estimated serum levels of anti-CCP2 and anti-MCV were significantly higher in patients compared to controls (p < 0.001). There were no significant correlations between anti-MCV levels and age, disease duration, duration of morning stiffness, number of swollen and tender joints, HAQ or ESR in patients with RA, while serum levels correlates significantly with DAS28, VAS and CRP (p < 0.05). Anti-CCP2 correlates significantly with DAS28, VAS and CRP and ANA (p < 0.05). Serum anti-MCV and anti-CCP2 were significantly correlated with each other (r = 0.483; p < 0.001). The receiver operating characteristic (ROC) curve was drawn and it showed that anti-MCV had diagnostic specificity, sensitivity of 93.3%, 75.5%, respectively, while anti-CCP2 specificity, sensitivity of 98.1%, 85%, respectively. Conclusion: Serum anti-MCV as well as the anti-CCP-2 assay perform comparably well in the diagnosis of RA. In the high-specificity range, however, the anti-CCP2 assay appears to be superior to the antiMCV test.
1. Introduction
Rheumatoid arthritis (RA) is a chronic, inflammatory, autoimmune, systemic disease, in which various joints in the body are inflamed, leading to swelling, pain, stiffness, and the possible loss of function [1]. Early diagnosis and treatment may prevent joint destruction and suppress disease progression [2].
Several different autoantibodies with varying have been found in serum from patients with RA [3], but rheumatoid factor (RF) is at present the only autoantibody included in the 1987 American College of Rheumatology (ACR) classification criteria [4].
Highly specific and sensitive tests are necessary to diagnose early RA and initiate aggressive treatment regimens to prevent the irreversible joint damage. Testing for RF alone is not enough. It can be found in only 70% - 80% of RA patients. It is also present in other rheumatic disorders and healthy individuals [5].
Antibodies recognizing citrullinated proteins/peptides, especially the peptide mixture designated cyclic citrullinated peptides (CCP), have reasonable sensitivity and high specificity for RA and are incessantly used in the evaluation of patients with RA [6,7]. It is found to be highly specific for early diagnosis (97%) as well as prognosis of [8]. Vimentin is an intermediate filament that is widely expressed by mesenchymal cells and macrophages and easy to detect in the synovium. Modification of the protein occurs in macrophages undergoing apoptosis, and antibodies to citrullinated vimentin may emerge if the apoptotic material is inadequately cleared [9]. Recently, anti-mutated citrullinated vimentin (anti-MCV) antibodies have been recommended to be better diagnostic marker for early arthritis [10].
Some studies showed that anti-MCV antibody is more sensitive compared to other antibodies against citrullinecontaining epitopes for RA diagnosis, and that anti-MCV antibody was present even earlier in the course of RA than anti-CCP, and therefore was a better marker of early RA [11]. Besides the higher sensitivity it has been shown that anti-MCV antibody is a better marker for early RA, and it correlates well with the disease activity score (DAS). The presence of anti-MCV antibodies at disease onset is associated with a more severe disease course, measured as higher level of inflammatory activity compared with anti-CCP [12].
Several studies demonstrated that anti-MCV antibodies have the same specificity as anti-CCP antibodies, but with better sensitivity [13,14]. Like anti-CCP antibodies, anti-MCV antibodies are also suitable for the early diagnosis of RA, with comparable sensitivity and specificity [15].
This work was aimed to evaluate the role of anti-mutated citrullinated vimentin antibodies (anti-MCV) and anti-CCP2for the diagnosis of RA.
2. Patients and Methods
This case control study was conducted on RA patients attending the outpatient clinic of Rheumatology and Rehabilitation Department, Sohag University Hospital, in the period between May 2012 and September 2012. Forty patients (female/male = 26/14) fulfilling the 1987 American College of Rheumatology (ACR) criteria for a diagnosis of RA with a mean age of 46.95 ± 11.60 years were studied. Thirty healthy control subjects (female/male = 18/12). The mean age of the control subjects was 43.7 ± 9.9 years. Informed consent was obtained from patients and controls participating in the study. Age, sex, disease duration, Body Mass Index (BMI) (kg/m2), swollen and tender joint counts of the RA patients were recorded. Duration of morning stiffness, Visual Analog Scale of pain (VAS) [16] disease activity index 28 (DAS28) [17], health assessment questionnaire (HAQ), erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) were used to assess disease activity. Disease Activity-Score 28 (DAS28) as a validated index of RA activity. It consists of four measures: 28 tender (TJC28) and swollen joint counts (SJC28), ESR, and patient global health assessment (VAS). Patients were grouped according to DAS28 scores as having high (DAS28 > 5.1) or mild to moderate (DAS28 < 5.1) disease activity.
3. Laboratory Methods
Six mL of peripheral venous blood were withdrawn aseptically from each patient and from each control subject. Two mL blood were left to clot for 15 minutes then centrifuged and sera were put into aliquots and stored at −20˚C until assayed for anti-MCV and anti CCP2 antibodies for both patients and controls. The remaining 3 ml were used for other investigations done to patients:
1) Complete blood picture CBC was performed on The CELL-DYN 3700 automated hematology analyzer.
2) Renal and liver function tests were performed on Autoanalyzer Bechman Synchron cx5 system.
3) Measurement of ESR by the Westergren method.
4) Serum CRP concentrations were determined by immunonephelometry methods on a Turboxnephelometer (Orion Diagnostica, Finland). The titer of 6 mg/l was considered positive for CRP.
5) Rheumatoid factor IgMisotype was analyzed using the ELISA kit for RF IgM quantitation (Orgentec Diagnostika GmbH, Germany) according to the manufacturer’s instructions. The titer of 20 IU/ml was regarded as positive [18].
6) Measurement of anti-nuclear antibody (ANA) was detected by the Fluro-kit supplied by DiaSorine Catalog No 1740 based on indirect immunoflorescent for the screening and titration ANA.
7) Anti-CCP2 antibodies were detected by enzymelinked immunosorbent assay Kit supplies by INOVA Diagnostica Cat. NO 570139 is a semiquantitative enzymelinked immunosorbent assay for the detection of IgG anti-CCP2 (Cyclic Citrullinated Peptide 2) antibodies in patient sera. The antigen is bound to the surface of a microwell plate, allowing any CCP2IgG antibodies present to bind to the immobilized antigen. A second incubation allows the enzyme labeled antihuman IgG to bind to any patient antibodies that have been attached to the microwells. After washing away any unbound enzyme labeled anti-human IgG, the remaining enzyme activity is measured by adding a chromogenic substrate and measuring the intensity of the color that develops spectrophotometrically. A titer above 20 units was considered as positive.
8) Determination of Anti-MCV antibodies were measured using ELISA kits (provided by ORGENTEC Diagnostica GmbH, Mainz, Germany) according to the manufacturer’s instructions [19]. Serum samples were diluted 1:100 and incubated on MCV coated microtiter wells for 30 minutes at room temperature. Plates were washed three times and incubated with peroxidase-labeled antihuman IgG-conjugate for 15 minutes. Then substrate was incubated for 15 minutes after additional washing. Color development was stopped with 1 M HCl solution, and the optical density was measured. Results are expressed in U/ml using a simple point-to-point curve-fitting method. Values of 20.0 U/ml or greater were considered to be abnormal according to manufacturer’s recommendations.
4. Statistics
The results were analyzed by IBM-SPSS (version 19). Results were given as means and standard deviation. Student’s t-test for continuous variables was used to examine the significance of differences between RA and control groups. P-value less than 0.05 was regarded as significant. The correlation between anti -MCV, anti-CCP2 levels and age, disease duration, duration of morning stiffness, swollen and tender joint counts, VAS, DAS28, HAQ, ESR, CRP and BMI was analyzed by Pearson correlation analyses. Receiver operator characteristic curve (ROC) was drawn to find out the best cut-off value of anti-MCV in diagnosing RA. p value > 0.05 was considered insignificant, p < 0.05 was significant and p < 0.001 was highly significant.
5. Results
Age, sex and BMI did not show statistically significant differences between RA patients and control subjects (p > 0.05).Patients with RA had mild to moderate (DAS28 < 5.1) disease activity. The demographic and clinical characteristics of patients with RA and of control subjects are shown in Table 1. The mean serum Anti-CCP2 in patients with RA (89.2 ± 11.3 U/ml) was significantly higher (p < 0.001) than controls (14.8 ± 3.21 U/mL).
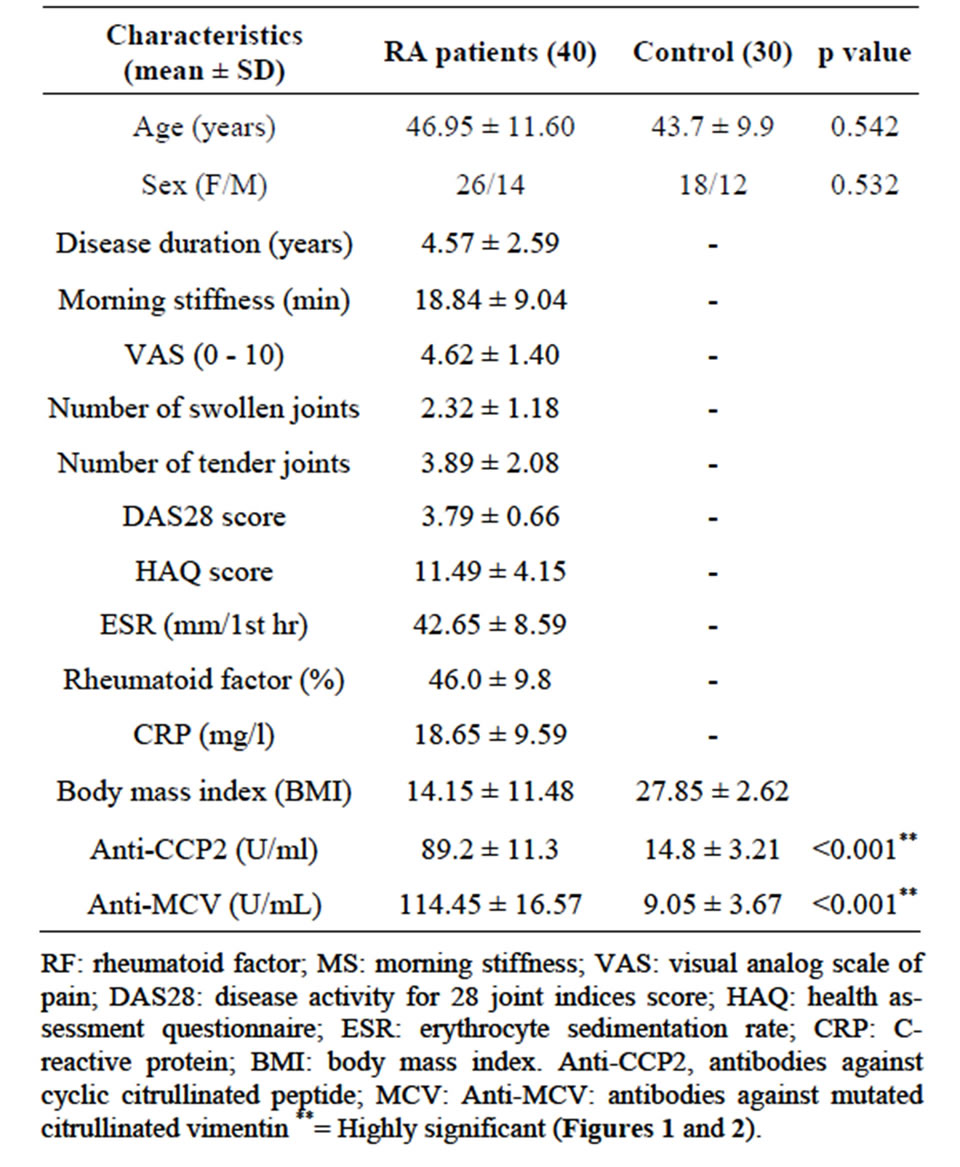
Table 1. Demographic and clinical characteristics of rheumatoid arthritis (RA) patients and control.
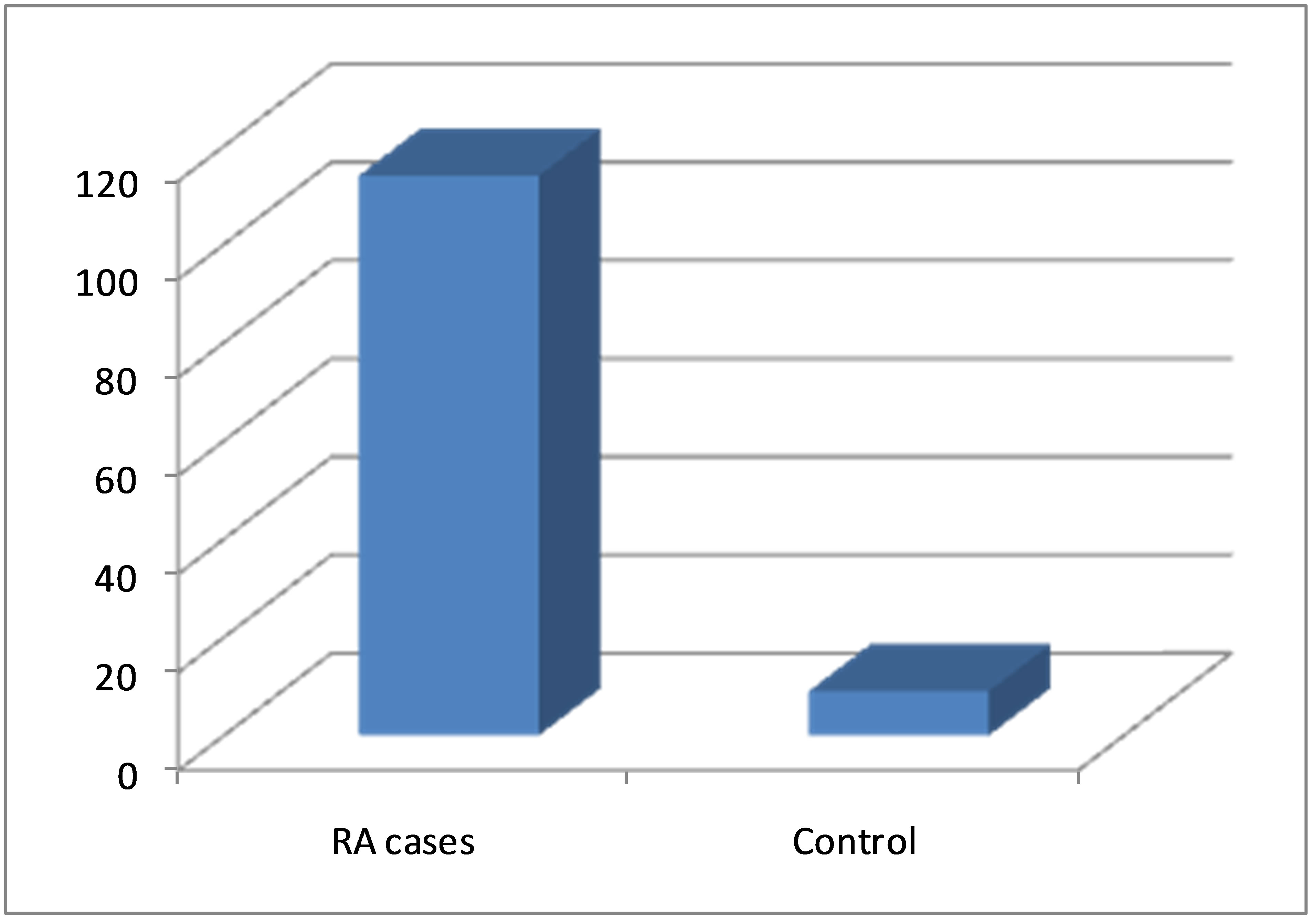
Figure 1. Serum anti-MCV levels in patients with RA and in controls.
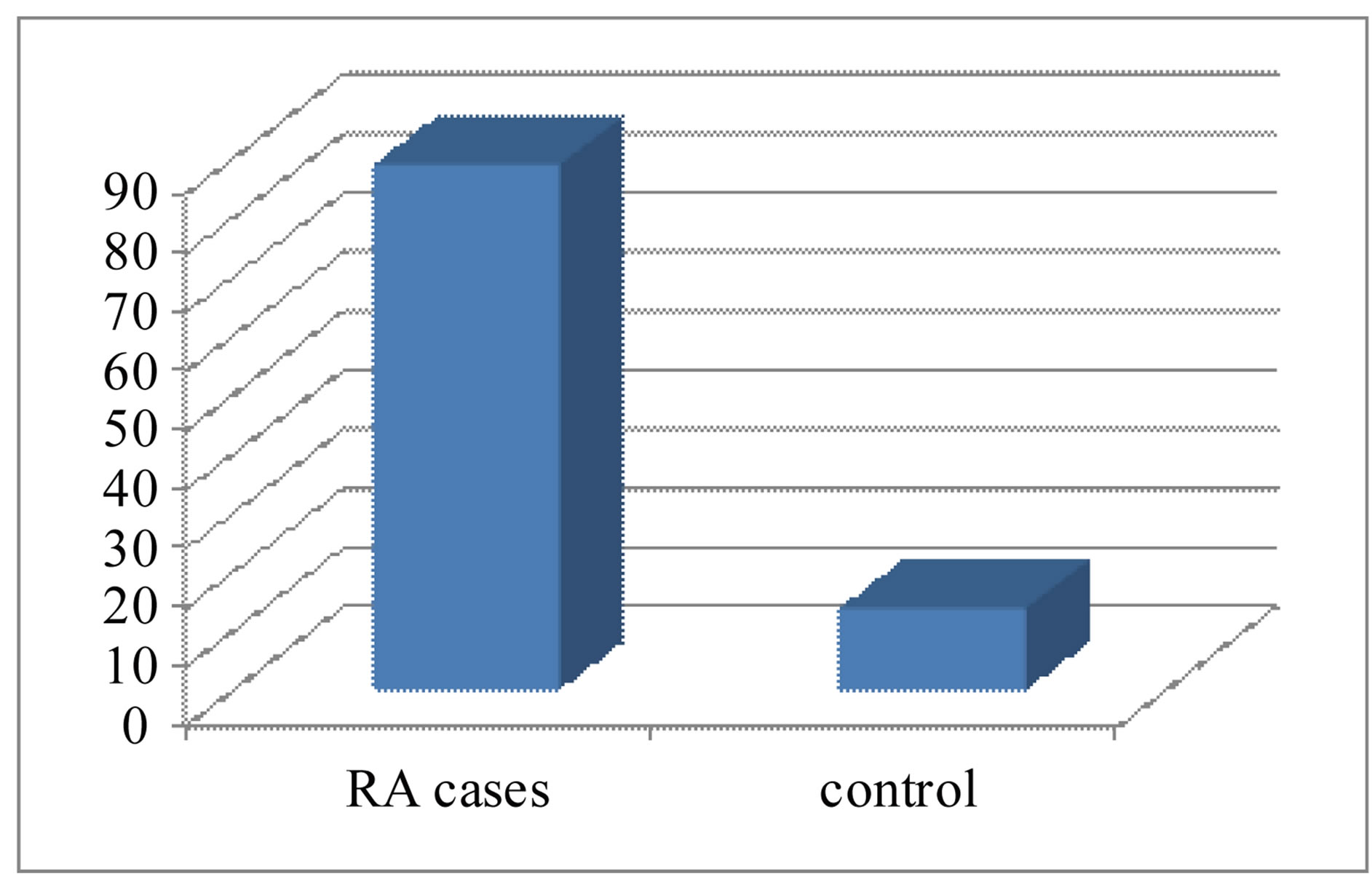
Figure 2. Serum anti-CCP2 levels in patients with RA and in controls.
Also, the anti-MCV levels (114.45 ± 16.57 U/ml) were higher (p < 0.001) in RA patients compared to healthy control subjects (9.05 ± 3.6 U/ml) Table 1.
Using Spearman’s rank correlation coefficient, serum levels of anti-MCV correlated positively but insignificantly with age, onset of RA, duration of the disease, number of tender joints and ESR. (p > 0.05) and correlated negatively with the number of swollen joints (r = −0.053, p > 0.05) in RA patients. The anti-MCV level significantly correlated with CRP, VAS and DAS 28 (r.0.084, 0.747 and 0.802, p < 0.05). The level of antiCCP2 correlated positively but insignificantly with age, onset of RA, duration of the disease, number of tender joints (p < 0.5) and significantly with VAS, DAS28, CRP, RF and ANA (p < 0.05). Anti-MCV and anti-CCP2 were significantly, correlated with each other (correlation coefficient = 0.483; p < 0.001) Table 2.
For direct comparison of the diagnostic values of the anti-MCV and the anti-CCP2 assays, we performed Receiver operator characteristic curve ROC analysis. The best cut-off value for the anti-MCV test was found at 21.5 U/mL (Figure 3). The area under the curve (AUC) was 0.893 at the 95% CI, Sensitivity was 75.6% and specificity was 93.3% indiagnosing RA patients, both positive and negative predictive values were (82.8% and 87.4%, respectively). At cut-off value 20 U/mL for the anti CCP2 test it was found that, the area under the curve was AUC 0.926 at the 95% CI, Sensitivity was 85.71% and specificity was 98.1% in diagnosing RA patients positive and negative predictive values were (86% and 93%, respectively) (Table 3 and Figure 4).
The sensitivity of each test is plotted against one minus specificity for varying cutoffs (values lower than the cutoff were considered negative, and other values were considered positive) (n = 40).
6. Discussion
Therapeutic intervention early in the course of RA leads to more efficient disease control, less joint damage, and better prognosis of disease outcome [20]. So, it is of a particular importance to identify a sensitive diagnostic marker which permits early definitive diagnosis of RA. Several reports have demonstrated high diagnostic value of antibodies directed against citrullinated proteins in the
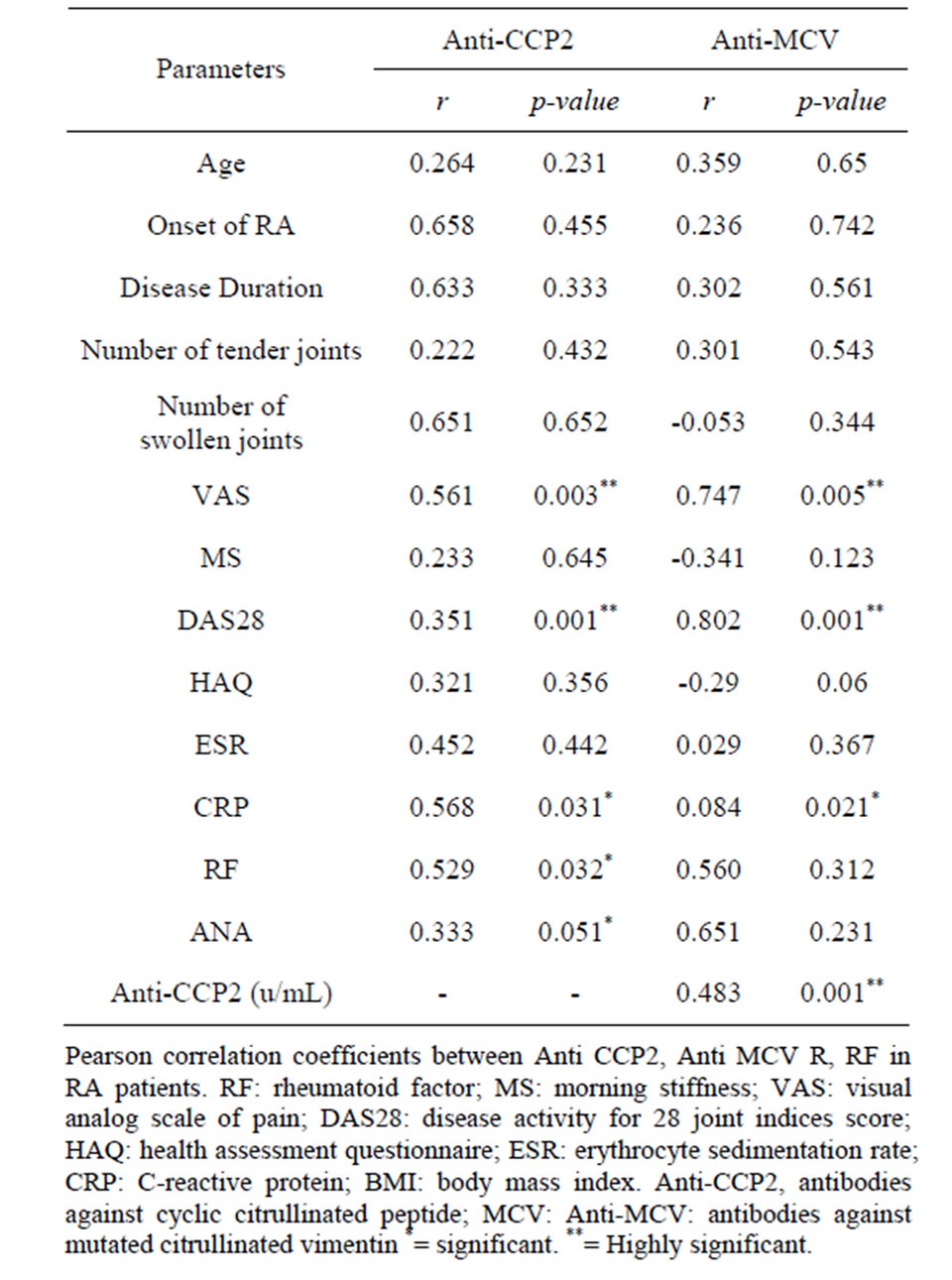
Table 2. Correlations between serums Anti CCP2, AntiMCV and patients’ characteristics.
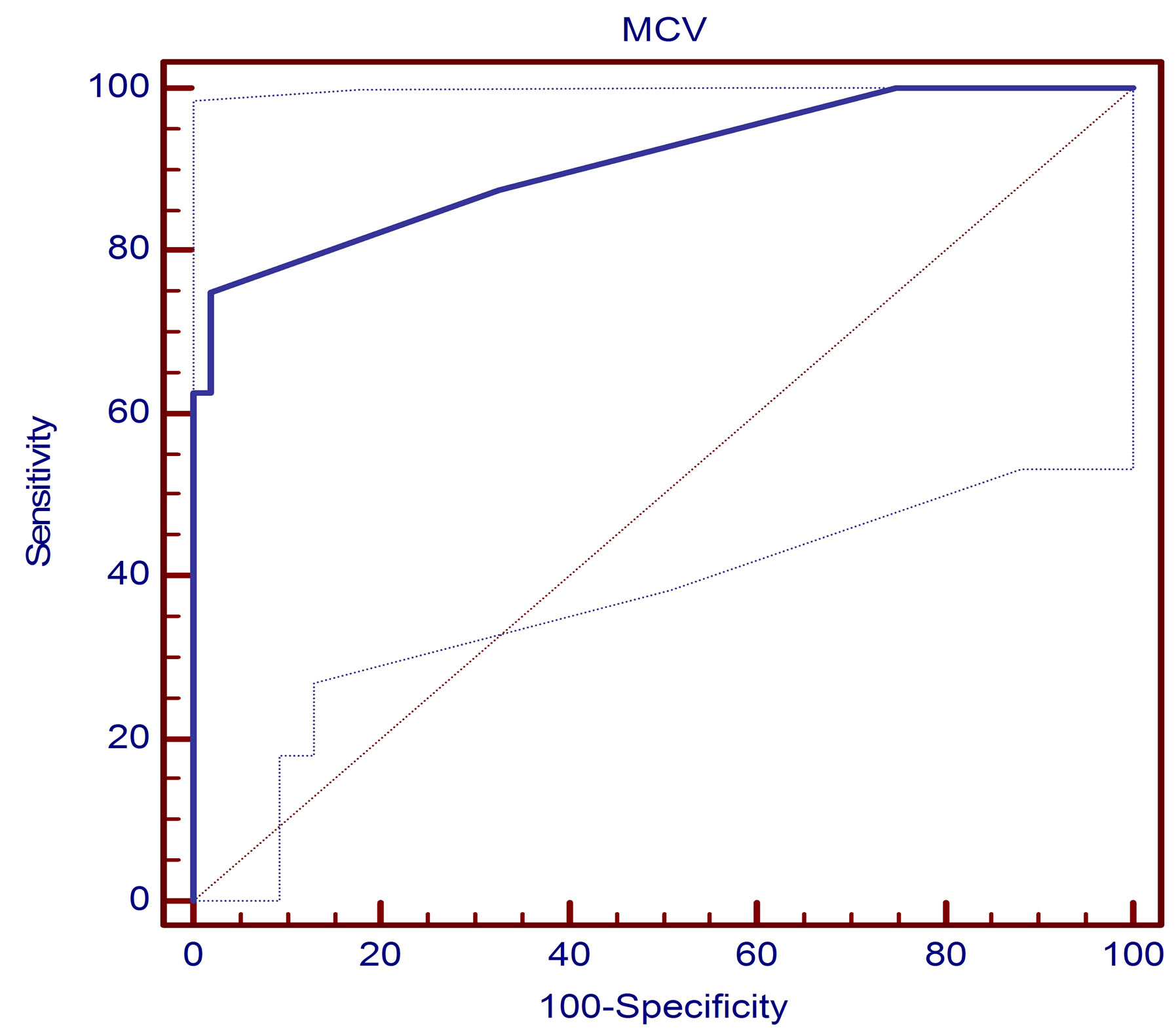
Figure 3. Receiver operator characteristic curve (ROC) assessing the validity of the anti-MCV test in diagnosing RA. The area under the curve was 0.893 at the 95% CI. The sensitivity of each test is plotted against one minus specificity for varying cutoffs (values lower than the cutoff were considered negative, and other values were considered positive) (n = 40).
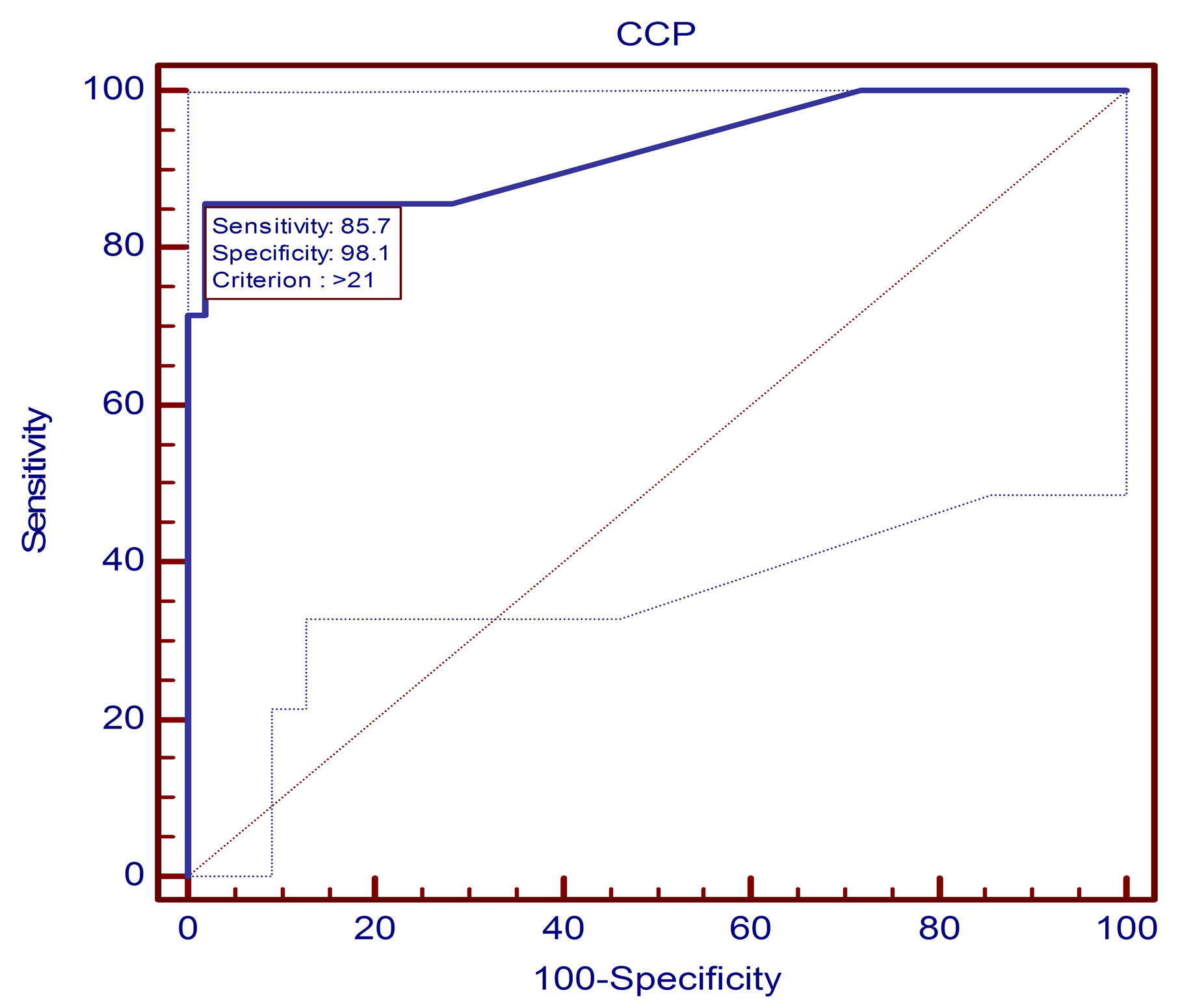
Figure 4. Receiver operator characteristic curve (ROC) assessing the validity of the anti-CCP2 test in diagnosing RA. The area under the curve was 0.923 at the 95% CI. The sensitivity of each test is plotted against one minus specificity for varying cutoffs (values lower than the cutoff were considered negative, and other values were considered positive) (n = 40).
diagnosis of RA. Although the specificity was more than 90% in most studies, the sensitivity of the same antibodies varied between 33% and 87.2%, possibly reflecting diverse genetic backgrounds and/or methodological differences in diverse antigen preparations and detection techniques applied [21].
In our study, we have shown that, the levels of antiCCP2 were significantly increased in the serum of the
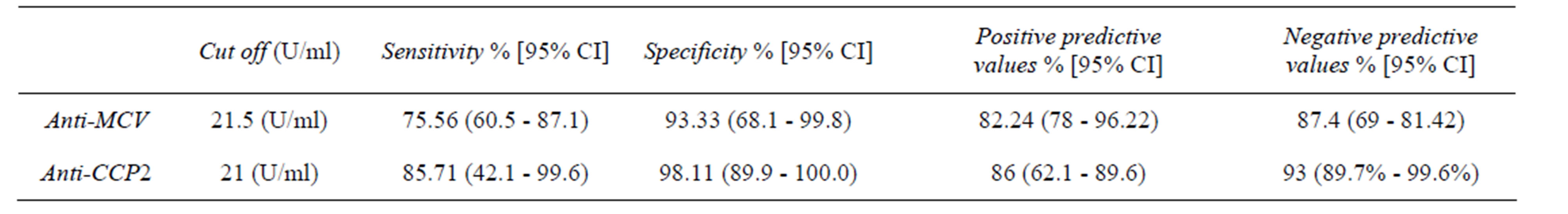
Table 3. Diagnostic effectiveness of the anti-MCV test and the anti-cyclic citrullinated peptide in RA patients.
patients with RA in comparison with the controls (p < 0.001). This results in agreement with other studies [22, 23].
The present study revealed a significantly higher serum anti-MCV antibody level in RA patients when compared to healthy controls (p < 0.001). This can be explained by the hypothesis that vimentin might trigger the initial immune response in RA [24]. It activates T lymphocytes by binding on HLA-DR4 on the surface of antigen presenting cells and may contribute to certain pathways in the pathogenesis of RA. Several studies demonstrated significant elevation in serum anti-MCV in RApatients versus controls [25-27].
In contrast to this finding, Morbach et al., [27], found no significant difference, a finding which can be explained by the fact that vimentin contains 43 arginine residues with 10 citrullination sites experimentally confirmed and anti-MCV antibodies are considered a heterogenous group of antibodies directed against different epitopes on the citrulline molecule [13].
In the present work, anti-MCV titer was significantly correlated with DAS-28, VAS and CRP (p < 0.05). Our finding is in consistent with the report of Innala et al., [28] who concluded that anti-MCV titer was significantly correlated with DAS-28, VAS and ESR. Similarly, in a three-year follow-up study of 427 RA patients, Keskin et al., [23] found that patients with active RA were found to have higher anti-MCV titers than patients with inactive disease, while, anti-CCP2 titer failed to show this correlation (a non-significant difference in anti-CCP2 titer between patients with active or inactive disease). Similarly he found that the mean serum anti-MCV levels were correlated with DAS 28, serum CRP levels, serum RF levels, swollen joints number and tender joints number (p = 0.001).
In a trial to assess the diagnostic performance of serum anti MCV antibodies in RA, the ROC curve was plotted. We found that at a cut off value of 21.5 u/ml serum anti MCV antibody had specificity and a sensitivity of 93.3% and 75.6% respectively and positive and negative predictive values were (82.8% and 87.4%, respectively). This is in consistent with the results of previous reports conducted by Damjanovska et al., Soos et al., and Mathsson et al., [26,29,30]. So, detection of anti-MCV antibodies has been shown to provide a sensitivity of 62% - 84% and specificity of 83% - 95% for the diagnosis of RA. Poulsom and Charles [10] found a specificity and sensitivity of 87% and 84% respectively, while Dejaco and associates [15] reported a result of 90.8% and 69.5% respectively. However, at a cut off value of 25 u/ml, Bang and colleagues [13] reported the specificity and sensitivity to be 88% and 82% respectively. Variation in the sensitivity and specificity can be attributed to the fact that some of the studies included patients with undifferentiated arthritis and psoriatic arthritis [31].
In the present study, out of 40 RA patients, 31 (77.5%) tested positive for anti-MCV. Anti-MCV sensitivity and specificity were 75.6% and 93.3%, respectively while anti-CCP2 was of sensitivity was 85.71% and specificity was 98.1% in diagnosing RA patients, similar to our finding a cutoff value of 20.0 U/ml (as recommended by the manufacturer), sensitivity and specificity of the anti-MCV ELISA were 69.5% and 90.8%, respectively, compared with 70.1% and 98.7% of the anti-CCP2 assay Dejaco et al., [15].
While, Egerer et al., [19] at cut-off value 20.5 found that anti-MCV antibodies in 1151 RA patients, have the same specificity as anti-CCP2 antibodies but with much better sensitivity (82% versus 72%). Similarly, Mathsson et al., and Gross et al., [20,26] who concluded that the anti-MCV assay extends the diagnostic spectrum for RA with higher sensitivity and prognostic value concerning radiological progression than anti-CCP2 antibodies (71% versus 58%, respectively).
7. Conclusion
In conclusion, Anti-MCV antibodies can be regarded as a promising diagnostic marker in RA patients with high sensitivity and specificity Autoantibodies to citrullinated proteins are specific for the diagnosis of RA. Antibodies directed against a modified citrullinated vimentin that demonstrates comparable overall diagnostic performance in relation to the anti-CCP2. In the high specificity range of both tests, which is clinically the most relevant, the antiCCP2 appears to be superior to the anti-MCV assay. Serum anti-MCV antibody could be a promising tool in diagnosing RA, show a significant link with disease activity.
REFERENCES
- J. H. Pedersen-Lane, R. B. Zurier and D. A. Lawrence, “Analysis of the Thiol Status of Peripheral Blood Leukocytes in Rheumatoid Arthritis Patients,” Journal of Leukocyte Biology, Vol. 81, No. 4, 2007, pp. 934-941. http://dx.doi.org/10.1189/jlb.0806533
- G. Serre, “Autoantibodies to Filaggrin/Deiminated Fibrin (AFA) Are Useful for the Diagnosis and Prognosis of Rheumatoid Arthritis, and Are Probably Involved in the Pathophysiology of the Disease,” Joint Bone Spine, Vol. 68, No. 2, 2001, pp. 103-105. http://dx.doi.org/10.1016/S1297-319X(01)00259-7
- T. Mottonen, L. Paimela, M. Leirisalo-Repo, H. Kautiainen, J. Ilonen and P. Hannonen, “Only High Disease Activity and Positive Rheumatoid Factor Indicate Poor Prognosis in Patients with Early Rheumatoid Arthritis Treated with ‘Sawtooth’ Strategy,” Annals of the Rheumatic Diseases, Vol. 57, No. 9, 1998, pp. 533-539. http://dx.doi.org/10.1136/ard.57.9.533
- F. C. Arnett, S. M. Edworthy, D. A. Bloch, et al., “The American Rheumatism Association 1987 Revised Criteria for the Classification of Rheumatoid Arthritis,” Arthritis & Rheumatism, Vol. 31, No. 3, 1988, pp. 315-324. http://dx.doi.org/10.1002/art.1780310302
- I. Vallbracht, J. Rieber, M. Oppermann, F. Forger, U. Siebert and K. Helmke, “Diagnostic and Clinical Value of Anti-Cyclic Citrullinated Peptide Antibodies Compared with Rheumatoid Factor Isotypes in Rheumatoid Arthritis,” Annals of the Rheumatic Diseases, Vol. 63, No. 9, 2004, pp. 1079-1084. http://dx.doi.org/10.1136/ard.2003.019877
- G. A. Schellekens, B. A. de Jong, F. H. van den Hoogen, L. B. van de Putte and W. J. van Venrooij, “Citrulline Is an Essential Constituent of Antigenic Determinants Recognized by Rheumatoid Arthritis-Specific Autoantibodies,” The Journal of Clinical Investigation, Vol. 101, No. 1, 1998, pp. 273-281. http://dx.doi.org/10.1172/JCI1316
- B. Harrison, W. Thomson, D. Symmons, et al., “The Influence of HLA-DRB1 Alleles and Rheumatoid Factor on Disease Outcome in an Inception Cohort of Patients with Early Inflammatory Arthritis,” Arthritis & Rheumatism, Vol. 42, No. 10, 1999, pp. 2174-2183. http://dx.doi.org/10.1002/1529-0131(199910)42:10<2174::AID-ANR19>3.0.CO;2-G
- E. Lindqvist, K. Eberhardt, K. Bendtzen, D. Heinegard and T. Saxne, “Prognostic Laboratory Markers of Joint Damage in Rheumatoid Arthritis,” Annals of the Rheumatic Diseases, Vol. 64, No. 2, 2005, pp. 196-201. http://dx.doi.org/10.1136/ard.2003.019992
- E. R. Vossenaar, T. R. Radstake, A. van der Heijden, et al., “Expression and Activity of Citrullinating Peptidylarginine Deiminase Enzymes in Monocytes and Macrophages,” Annals of the Rheumatic Diseases, Vol. 63, No. 4, 2004, pp. 373-381. http://dx.doi.org/10.1136/ard.2003.012211
- H. Poulsom and P. J. Charles, “Antibodies to Citrullinated Vimentin Are a Specific and Sensitive Marker for the Diagnosis of Rheumatoid Arthritis,” Clinical Reviews in Allergy & Immunology, Vol. 34, No. 1, 2008, pp. 4-10. http://dx.doi.org/10.1007/s12016-007-8016-3
- J. Ursum, M. M. Nielen, D. van Schaardenburg, et al., “Antibodies to Mutated Citrullinated Vimentin and Disease Activity Score in Early Arthritis: A Cohort Study,” Arthritis Research & Therapy, Vol. 10, No. 1, 2008, p. R12. http://dx.doi.org/10.1186/ar2362
- J. T. Cassidy, J. E. Levinson, J. C. Bass, et al., “A Study of Classification Criteria for a Diagnosis of Juvenile Rheumatoid Arthritis,” Arthritis & Rheumatism, Vol. 29, No. 2, 1986, pp. 274-281. http://dx.doi.org/10.1002/art.1780290216
- H. Bang, K. Egerer, A. Gauliard, et al., “Mutation and Citrullination Modifies Vimentin to a Novel Autoantigen for Rheumatoid Arthritis,” Arthritis & Rheumatism, Vol. 56, No. 8, 2007, pp. 2503-2511. http://dx.doi.org/10.1002/art.22817
- R. Sghiri, E. Bouajina, D. Bargaoui, et al., “Value of Anti-Mutated Citrullinated Vimentin Antibodies in Diagnosing Rheumatoid Arthritis,” Rheumatology International, Vol. 29, No. 1, 2008, pp. 59-62. http://dx.doi.org/10.1007/s00296-008-0614-8
- C. Dejaco, W. Klotz, H. Larcher, C. Duftner, M. Schirmer and M. Herold, “Diagnostic Value of Antibodies against a Modified Citrullinated Vimentin in Rheumatoid Arthritis,” Arthritis Research & Therapy, Vol. 8, No. 4, 2006, p. R119. http://dx.doi.org/10.1186/ar2008
- R. K. Mallya and B. E. Mace, “The Assessment of Disease Activity in Rheumatoid Arthritis Using a Multivariate Analysis,” Rheumatology and Rehabilitation, Vol. 20, No. 1, 1981, pp. 14-17. http://dx.doi.org/10.1093/rheumatology/20.1.14
- M. L. Prevoo, M. A. van ‘t Hof, H. H. Kuper, M. A. van Leeuwen, L. B. van de Putte and P. L. van Riel, “Modified Disease Activity Scores That Include Twenty-EightJoint Counts. Development and Validation in a Prospective Longitudinal Study of Patients with Rheumatoid Arthritis,” Arthritis & Rheumatism, Vol. 38, No. 1, 1995, pp. 44-48. http://dx.doi.org/10.1002/art.1780380107
- G. Kleveland, T. Egeland and T. Lea, “Quantitation of Rheumatoid Factors (RF) of IgM, IgA and IgG Isotypes by a Simple and Sensitive ELISA. Discrimination between False and True IgG-RF,” Scandinavian Journal of Rheumatology, Vol. 75, 1988, pp. 15-24.
- K. Egerer, E. Feist and G. R. Burmester, “The Serological Diagnosis of Rheumatoid Arthritis: Antibodies to Citrullinated Antigens,” Deutsches Ärzteblatt International, Vol. 106, No. 10, 2009, pp. 159-163.
- W. L. Gross, F. Moosig and P. Lamprecht, “Anticitrullinated Protein/Peptide Antibodies in Rheumatoid Arthritis,” Deutsches Ärzteblatt International, Vol. 106, No. 10, 2009, pp. 157-158.
- T. Mimori, “Clinical Significance of Anti-CCP Antibodies in Rheumatoid Arthritis,” Internal Medicine, Vol. 44, No. 11, 2005, pp. 1122-1126. http://dx.doi.org/10.2169/internalmedicine.44.1122
- T. R. Mikuls, J. R. O’Dell, J. A. Stoner, et al., “Association of Rheumatoid Arthritis Treatment Response and Disease Duration with Declines in Serum Levels of IgM Rheumatoid Factor and Anti-Cyclic Citrullinated Peptide Antibody,” Arthritis & Rheumatism, Vol. 50, No. 12, 2004, pp. 3776-3782. http://dx.doi.org/10.1002/art.20659
- G. Keskin, A. Inal, D. Keskin, et al., “Diagnostic Utility of Anti-Cyclic Citrullinated Peptide and Anti-Modified Citrullinated Vimentin Antibodies in Rheumatoid Arthritis,” Protein & Peptide Letters, Vol. 15, No. 3, 2008, pp. 314-317. http://dx.doi.org/10.2174/092986608783744153
- A. J. Zendman, W. J. van Venrooij and G. J. Pruijn, “Use and Significance of Anti-CCP Autoantibodies in Rheumatoid Arthritis,” Rheumatology (Oxford), Vol. 45, No. 1, 2006, pp. 20-25. http://dx.doi.org/10.1093/rheumatology/kei111
- X. Liu, R. Jia, J. Zhao and Z. Li, “The Role of Anti-Mutated Citrullinated Vimentin Antibodies in the Diagnosis of Early Rheumatoid Arthritis,” Journal of Rheumatology, Vol. 36, No. 6, 2009, pp. 1136-1142. http://dx.doi.org/10.3899/jrheum.080796
- L. Mathsson, M. Mullazehi, M. C. Wick, et al., “Antibodies against Citrullinated Vimentin in Rheumatoid Arthritis: Higher Sensitivity and Extended Prognostic Value Concerning Future Radiographic Progression as Compared with Antibodies against Cyclic Citrullinated Peptides,” Arthritis & Rheumatism, Vol. 58, No. 1, 2008, pp. 36-45. http://dx.doi.org/10.1002/art.23188
- H. Morbach, H. Dannecker, T. Kerkau and H. J. Girschick, “Prevalence of Antibodies against Mutated Citrullinated Vimentin and Cyclic Citrullinated Peptide in Children with Juvenile Idiopathic Arthritis,” Clinical and Experimental Rheumatology, Vol. 28, No. 5, 2010, p. 800.
- L. Innala, H. Kokkonen, C. Eriksson, E. Jidell, E. Berglin and S. R. Dahlqvst, “Antibodies against Mutated Citrullinated Vimentin Are a Better Predictor of Disease Activity at 24 Months in Early Rheumatoid Arthritis than Antibodies against Cyclic Citrullinated Peptides,” Journal of Rheumatology, Vol. 35, No. 6, 2008, pp. 1002-1008.
- L. Damjanovska, M. M. Thabet, E. W. Levarth, et al., “Diagnostic Value of Anti-MCV Antibodies in Differentiating Early Inflammatory Arthritis,” Annals of the Rheumatic Diseases, Vol. 69, No. 4, 2010, pp. 730-732. http://dx.doi.org/10.1136/ard.2009.108456
- L. Soos, Z. Szekanecz, Z. Szabo, et al., “Clinical Evaluation of Anti-Mutated Citrullinated Vimentin by ELISA in Rheumatoid Arthritis,” Journal of Rheumatology, Vol. 34, No. 8, 2007, pp. 1658-1663.
- R. E. Petty, T. R. Southwood, P. Manners, et al., “International League of Associations for Rheumatology Classification of sion, Edmonton, 2001,” Journal of Rheumatology, Vol. 31, No. 2, 2004, pp. 390-392.

