World Journal of Cardiovascular Surgery
Vol.3 No.2(2013), Article ID:32775,4 pages DOI:10.4236/wjcs.2013.32005
Factors Predictive of Right Internal Jugular Vein Cross-Sectional Area Change in Response to Trendelenburg Positioning*
Department of Anesthesiology, Division of Cardiothoracic Anesthesiology, Miller School of Medicine, University of Miami, Miami, USA
Email: #emaratea@med.miami.edu, ccastillo3@med.miami.edu, LCooper@med.miami.edu, HOlivera@med.miami.edu, EGologorsky@med.miami.edu
Copyright © 2013 Edward Maratea et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received March 22, 2013; revised April 22, 2013; accepted April 30, 2013
Keywords: Cannulation; Cardiac Surgery; Jugular Vein
ABSTRACT
Background/Purpose: The right internal jugular vein (RIJV) is the most commonly accessed central venous site in the cardiac operating room. The Trendelenburg position is frequently used to increase the cross-sectional area (CSA) of the RIJV to facilitate its cannulation. However, the extent of change of RIJV CSA in response to Trendelenburg positioning in anesthetized patients and its predictive factors remain unknown. Methods: Thirty-seven patients presented for the cardiac surgery, and 20 ASA I and II surgical patients without a history of cardiac disease (control) were studied. After induction of anesthesia, RIJV CSA was measured both at supine level position and in 10-degree Trendelenburg using vascular ultrasonography. Central venous pressure was measured in cardiac surgery patients only, since the patients in control group did not require invasive lines placement. Results and Conclusions: Body-surface area, central venous pressure, type of surgery and ejection fraction did not show any correlation with the degree of RIJV CSA change. RIJV dilation in response to Trendelenburg was significantly less pronounced, and more variable, in female patients.
1. Introduction
In cardiac surgical patients, the right internal jugular vein (RIJV) is the most commonly central venous access site [1]. Trendelenburg position is commonly used to promote venous distention to facilitate the vascular cannulation [2-8]. Jugular veins are thought to be more responsive to Trendelenburg positioning than subclavian [9]. However, factors predictive of the degree of venous dilation are largely unknown. A systematic search of the National Library of Medicine (PubMed) revealed no studies of factors predictive of, or associated with, the responsiveness of jugular veins to Trendelenburg maneuver. We, therefore, studied the factors likely associated with the jugular venous responsiveness to Trendelenburg position in anesthetized patients.
2. Methods
Institutional Review Board approval was granted for the study, and a signed consent form was obtained from each patient. Thirty-seven patients scheduled to undergo coronary artery bypass or valve surgery (cardiovascular disease (CVD) group), and 20 ASA I and II patients presented for non-cardiothoracic surgery, were enrolled over an 8-month period. Exclusion criteria included conditions that could have interfered with the responsiveness of RIJV or the ability to measure it, such as indwelling catheters, neck pathology, previous neck surgery, renal failure and congestive heart failure.
Imaging and measurement of the RIJV cross-sectional area (CSA) was accomplished using vascular ultrasonography (Sonosite, Inc., Bothwell, WA) in the supine position after induction of general anesthesia and initiation of mechanical ventilation. Measurements were obtained in level position and repeated in the 10-degree Trendelenburg. CSA of RIJV was used in lieu of its diameter due to its irregular complex shape [10]. Planimetric CSA measurements were made from the RIJV sort-axis view in the apex of the anterior triangle formed by the bifurcation of the clavicular and sternal heads of the sternocleidomastoid muscle, a site typically chosen for RIJV cannulation.
RIJV was cannulated as part of anesthetic plan in cardiac surgical patients. The central venous pressure (CVP) was measured and recorded at end-expiration in level supine position with the transducer at the level of midaxillary line. Perioperative transesophageal echocardiography (TEE) was used to estimate the left ventricular contractility, and recorded as ejection fraction (EF). No measurements of EF and CVP were made in the control group, as central venous cannulation and TEE were not indicated.
3. Statistical Methods
Categorical and continuous data are presented in Tables 1 and 2 as frequencies and percentages or means and standard deviations, respectively. Chi-square and Student’s t-test were used to compare the CVD and control groups. A linear mixed model was used to perform a repeated measures analysis of variance for the RIJV CSA
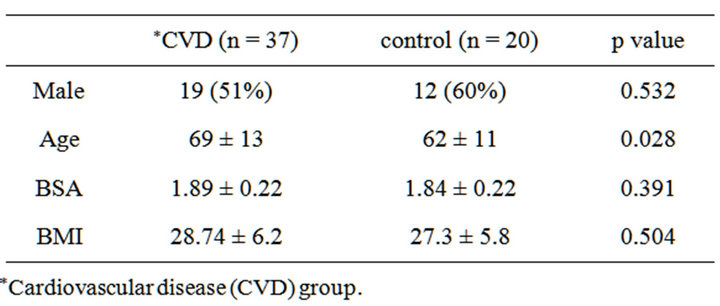
Table 1. Patient characteristics.

Table 2. CVD group: procedure, CVP, and TEE results.
data. The model included fixed effects for both groups (CVD and control) as the between-subjects factor, conditions (level and Trendelenburg) as the within-subjects factor, and the interaction between groups and conditions. Fixed covariates for age, sex, and interactions of group and condition with gender were also included in the model to control for their possible confounding effects. Subjects nested within groups were included as the random factor. An unstructured covariance matrix was used for the correlated error structure. Contrasts were used to compare conditions within groups and groups within conditions. The data are presented as adjusted means and standard errors in Table 3. SAS 9.2 (SAS Institute, Inc., Cary, NC) was used for all analyses. p = 0.05 probability level was used to determine the statistical significance of test results. Power analysis showed the required sample size of 34 to detect a significant moderate effect size of 0.5 in the change in CSA from supine to Trendelenburg at the two-tailed 0.05 level with 80% power.
4. Results
CVD and control groups were similar with regard to gender, body surface area (BSA) and body mass index (BMI). CVD patients were older than control patients, 69 vs. 62 years (Table 1). Surgical procedures, CVP and TEE findings for the CVD group are displayed in Table 2.
RIJV CSA data are shown in Table 3. In the CVD group, the CSA increased from 1.79 ± 0.14 to 2.01 ± 0.15 cm2 (p = 0.001), and in the control group from 1.82 ± 0.20 to 2.04 ± 0.23 cm2 (p = 0.013). There were no significant differences in CSA between the CVD and control groups in the supine (p = 0.905) or Trendelenburg (p = 0.935) positions, nor was there a significant difference in the CSA positional change between groups (p = 0.995). Central venous pressure had no independent effect on the size of the RIJV or its response to 10-degrees Trendelenburg. There was no correlation between the baseline CSA of the RIJV, or the dilatory response to Trendelenburg, in relation to patient age, BSA, BMI, or cardiac function. Notable was the wide inter-subject variability of the baseline (level) size of the RIJV. The observed values ranged 0.45 - 3.49 cm2 in the cohort of interest, and
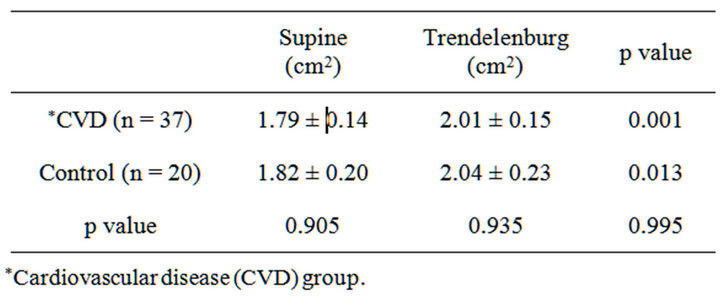
Table 3. Supine and Trendelenburg cross-sectional area for CVD and control groups.
0.55 to 3.82 cm2 in control. The response to 10-degree Trendelenburg was unpredictable and variable, ranging from −11.4% to 57.5% in the cardiac patients and from −16.9% to 66.1% in control.
Although both groups showed a statistically significant dilatory response to 10-degree Trendelenburg position, only 24% of cardiac patients and 35% of controls had a dilatory response of greater than 20%, and less than half (49% of cardiac patients and 45% of the controls) exhibited dilatory responses of greater than 10%.
Gender differences in supine CSA and in response to Trendelenburg are summarized in Table 4. Controlled for BSA and age, male patients had significantly larger RIJV, both at level (p = 0.045) and in the 10-degree Trendelenburg position (p = 0.011). In male patients increase in RIJ SCA was significant (2.09 ± 0.16 cm2 to 2.43 ± 0.18 cm2, p < 0.001). In female patients no significant dilatory response to 10-degree Trendelenburg was observed (1.51 ± 0.20 cm2 to 1.62 ± 0.22 cm2, p = 0.167).
5. Discussion
Our data suggest that the change of RIJ CSA in response to Trendelenburg position is variable and largely unpredictable, particularly in female patients. Therefore, the risks of Trendelenburg position [11-14] should be considered in the light of its inconsistent venodilatory effects and unpredictable efficacy. Potentially deleterious hemodynamic and cerebrovascular effects of Trendelenburg maneuver include increased arterial, central venous, intracerebral and intraocular pressures, decreased cerebral perfusion pressure, and the potential for respiratory and cardiac compromise, especially in patients with pulmonary hypertension and abnormal respiratory mechanics [2,13,15,16].
Our findings are consistent with previous studies [1,8, 12]. Marcus et al. [1] studied effects of various levels of PEEP and 20-degree Trendelenburg position in 50 cardiothoracic surgical patients. A 20% increase in the CSA was defined as a clinically significant response—an effect achieved only in 54% of studied patients.
We were not able to demonstrate any correlation between the CVP and RIJV responsiveness. We believe our
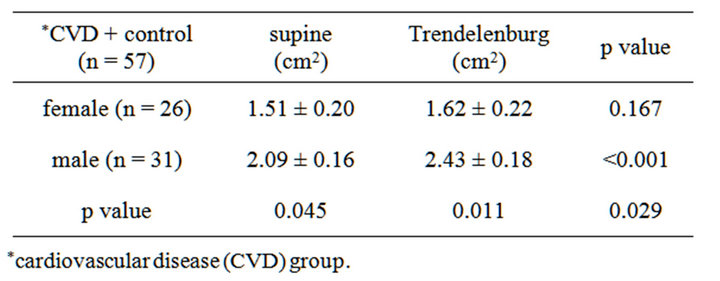
Table 4. Gender difference in supine CSA and response to Trendelenburg; CVD and control groups.
observations further support the limited value of singlepoint measured CVP in assessment of intravascular volume [17].
Our data indicate a great variability in the size of the RIJV and the absence of any correlation between the degree of RIJ CSA change with Trendelenburg maneuver and commonly assumed factors such as body habitus, CVP or level size of RIJ. Therefore, our results support the ultrasound guidance as the only evidence-supported means of increasing the success rate of RIJ cannulation and decreasing the risk of complications [9,18,19]. The ultrasound examination allows the determination not only of the baseline level size of the RIJV (commensurate with the intended cannulation), but of the dilatory response to the Trendelenburg maneuver.
REFERENCES
- H. E. Marcus, E. Bonkat, O. Dagtekin, et al., “The Impact of Trendelenburg Position and Positive End-Expiratory Pressure on the Internal Jugular Cross-Sectional Area,” Anesthesia & Analgesia, Vol. 111, No. 2, 2010, pp. 432- 436. doi:10.1213/ANE.0b013e3181e2fe41
- M. Doi and Y. Kawai, “Mechanisms of Increased Intracranial Pressure in Rabbits Exposed to Head-Down Tilt,” The Japanese Journal of Physiology, Vol. 48, No. 1, 1998, pp. 63-69. doi:10.2170/jjphysiol.48.63
- P. J. Armstrong, R. Sutherland and D. H. Scott, “The Effect of Position and Different Manoeuvres on Internal Jugular Vein Diameter Size,” Acta Anaesthesiologica Scandinavica, Vol. 38, No. 3, 1994, pp. 229-231. doi:10.1111/j.1399-6576.1994.tb03879.x
- C. Terai, H. Anada, S. Matsushima, S. Shimizu and Y. Okada, “Effects of Mild Trendelenburg on Central Hemodynamics and Internal Jugular Vein Velocity, Cross-Sectional Area, and Flow,” The American Journal of Emergency Medicine, Vol. 13, No. 3, 1995, pp. 255-258. doi:10.1016/0735-6757(95)90194-9
- E. B. Lobato, C. A. Sulek, R. L. Moody and T. E. Morey, “Cross-Sectional Area of the Right and Left Internal Jugular Veins,” Journal of Cardiothoracic and Vascular Anesthesia, Vol. 13, No. 2, 1999, pp. 136-138. doi:10.1016/S1053-0770(99)90075-7
- S. J. Schreiber, U. K. Lambert, F. Doepp and J. M. Valdueza, “Effects of Prolonged Head-Down Tilt on Internal Jugular Vein Cross-Sectional Area,” The British Journal of Anaesthesia, Vol. 89, No. 5, 2002, pp. 769-771. doi:10.1093/bja/89.5.769
- S. Clenaghan, R. E. McLaughlin, C. Martyn, S. McGovern and J. Bowra, “Relationship between Trendelenburg Tilt and Internal Jugular Vein Diameter,” Emergency Medicine Journal, Vol. 22, No. 12, 2005, pp. 867-868. doi:10.1136/emj.2004.019257
- P. Beddy, T. Geoghegan, N. Ramesh, et al., “Valsalva and Gravitational Variability of the Internal Jugular Vein and Common Femoral Vein: Ultrasound Assessment,” European Journal of Radiology, Vol. 58, No. 2, 2006, pp. 307-309. doi:10.1016/j.ejrad.2005.11.005
- C. A. Troianos, G. S. Hartman, K. E. Glas, et al., “Guidelines for Performing Ultrasound Guided Vascular Cannulation: Recommendations of the American Society of Echocardiography and the Society of Cardiovascular Anesthesiologists,” Journal of the American Society of Echocardiography, Vol. 24, No. 12. 2011, pp. 1291-1318. doi:10.1016/j.echo.2011.09.021
- M. A. Bellazzini, P. M. Rankin, R. E. Gangnon and L. P. Bjoernsen, “Ultrasound Validation of Maneuvers to Increase Internal Jugular Vein Cross-Sectional Area and Decrease Compressibility,” The American Journal of Emergency Medicine, Vol. 27, No. 4, 2009, pp. 454-459. doi:10.1016/j.ajem.2008.03.034
- A. Falabella, E. Moore-Jeffries, M. J. Sullivan, R. Nelson and M. Lew, “Cardiac Function during Steep Trendelenburg Position and CO2 Pneumoperitoneum for RoboticAssisted Prostatectomy: A Trans-Oesophageal Doppler Probe Study,” The International Journal of Medical Robotics + Computer Assisted Surgery, Vol. 3, No. 4, 2007, pp. 312-315. doi:10.1002/rcs.165
- E. B. Lobato, O. G. Florete Jr., G. B. Paige and T. E. Morey, “Cross-Sectional Area and Intravascular Pressure of the Right Internal Jugular Vein during Anesthesia: Effects of Trendelenburg Position, Positive Intrathoracic Pressure, and Hepatic Compression,” Journal of Clinical Anesthesia, Vol. 10, No. 1, 1998, pp. 1-5. doi:10.1016/S0952-8180(97)00189-X
- A. F. Kalmar, L. Foubert, J. F. Hendrickx, et al., “Influence of Steep Trendelenburg Position and CO(2) Pneumoperitoneum on Cardiovascular, Cerebrovascular, and Respiratory Homeostasis during Robotic Prostatectomy,” British Journal of Anaesthesia, Vol. 104, No. 4, 2010, pp. 433-439. doi:10.1093/bja/aeq018
- E. Y. Park, B. N. Koo, K. T. Min and S. H. Nam, “The Effect of Pneumoperitoneum in the Steep Trendelenburg Position on Cerebral Oxygenation,” Acta Anaesthesiologica Scandinavica, Vol. 53, No. 7, 2009, pp. 895-899. doi:10.1111/j.1399-6576.2009.01991.x
- G. Murthy, R. J. Marchbanks, D. E. Watenpaugh, J. U. Meyer, N. Eliashberg and A. R. Hargens, “Increased Intracranial Pressure in Humans during Simulated Microgravity,” The Physiologist, Vol. 35, No. 1, 1992, pp. 184- 185.
- P. Mavrocordatos, B. Bissonnette and P. Ravussin, “Effects of Neck Position and Head Elevation on Intracranial Pressure in Anaesthetized Neurosurgical Patients: Preliminary Results,” Journal of Neurosurgical Anesthesiology, Vol. 12, No. 1, 2000, pp. 10-14. doi:10.1097/00008506-200001000-00003
- R. C. Roy, “Central Venous Pressure in Clinical Care Algorithms: Are Anesthesiologists and Intensivists Ready?” Anesthesia and Analgesia, Vol. 11, No. 3, 2010, pp. 591- 592. doi:10.1213/ANE.0b013e3181e9f64f
- W. R. Fry, G. C. Clagett and P. T. O’Rourke, “Ultrasound-Guided Central Venous Access,” Archives of Surgery, Vol. 134, No. 7, 1999, pp. 738-740. doi:10.1001/archsurg.134.7.738
- B. G. Denys, B. F. Uretsky and P. S. Reddy, “Ultrasound-Assisted Cannulation of the Internal Jugular Vein. A Prospective Comparison to the External LandmarkGuided Technique,” Circulation, Vol. 87, No. 5, 1993, pp. 1557-1562. doi:10.1161/01.CIR.87.5.1557
NOTES
*Conflict of Interest: None; Funding: The authors have no financial disclosures as it pertains to this body of work.
#Corresponding author.

