Natural Resources
Vol.3 No.2(2012), Article ID:20218,7 pages DOI:10.4236/nr.2012.32006
Use of Prosopis laevigata Seed Gum and Opuntia ficus-indica Mucilage for the Treatment of Municipal Wastewaters by Coagulation-Flocculation
![]()
1Department of Bioprocesses, Unidad Profesional Interdisciplinaria de Biotecnología UPIBI-Instituto Politécnico Nacional, Mexico City, Mexico; 2Departamento de Energía, Universidad Autónoma Metropolitana, Unidad Azcapotzalco, Mexico City, Mexico.
Email: ltorresbustillos@gmail.com
Received December 6th, 2011; revised February 14th, 2012; accepted February 22nd, 2012
Keywords: Biopolymers; Coagulation; Flocculation; Opuntia ficus Mucilage; Prosopis laevigata Seed Gum; Wastewaters
ABSTRACT
Prosopis laevigata and Opuntia ficus-indica grow in arid and semiarid regions of Mexico and other countries. Both produce biopolymers with interesting characteristics from the rheological point of view as well as because of their coagulating-flocculating capabilities. Prosopis produce galactomannans inside the endosperm, very similar to those found in guar, locust bean, and tara gums. Opuntia sp. produces mucilage that contains polygalacturonic acid and five neutral sugars. Prosopis seed gum has not been proposed to be used as coagulant-flocculant before. In the case of Opuntia mucilage, some authors have suggested its use in the treatment of waters, using either the mucilage or the whole cladode powder. The use of these products in the treatment of municipal or even industrial wastewaters could give rise to diverse benefits. From the environmental point of view, treated waters with neither Fe nor Al, nor synthetic polymers would be obtained (with less toxicity risk). Besides, the produced sludges would be smaller in amount, with better biodegradability, and lower metals content. From the economical point of view, the use of these biopolymers would give an added value to the Opuntia and Prosopis culture in Mexico, helping small communities to enhance their incomes by producing environmental-friendly products. This work shows that both Prosopis galactomannan and Opuntia mucilage can be used to treat municipal wastewaters with an initial organic charge of about 827 mg/L as COD by the coagulation-flocculation process, with COD removals for the mesquite seed gum of up to 90% (pH 10, dose of 75 mg/L) and of 60% (pH 7, doses of 50 and 150 mg/L). In the case of mucilage, 65% of the initial COD was removed at pH 10 (dose of 50 mg/L). These figures are very promising for the treatment of wastewaters, with environmental-friendly products.
1. Introduction
Prosopis laevigata (Humb. Et Bonpl. Ex Wild), also known as Prosopis dulcis, Mimosa rotundata, Neltuma laevigata and Acacia laevigata (among others), is a tree with maximum height of up to 13 m and a diameter of 0.8 m (Figure 1). This tree is widely distributed in South America (Venezuela and Colombia), Panama, The Antilles and Mexico. In Mexico, Prosopis sp. is found in the pacific coast from Michoacan down to Oaxaca and near the Gulf of Mexico (i.e., in Nuevo Leon, Tamaulipas and the north of Veracruz). It is also distributed in central regions up to 2300 masl, such as San Luis Potosí, Guanajuato, Zacatecas, Durango, Coahuila and Hidalgo [1]. This plant has been introduced into India and spread all over the country particularly in the semi-arid and wastelands [2].
The whole tree is used as a source of firewood and its pods are used as fodder for cattle (sheep and goats). The endosperm portion of the seed contains galactomannan gum, very similar to guar gum.


Figure 1. Prosopis laevigata tree and pods.
The two best studied galactomannans-producing Prosopis species are P. juliflora [2,3] and P. pallida [4]. In Mexico, this species is known as mesquite. In order to differentiate the plant exudates and the gum contained inside the endosperm, this gum will be called seed gum.
The Prosopis galactomannans share many characteristics with other related galactomannans such as locust bean, guar and tara gums [4]. These characteristics include its capabilities as thickening agents, the low surface tension of gum dispersions, the tendency to form gels alone or when combined with other gums (such as carrageenan, agar and xanthan gum). Finally, galactomannans can act as a coagulant-flocculant agent for treating wastewaters and waters for human consumption [5].
Galactomanans have been obtained traditionally from Cyamopsis tetragonolobo (guar gum), Caesalipina spinosa (tara gum), and Ceratonia siliqua (locust bean gum). Besides, there are reports of other species of Leguminosae galactomannan producers such as Adenanthera pavonina, Caesalpinia pulcherrrima, Gleditsia triacanthos, and Sophora japonica [6].
Other known legume galacomannan-polysaccharides are fenugreek (from Trigonellafoenum graecum), cassia (from Casia tora), lucerne (from Medicago sativa), and clover (from Trifolium pretense) [2].
Opuntia ficus-indica is a cactaceae from arid and semiarid regions, in the form of shrub or tree up to 5 m tall, forming a sturdy trunk when aging (Figure 2). This species is native from Mexico, but it was introduced into Southern Europe, Africa and India a long time ago [7]. Traditionally, it is used for defensive hedge, as support for cochineal production of dyes (acaraminic acid), fodder, and its edible fruit, known as prickly pear, is consumed widely in Mexico. The boiled cladodes are edible and very frequently used in the Mexicans diet in dishes such as salads, soups or main dish, combined with hot sauces and meat.

Figure 2. Opuntia ficus indica shrub and fruit.
The biophysical limits for Opuntia sp. are the following. Altitude 0 - 2600 masl. Mean annual temperature 18˚C - 26˚C. Mean annual rainfall from 150 - 600 mm [8].
The mucilage extracted from the cladodes of Opuntia sp. contains basically polygalacturonic acid (very similar to pectin structure), plus residues of some sugars such as D-galactose, D-xylose, L-arabinose, L-rhamnose, and D-galacturonic acid [9]. Some authors have already suggested that Opuntia sp. mucilage has a functional component with industrial perspectives [10], and have reported papers regarding its extraction and characterization [11].
Reference [12] have reported an excellent review about pectins from Opuntia sp. Authors have established the pectin content of some species and of some fruits (for comparison purposes), as well as the sugar composition of Opuntia mucilage extracted by an alkaline process. Finally, they have discussed the properties of the obtained mucilage including gelling, rheological and physiological properties of pectin.
The use of Opuntia sp. as coagulant in water treatment has been reported by some authors [10,13]. Besides, [14] have reported the use of Opuntia streptacantha as a low cost biosorbent for lead in water treatment.
Though the use of Opuntia sp. mucilage (or the whole cladode dry powder) has been proposed as a coagulantflocculant agent, most of these works used real or simulated wastewaters (most of them simulated ones) where only changes in turbidity have proven the efficiency of Opuntia mucilage as coagulant-flocculant agent [13,15]. In another work [16], the whole cladode, dried and milled was used as a coagulant-flocculant agent with excellent results.
The coagulation-flocculation process has been applied to treat municipal and industrial wastewaters. The system has many advantages over other treatment systems. One problem associated with this methodology is the generation of residual sludges. Very frequently these sludges contain high amount of metals, since the preferred coagulants are salts of Al and Fe. These metals make the sludges difficult to treat by biological methods. Biochemical to chemical oxygen demand ratios, BOD/COD, could be low for these sludges.
Preliminary results [17] indicate that biopolymers work as coagulant-flocculant elements, producing fair values of COD, turbidity and salts removal, with slight changes in the final pH. In comparison with the use of ferric chloride, the use of polysaccharides (in particular, mesquite seed gum and Opuntia mucilage) seems to be very promising.
The use of natural coagulant-flocculants will promote more biodegradable sludges at the end of the process. In this work, the use of natural polymers such as guar, locust bean, and mesquite seed gum, as well as Opuntia indica mucilage is proposed. Guar and locust bean gums are galactomannans produced by plants from the Leguminosae genus.
Finally, the coagulation-flocculation capabilities of all these biopolymers were compared to the use of FeCl3, a chemical coagulant very frequently employed in wastewater treatment. It is important to remark that no synthetic polymer will be added after FeCl3 addition. This fact would seem disadvantageous for FeCl3, but it has been reported that the use of a synthetic polymer can promote additional COD removals of around 10% - 15% to that obtained only with FeCl3 [18].
The use of these seed gum and mucilage products obtained from plants widely distributed in arid zones of Mexico will contribute to give added value to the culture of Opuntia ficus indica and Prosopis laevigata.
2. Materials and Methods
2.1. Biopolymers
Biopolymers guar and locust bean gums (LBG) were purchased at Drogueria Cosmopolita (Mexico City, Mexico). HTPCor cosmedia guar (a cationic derivative of guar gum) was purchased at Grupo Lar (Mexico, D. F., Mexico).
Opuntia cladodes without spines were purchased in a public market (Mexico City, Mexico). Cladodes were washed repeatedly with tap water. Mucilage was produced by boiling the cladodes cut in small pieces, until the material was light green and soft (20 - 30 min). Cladodes cuts were separated from the mucilage solution using a rough sieve. Total (TS) and volatile solids (VS) were determined according to [19]. VS were used to calculate mucilage concentration.
Mesquite seed gum was produced as follows. Pods were collected in an arid region of the state of Guanajuato (Mexico). The endosperms are inside the pods, covered by a stiff layer, very similar to lentils. Endosperms were extracted using NaOH-diluted solutions. Afterwards, endosperms were milled and sieved. The white-creamy powder was washed using ethanol in a Soxhlet system, until no oil remained. The powder was dried at environmental temperature and stored in a glass flask until its use.
2.2. Jar Test Experiments
All biopolymers were employed in solutions with concentrations from 50 to 150 mg/L. Ferric chloride (J. T. Baker, Mexico) was employed for comparison purposes at the same concentrations. Jar-test equipment was used with beakers containing 1 L of wastewater. The removals of COD, turbidity and dissolved salts (measured as electrical conductivity), as well as the changes in the pH values, were measured following [19]. Municipal wastewaters were sampled from San Juan Ixhuatepec wastewater treatment plant (Estado de Mexico) at the influent, after the screens that remove large particles and big plastic materials. Sludge volumes were measured using 1-L Imhoff cones during 60 min.
3. Results and Discussion
3.1. Characterization of the Wastewaters
General characteristics of the actual municipal wastewaters are those presented in Table 1. The COD value of the stream (827 mg/L) resulted quite high for a municipal wastewater. The ratio BOD/COD resulted in 0.53, which means that about half of the present material could be degraded by microbial means. In other measurements (data not shown), it was determined that CODT/CODs is about 0.56, CODT and COD represent the total, CODs and the dissolved fraction, respectively.
The pH value of wastewater was rather acid, and conductivity was about 1900 μS. Hardness was 288 mg/L, MBAS was 4.1 mg/L and grease and oils were 230 mg/L. All metals evaluated were present at rather low values, except Cr, which was below the detection limit.
3.2. Effect of Concentration in Coagulation-Flocculation Experiments
Three experiments were carried out using the jar-test system. Four different concentrations of the biopolymer (or FeCl3) were studied (50, 75, 125, and 150 mg/L). In a second experiment, the dose was fixed at 75 mg/L and pH was modified. In a third experiment, pH was adjusted to 10 and the dose of coagulant-flocculant agents was modified.
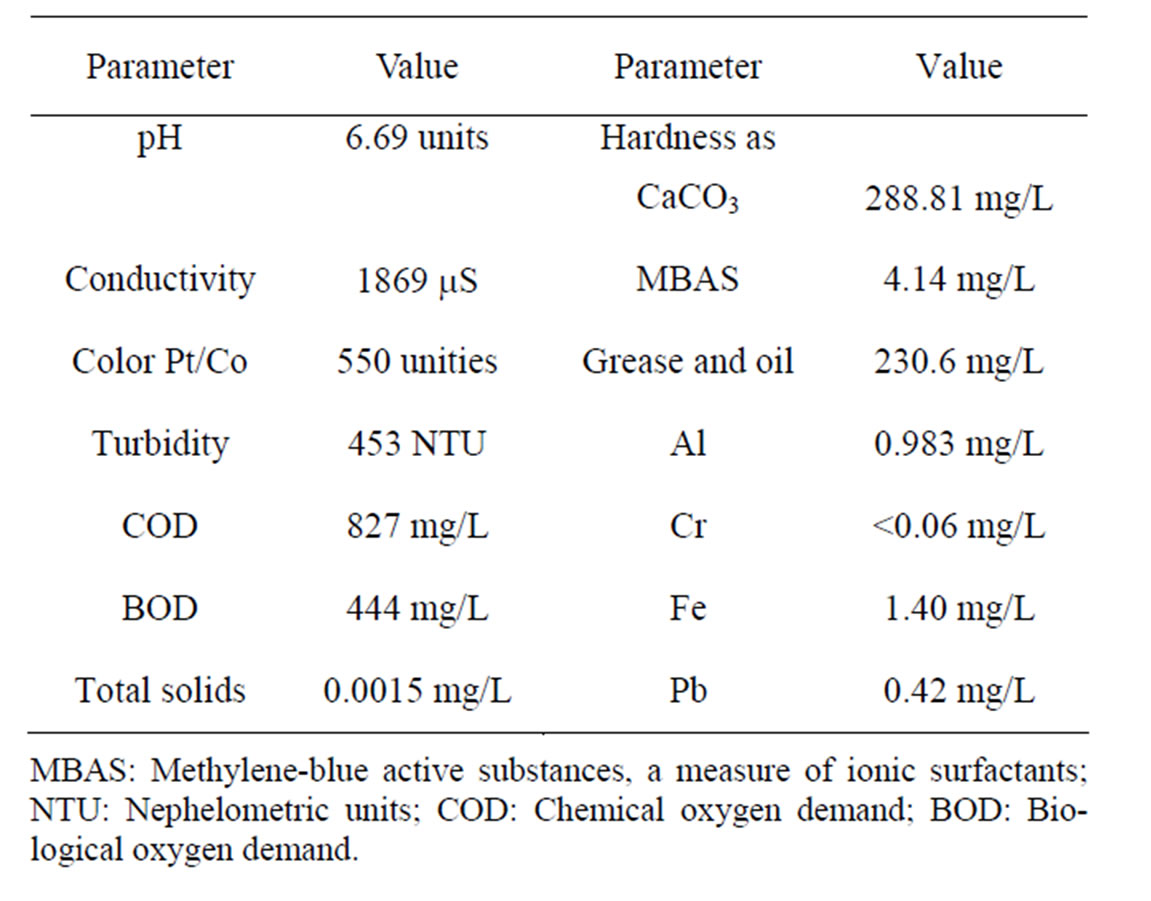
Table 1. Wastewater initial conditions.
Initial wastewater characteristics were: pH = 6.69, conductivity = 1869 mS, turbidity 453 units, and COD = 827 mg/L. Turbidity removals in the coagulation-flocculation assessments were as large as 37.2% for FeCl3 and 31.7% for guar gum and mucilage with 125 mg/L. The turbidity removal with 50 mg/L of LBG was of 26.83% and 52% for mucilage.
Results regarding COD removal (Figure 3) were as follows. Best results were achieved using FeCl3 (93%) at 150 mg/L. Regarding the natural-gums, the best result was obtained with 150 mg/L of mesquite seed gum (57.7%). Mesquite gum at 50 and 75 mg/L resulted in fairly good COD removals (57.7% and 53.2%). Note that for the two lower concentrations, the COD removal for mesquite seed gum was inversely proportional to its concentration. The latter is a good remark, since the lower the coagulant dose, the lower the wastewater treatment total cost. Using guar gum, COD removals higher than 20% at polymer concentrations of 50 and 75 mg/L were achieved. The COD removal with Opuntia mucilage was very similar to that obtained with mesquite seed gum under these conditions, except at a concentration of 150 mg/L. The concentration, 75 mg/L seems to be the optimum under the described conditions.
Although salinity removal is not a feature of the coagulation-flocculation process, in some cases dissolved salts were removed by a drag phenomenon (data not shown). Salinity removals were a function of the polymer/salt concentration. Values were quite low, i.e., between 0 and 5%. Best salinity removals were observed when using guar and locust bean gums (150 mg/L) and mesquite gum (125 mg/L).
pH is a very important issue for coagulation-flocculation processes. First, it is desirable that no pH change is necessary for the initial wastewaters treatment. Second, it is desirable that final pH values are near neutrality after
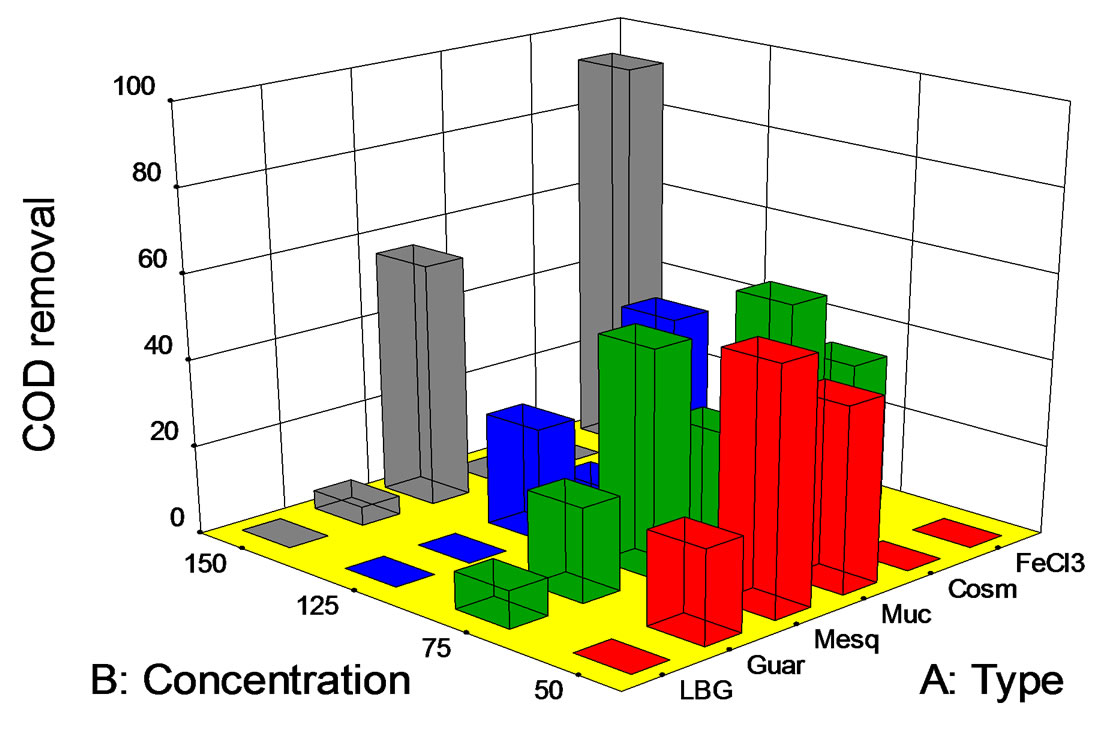
Figure 3. COD removal (%) at pH 7 vs. biopolymer concentration (mg/L). LBG: locust bean gum; Guar:guar gum; Mesq: mesquite gum; Muc: Opuntia mucilage; Cosm: cosmedia guar; FeCl3: ferric chloride.
the coagulation-flocculation processes. In this respect, galactomannans, as well as FeCl3, slightly increased the pH value of the treated wastewaters. On the other hand, Opuntia mucilage promoted a diminution of the pH value, up to 5.94 units when using a 125 mg/L concentration (data not shown).
Sludge production of wastewater (in mL/L) is presented in Figure 4. It can be noted that for all the polymers and FeCl3, sludge production was higher as the polymer/salt concentration increased. Unexpectedly, mucilage showed a slight COD and turbidity removal at the assessed concentrations. In fact, mucilage assessments showed the highest sludge productions at concentrations of 125 and 150 mg/L (more than 2.5 mL/L). Other important sludge productions were found when using guar gum (150 mg/L) and mesquite seed gum (125 mL/L). This issue is very interesting, since the amount of sludge produced in a real-scale process is determinant for the process. Produced sludges could be very light (low density) or heavy (high density), but useful design information is not complete if only the amount of produced sludge is reported.
It is important to remember [20] that FeCl3 works better at acidic pH values (4-5), so these assessments were influenced by that fact. In the future, new tests will be carried out using acidic values for wastewaters pH values.
3.3. Effect of pH at 75 mg/L
Note that for a fixed amount of coagulant-flocculant (75 mg/L) (Figure 5), the best result at pH = 5 for biopolymers was observed for Opuntia mucilage with approximately a 50% COD removal. In the case of pH = 7, the best result was for guar gum with a COD removal of 70%, whereas, at pH = 9, both guar and LBG obtained a 65% of COD removal. At last, for pH = 10, the best results were obtained using mesquite seed gum, with a 90% COD removal.
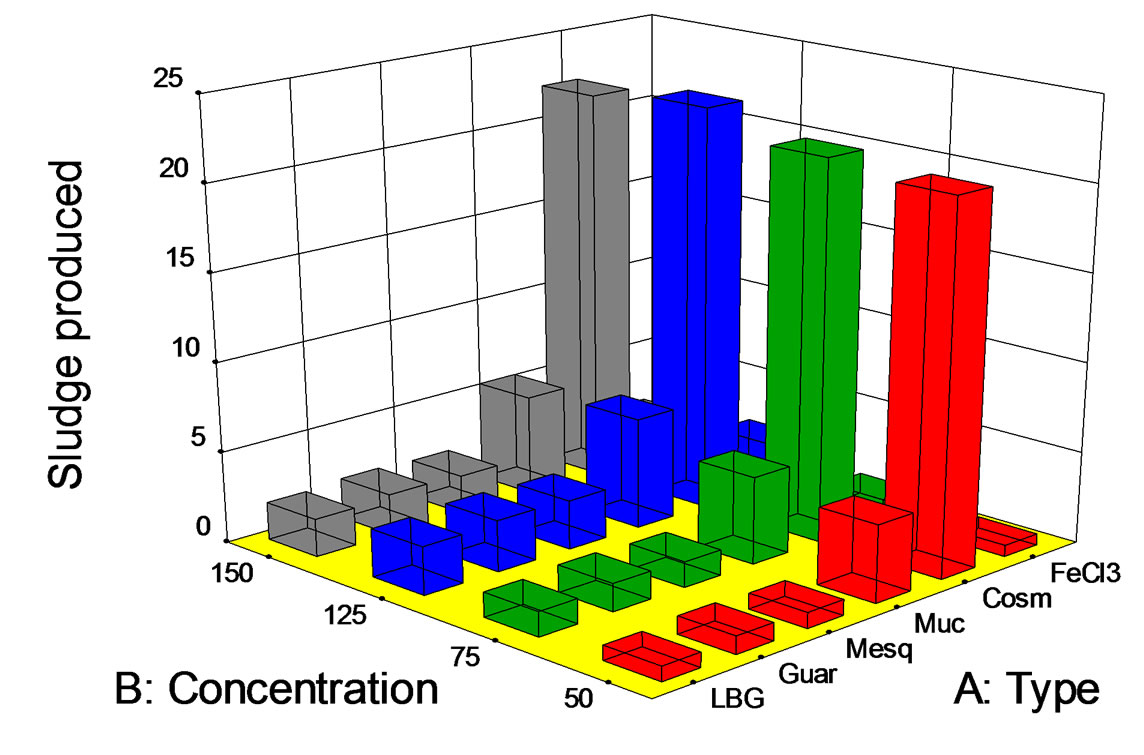
Figure 4. Sludge produced (mL/L) at pH 7 vs. biopolymer concentration (mg/L).
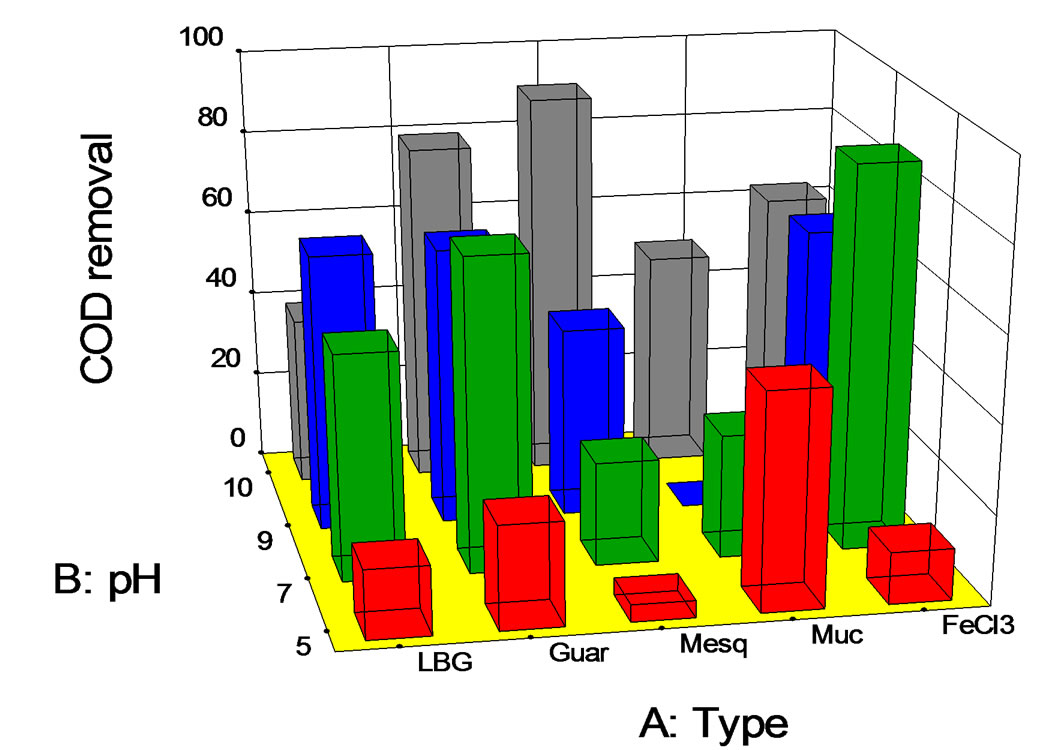
Figure 5. COD removal (%) at fixed dose of 75 mg/L vs. pH.
Sludge production for those experiments is shown in Figure 6. Sludge volumes were directly proportional to the pH value, but the relationship was not linear. For pH between 5 and 7, sludge volumes of about 10 mL/L were observed. For an alkaline pH value (9), these volumes were between 10 and 20 mL/L. Finally, for a pH value of 10, sludges were up to 40 - 75 mL/L. We hypothesize that this amount of sludge is due to the effect of the pH on the biopolymer and the interaction with colloidal material present in the wastewaters, or due to the effect of pH on the colloidal material directly. Although data are not shown, modification of the wastewater pH value promoted sedimentation of quite high amounts of solids without the addition of any salt or polymer. This could imply that the sludge production was more related with the instability of the colloidal material at alkaline pH values.
3.4. Effect of Concentration at pH = 10
The following experiments were carried out at a pH = 10 with different salt or biopolymer doses. At this point it is important to remember that modification of wastewaters pH is feasible, but it represents an operational cost. So, it will be necessary to take this into account when discussing COD removals at pH values different from the original pH value. The results of experiments at pH = 10 are presented in Figure 7. It is noticeable that COD removals were, in general, much better than those observed for the experiment with pH = 7. Secondly, it seems that all COD removals are an inverse function of salt or polymer dose.
If we take the case of Opuntia mucilage, very good COD removals were observed, i.e., 60%, 50%, and 40% for polymer doses of 50, 75, and 100 mg/L, respectively. In the case of guar gum, removals of 70%, 80% and 70% were obtained for doses of 50, 75, and 150 mg/L. Obviously, the 150 mg/L is not a valid option. On the other hand for a dose of 125 mg/L, inexplicably no COD removal was observed (Figure 7).
In the case of mesquite seed gum, attractive COD removals were obtained. For doses of 50, 75, 100, and 150 mg/L, removals of 60%, 90%, 80%, and 50% were obtained. The use of 75 mg/L of mesquite seed gum at pH = 10 yielded the best result for COD removal in the entire study.
The results of sludge production under the same conditions can be observed in Figure 8; again, sludge production (in mL/L) was very dependent on the coagulant-flocculant dose, reaching values between 40 and 85 mL/L for the whole set of experiments.
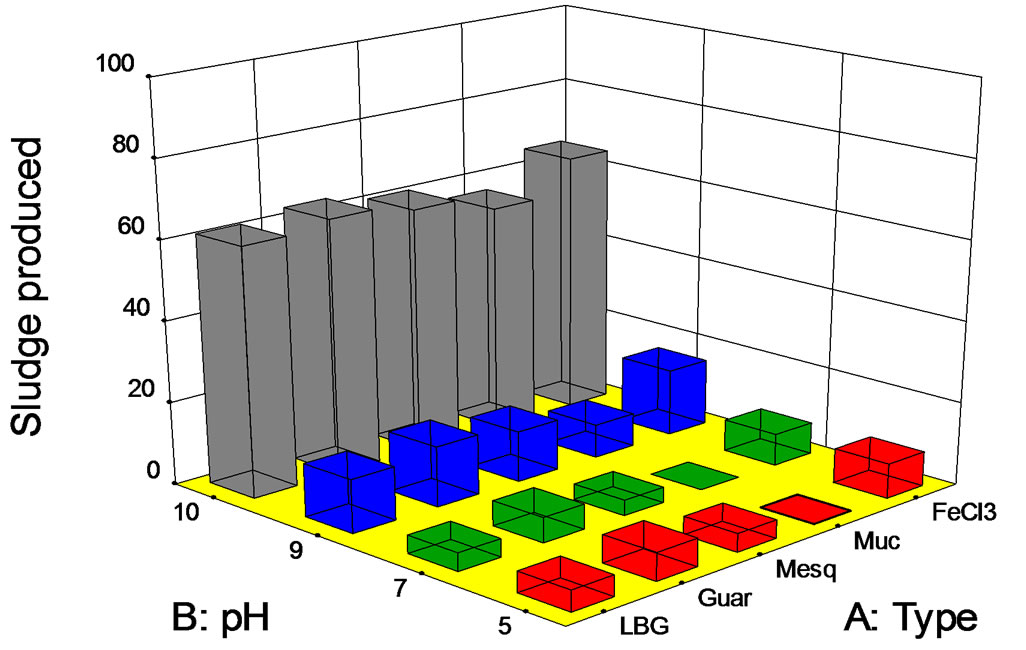
Figure 6. Sludge produced (mL/L) at fixed dose of 75 mg/L vs. pH.
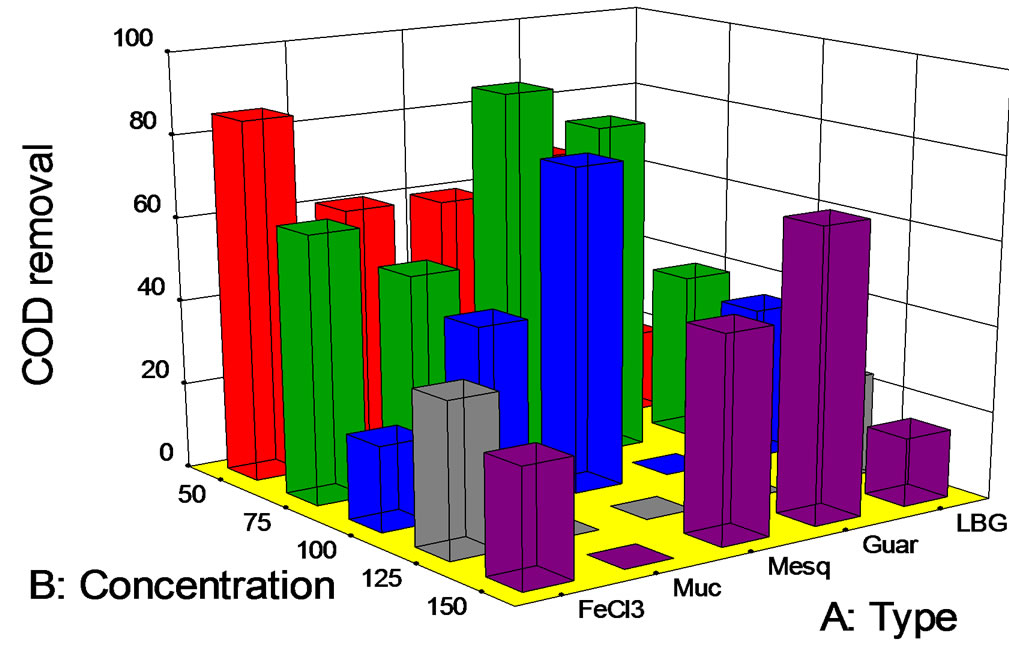
Figure 7. COD removal (%) at pH 10 vs. biopolymer concentration (mg/L).
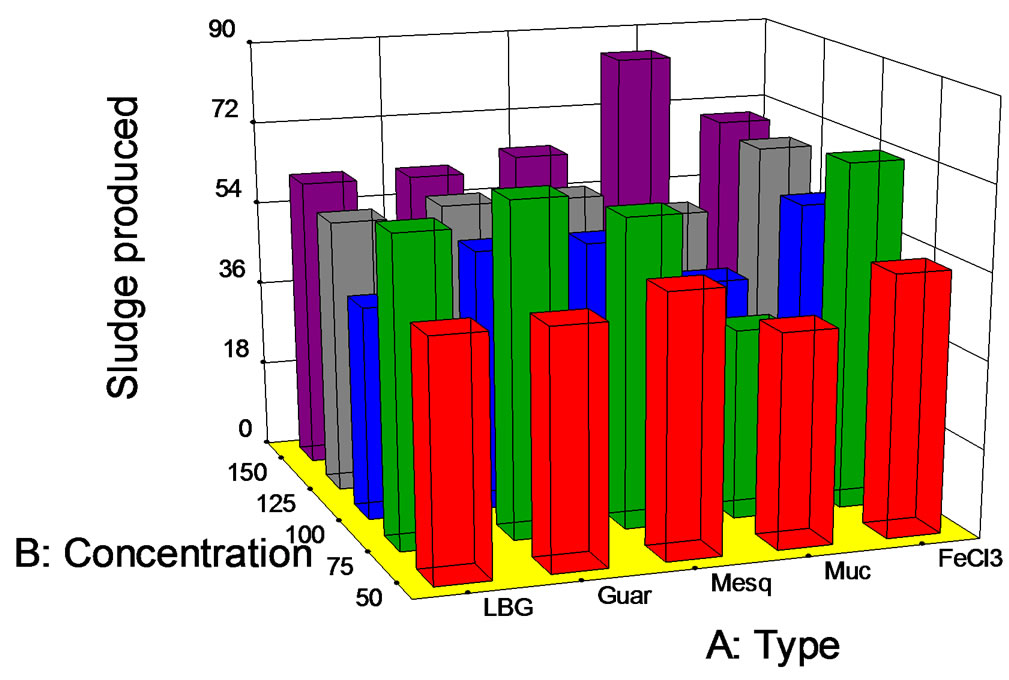
Figure 8. Sludge produced (mL/L) at pH 10 vs. biopolymer concentration (mg/L).
4. Conclusions
This work showed that all biopolymers guar, locust bean, and mesquite gums, as well as Opuntia mucilage, have potential to replace Fe or Al salts in the coagulation-flocculation process.
The COD, salt, and turbidity removals using biopolymers were quite good and comparable to those observed when using FeCl3. Sludge production was in general lower for biopolymers that those observed when using FeCl3, but it was very dependent on pH and amount of coagulant-flocculant employed.
Both Prosopis galactomannan and Opuntia mucilage were capable of treating municipal wastewaters with initial organic charges of about 827 mg/L as COD by coagulation-flocculation process with COD removals for mesquite seed gum of up to 90% (pH = 10, dose of 75 mg/L) and about 60% (pH = 7, doses of 50 and 150 mg/L). In the case of mucilage, 65% of the initial COD was removed at pH =10 (dose of 50 mg/L). These figures are very promising for the treatment of wastewaters, with environmental-friendly products.
In agreement with Yin (2010), it was corroborated that plant based coagulants can provide environmental benefits and numerous laboratory and scale studies are proving that the utilization of these products is technically feasible.
5. Acknowledgements
Funding is thanked to ICyT-DF; Grant PICSO10-8 and SIP-IPN project 2011933. Raw wastewaters were collected at San Juan Ixhuatepec (Estado de Mexico) wastewater treatment plant. Authors thank to G. Cuevas (Universidad de Guanjuato) which collected the Prosopis pods. The solvent-washing of the Prosopis bean powder and the determination of some polymer characteristics were made by L. Corzo (UPIBI-IPN).
REFERENCES
- SIRE, 2010. http://sire.uca.es/sire/idex.do.html
- V. Mathur and N. K. Mathur, “Fenugreek and Other Lesser Known Legume Galactomannan-Polysaccharides: Scope for Developments,” Journal of Scientific and Industrial Research, Vol. 64, No. 7, 2005, pp. 475-481.
- E. G. Azero and C. T. Andrade, “Characterization of Prosopis juliflora Seed Gum and Effect of Its Addition to k-Carrageenan Systems,” Journal of the Brazilian Chemical Society, Vol. 17, No. 5, 2006, pp. 844-850. doi:10.1590/S0103-50532006000500005
- L. Chairez-Martínez, J. A. Salazar-Motoya and E. G. Ramos-Ramírez, “Physicochemical and Functional Characterization of the Galactomanan Obtained from Mesquite Seeds (Prosopis palida),” European Food Research Technology, Vol. 227, No. 6, 2008, pp. 1669-1676. doi:10.1007/s00217-008-0892-0
- C.-Y. Yin, “Emerging Usage of Plant-Based Coagulants for Water and Wastewater Treatment,” Process Biochemistry, Vol. 45, No. 9, 2010, pp. 1437-1444. doi:10.1016/j.procbio.2010.05.030
- M. A. Cerqueira, A. C. Pinheiro, B. W. S. Souza, A. M. P. Lima, C. Ribeiro, C. Miranda, J. A. Teixeira, R. A. Moreira, M. A. Coimbra, M. P. Goncalves and A. A. Vicente, “Extraction, Purification and Characterization of Galactomannans from Non-Traditioal Sources,” Carbohydrate Polymers, Vol. 75, No. 3, 2009, pp. 408-414. doi:10.1016/j.carbpol.2008.07.036
- PROSEA, Internet Resources, 2010. http://www.prosea.lipi.go.id/html
- “Agro Forestry Tree Database,” 2010. http://www.worlagroforestrycentry.org/sea/Products/AFDbases/af/index.asp
- D. McGarvie and H. Parolis, “The Mucilage of Opuntia ficus-indica,” Carbohydrate Research, Vol. 69, No. 1, 1979, pp. 171-179. doi:10.1016/S0008-6215(00)85762-6
- C. Saenz, E. Sepulveda and B. Matsuhiro, “Opuntia spp. Mucilage’s: A Functional Component with Industrial Perspectives,” Journal of the Arid Environments, Vol. 57, No. 3, 2004, pp. 275-290. doi:10.1016/S0140-1963(03)00106-X
- E. Sepúlveda, C. Saez, E. Aliaga and C. Aceituno, “Extraction and Characterization of Mucilage in Opuntia spp.,” Journal of Arid Environments, Vol. 68, No. 4, 2007, pp. 534-545. doi:10.1016/j.jaridenv.2006.08.001
- F. M. Goycoolea and A. Cardenas, “Pectins from Opuntia spp.: A Short Review,” Journal of the Professional Association for Cactus Development, Vol. 2, 2003, pp. 152-159.
- J. D. Zhang, F. Zhang, Y. H. Luo and H. Yang, “A Preliminaty Study on Cactus as Coagulant in Water Treatment,” Process Biochemistry, Vol. 41, No. 3, 2006, pp. 730-733. doi:10.1016/j.procbio.2005.08.016
- P. Miretzly, C. Muñoz and A. Carrillo-Chavez, “Experimental Binding of Lead to a Low Cost on Biosorbent: Nopal (Opuntia streptacantha),” Bioresource Technology, Vol. 99, No. 5, 2008, pp. 1211-1217. doi:10.1016/j.biortech.2007.02.045
- S. M. Miller, E. J. Furgate, V. O. Craver, J. A. Smith and J. B. Zimmerman, “Towards Understanding the Efficacy and Mechanism of Opuntia spp. as a Natural Coagulant for Potential Application in Water Treatment,” Environmental Science and Technology, Vol. 42, No. 12, 2008, pp. 4274-4279. doi:10.1021/es7025054
- E. R. Bandala, F. J. Camargo and L. G. Torres, “Application of Natural Surfactants in the Treatment of Wastewaters Generated in the Hydrocarbon-Contaminated SoilWashing,” Journal of Enviromental Science and Health: Part A, 2012.
- S. Carpinteyro-Urban, J. Yáñez and L. G. Torres, “Coagulation-Flocculation of Wastewaters Employing Guar, Locust Bean and Mesquige Gums, as well as Opuntia Mucilage,” Proceedings of the 2nd IWA Mexico Young Water Professional Conference, Queretaro, Mexico, 12-14 April 2010.
- P. M. Nacheva, L. T. Bustillos, E. R. Camperos, S. L. Armenta and L. C. Vigueros, “Characterization and Coagulation-Flocculation Treatability of Mexico City Wastewater Applying Ferric Chloride and Polymers,” Water Science and Technology, Vol. 34, No. 3-4, 1996, pp. 235- 247. doi:10.1016/0273-1223(96)00579-3
- APHA, AWWA, WPFC, “Standard Methods for the Examination of Water and Wastewater,” 18th Edition, American Public Health Association, Washington DC, 1995.
- L. K. Wang, Y.-T. Hung and N. K. Shammas, “Physicochemical Treatmet Processes,” Humana Press, New York City, 2005. doi:10.1385/159259820x

