World Journal of Cardiovascular Diseases
Vol.4 No.4(2014), Article ID:44663,8 pages DOI:10.4236/wjcd.2014.44020
The Effect of Renal Sympathetic Denervation (RSD) in Atrial Fibrillation (AF) Inducibility
Indra Prasad Upadhyay1, Jialu Hu2, Wugeti Naji Na1, Wang Kun1, Taji Di Linuer1, Zhao Li1, Alia1, Yuemei Hou3*
1Department of Cardiology, The First Teaching Hospital, Xinjiang Medical University, Urumqi, China
2Department of Cardiology, Zhongshan Hospital, Fudan University, Shanghai, China
3Department of Cardiology, The Sixth People’s Hospital (Southern) of Shanghai, Jiao Tong University, Shanghai, China
Email: *houyuemei@sina.com
Copyright © 2014 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/


Received 13 February 2014; revised 16 March 2014; accepted 24 March 2014
ABSTRACT
Objective: The purpose of this study is to investigate the effects of renal sympathetic nerve stimulation (RSN-S) and ablation (RSN-A) on atrial effective refractory period (ERP) and AF in normal canine heart. Atrial Fibrillation (AF) is a complex disease and one of the most frequent arrhythmias, especially in elderly patients. Multiple mechanisms are involved including interaction between the autonomic nervous system (ANS), electrophysiological properties of the atria, and vulnerability for AF. Cardiac overload increases the incidence of AF. In lone AF the triggers are in the pulmonary veins. AF caused by underlying disease has different mechanism. Atrial fibrillation (AF) is associated with activity of renin-angiotensin-aldosterone system (RAAS). Reduction in renal noradrenaline spillover could be achieved after renal sympathetic denervation (RSD). Methods: 1) Establish of atrial fibrillation model; 2) Ventricular rate analysis of AF; 3) Statistical analysis. Results: 1) The establishment of atrial fibrillation model; 2) Inducibility and duration of AF; 3) The changes of AERP dispersion. Conclusion: Left RSN-S shortened left atrial ERP, increased ERP dispersion, but did not change right atrial ERP. Bilateral RSN-A produced significant prolongation in both atrial ERP, but did not affect ERP dispersion. The on time of RD effect is at 4 hrs after RD procedure and the RD effect on AF will last for 20 hrs after RD procedure.
Keywords:Atrial Fibrillation, Renal Sympathetic Denervation, Catecholamine, Ablation

1. Introduction
Atrial fibrillation (AF) is one of the most frequent arrhythmias in adulthood, with its incidence increasing with age. The use of alcoholic beverages has been associated with AF—“holiday heart syndrome”. Atrial fibrillation is characterized by rapid and irregular activation of the atrium, and it is associated with increased morbidity and mortality.
The kidney receives a dense innervation of sympathetic and sensory fibres and can be both a target of sympathetic activity and a source of signals that drive sympathetic tone [1] . The renal sympathetic nerves have been identified as a major contributor to the complex pathophysiology of hypertension and atrial fibrillation in both experimental models and in humans. Patients with essential hypertension generally have increased efferent sympathetic drive to the kidneys, as evidenced by elevated rates of renal norepinephrine spillover, defined as the amount of transmitter that escapes neuronal uptake and local metabolism and thus “spills over” into the circulation. Hypertension is also characterized by an increased rate of sympathetic-nerve firing, possibly modulated by afferent signaling from renal sensory nerves.
The fundamental mechanisms underlying atrial fibrillation (AF) have long been debated, but electrical and structural remodelling are each important synergistic contributors to the AF substrate, and these changes further perpetuate the existence and maintenance of AF [2] -[4] . Much attention has been devoted in the past few years to assess the role of rennin-angiotensin-aldosterone system (RAAS) in AF [5] -[7] . Studies have shown that Angiotensin II (Ang II) and Aldosterone might be involved in atrial structural and electrical remodelling in patients with AF [8] [9] . The inhibition of the RAAS might have a protective effect on remodeling [9] . Studies have demonstrated that the cross talk between the kidney and the heart includes the upregulated sympathetic nervous system, activation of the RAAS and vasopressin release. It has been shown that compromised hemodynamics during AF leads to an increase in catecholamines and sympathetic tone with parasympathetic withdrawal [10] . The activation of sympathetic nervous system is related to the effects of circulating renin released from the kidneys. Previous study, Nakashima et al. showed that the inhibition of endogenous Ang II prevented atrial effective refractory period (AERP) shortening during rapid atrial pacing [11] .
2. Methods
The protocol of study was approved by the Ethical Committee of the Xinjiang Medical University School of Medicine, and animal handling was carried out according to the Xinjiang Directive for Animal Research. All dogs were premedicated with pentobarbital sodium (30 mg/kg IV), intubated and ventilated with room air supplemented with oxygen by a respirator (MAO01746, Harvard Apparatus Holliston, USA). Normal saline at 50 to 100 ml/h was infused to replace spontaneous fluid losses.
In 11 anesthetized dogs, Standard surface electrocardiographic leads (I, II, III, aVR, aVL and aVF) were continuously monitored, two multiple electrode catheters were placed & sutured at left and right ventricular free walls for recording. All the dogs were assigned into the RSN-S group (n = 5) and RSN-A group (n = 6). RSN-S (20 Hz, 2 ms) was delivered on the adventitia of left renal artery via an electrode at the voltage required to increase 10% of the systolic blood pressure (SBP). RSN-A was performed at more than four sites in each renal artery where high-frequency electrical stimulation (20 Hz, 2 ms) increased the SBP & obviously induced AF. Complete ablation was considered when stimulation of each target site no longer produced increase of blood pressure & induction of AF. ERP was measured at baseline and during RSN-S or after RSN-A, respectively.
2.1. Establish of Atrial Fibrillation Model
The left cervical artery and right cervical vein were dissected. A 4-Fr. sheath was inserted into the left cervical artery for condinuously monitor systolic blood pressure (via a pressure transducer). A 5-Fr. multi-electrode catheter was inserted into the right cervical vein and then advanced to the right atrial appendage for programmed stimulation and induction of AF. The AF induction rate,atrial effective refractory period (AERP), rate adapation of AERP, ventricular ejection fraction (EF), heart rate variability (HRV) were measured after pacing at 0 h, 4 h, 8 h, 12 h, 24 h. AF was defined as irregular atrial rates > 500 beats/min and a duration > 5 seconds associated with irregular atrioventricular conduction. AF lasting > 30 minutes was considered sustained.
We use WOV as a quantitative measure of AF inducibility. AF was defined as irregular atrial rates faster than 500 beats per minuters associated with irregular AV conduction lasting > 5 sec. During ERP measurements, if AF was induced by decremental S1S2 interval at which AF was induced were determined. The difference between the two was designated as the WOV. The SWOV was counted as the sum of WOV acquired all, the time for determining the ERP and WOV after each group of pacing was less than 8 min in most cases.
2.2. Ventricular Rate Analysis of AF
Ventricular rate was determined by 20 continuous R-R intervals, and then to calculate the average ventricular rate, and measure every group average ventricular rate in AF separately.
2.3. Statistical Analysis
Qualitative data was expressed as a ratio and measurement data was expressed as mean with SD. ANOVA for repeated measurements was used for comparisons of ERP and ERP dispersion among different pacing hours. The chi-square test was used to compare the AF induction rate. Values of P < 0.05 were considered statistically significant.
3. Results
3.1. The Establishment of Atrial Fibrillation Model
In RSN-S group, left RSN-S significantly shortened ERP in left atrium compared to baseline (BS) (P < 0.05 for all), but did not change ERP in right atrium (Figure 1(a)). Left RSN-S also increased atrial ERP dispersion compared to BS (P < 0.05) (Figure 1(b)). In RSN-A group, RSN-A signigicantly prolonged atrial ERP at all recording sites (P < 0.05 for all) (Figure 1(a)). However, RSN-A did not change ERP dispersion (Figure 1(b)).
The definition to induce atrial fibrillation: more than 10 times fast (>350 - 600 times/min), irregular, variable morphological atrial beat. (Figure 1)
3.2. Inducibility & Duration of AF
When compared with RSN-A (as the figure shows in AF group), in RSN-S group HRA pacing significantly increased the AF induction rate & AF duration from 8 hrs pacing to 24 hrs pacing, and the most AF induction rate was obtained at 24 hrs pacing; while no significant difference in AF induction rate was found in RSN-A (as the figure shows in control group) (Figure 2).
3.3. The Changes of AERP Adaptation & Dispersion
In RSN-S group, starting from 8 hrs of pacing the AERP decreased significantly and even more in 24 hrs of pacing. The adaptation of AERP reduced in RSN-S group compared with RSN-A group (Table 1). In the RSN-S group, the atrial ERP dispersion showed significant changes compared to the RSN-A at 8 h, 12 h, 16 h, 20 h, and 24 h (Table 2).
3.4. Atrium Diameter & Heart Functions Measurement
The atrium diameter was increased from 12 to 24 hrs of pacing in RSN-S group, but without difference compared with RSN-A group, (P > 0.05). EF decreased significantly from 12 hrs to 24 hrs compared with RSN-A (P < 0.05) (Table 3 and Table 4).
4. Discussion
This study demonstrated for the first time that episodes and duration of AF could be decreased by renal sympathetic denervation during short-time rapid atrial pacing. Decreased inducibility of AF might have associated with decreased activity of RAAS. Initial study on total efferent vagal denervation via epicardial fat pad ablation indicated that such a dedicated approach may prevent AF in dogs produced by atrial pacing [12] . Transvascular atrial parasympathetic nerve system modification by radiofrequency catheter ablation abolishes vagally mediated AF has been reported [13] . Recently, Tan et al. demonstrated that cryoablation of the superior cardiac branches of vagus nerve eliminated episodes of AF [14] . Rapid atrial pacing can facilitate the initiation and maintenance of AF with the prolonged time [15] . Previous studies have demonstrated that high-frequency pacing increases
 (a)
(a) 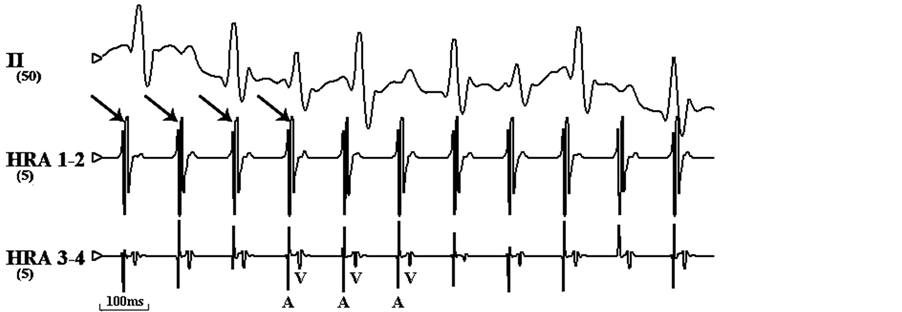 (b)
(b)
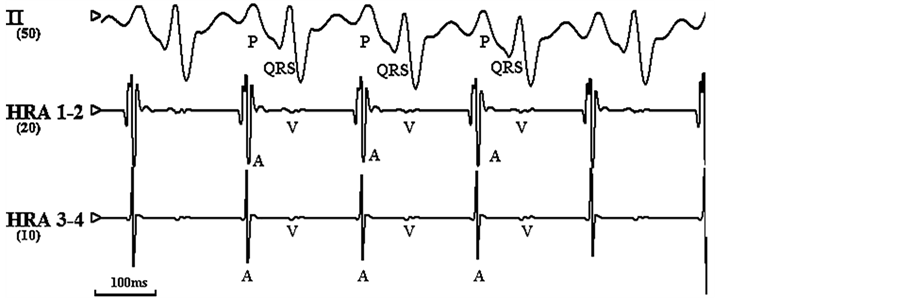
Figure 1. Electrocardiograms of surface atrial were simultaneously recorded. (a) Normal electrocardiogram of rapid before pacing. (b) Atrial fibrillation induced by rapid pacing on HRA.
Note: HRA: high right atrium; arrow means the stimulus signal.
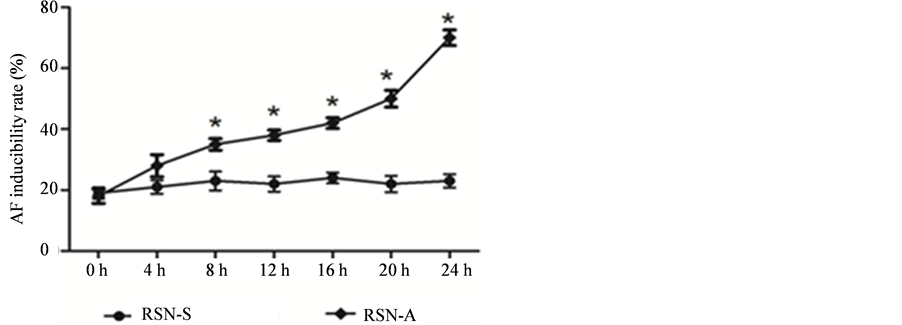
Figure 2. Inducibility of two groups at different time.
Note: compared with control group, P < 0.05.
intracellular calcium levels in cardiac myocytes, and intracellular calcium overload is thought to contribute to this phenomenon [2] [16] . Calcium channel blockade prevents AERP shortening during rapid atrial pacing [17] . It has been shown that Ang II increases the intracellular calcium concentration significantly in atrial myocytes in rat heart [18] . Recently, Laszlo et al. demonstrated that selective mineralocorticoid receptor antagonist (eplerenone) influenced ICa,L in the early tachycardia-induced atrial electrical remodelling [19] .
Table 1. AERP changes in two groups at different time (ms, ).
).
Note: AERP200, 150 indicate S1S2 cycle is 200 ms, 150 ms. note: *compare with control group, P < 0.05.
Table 2. The result of ERP dispersion ( , ms, n = 10).
, ms, n = 10).
Note: *compare with control group, P < 0.05.
Table 3. Left and right atrium diameter changes in mm, ( ).
).
Note: *compared with control group, P < 0.05.
Table 4. Heart function changes at different time (%, ).
).
Note: *compared with control group, P < 0.05.
4.1. Renal Denervation in the Treatment of AF
The kidney receives a dense innervation of sympathetic and sensory fibres and can be both a target of sympathetic activity and a source of signals that drive sympathetic tone. It is well-known that renal sympathetic stimulation induces renin release; conversely, Ang II appears to have important actions in modulating sympathetic nerve activity. The RAAS is involved in myocardial fibrosis and increased Ang II production causes marked atrial dilation with focal fibrosis and AF [8] [20] . Under AF, development and persistent sympathetic nerve and RAAS are activated and each condition predisposes to the other. Our study showed that the average duration & induced episodes of AF were longer significantly in RSN-S dogs than in renal sympathetic denervation dogs, while RD can significantly decrease AF induced by RSN-S. The onset time of RD in the treatment of AF and the lasting time of the RD effect on AF inducibility are the most clinical concern. In our study, the effect of RD on inhibition of AF induced by RSN-S started from 4 hrs of pacing and lasted till to 24 hrs pacing. In our study, RD inhibited AF induced by RSN-S could be explained by reversing atrial electrical remodeling through reducing ERP shortening and dispersion, as well as by reversing atrial structure remodeling through reducing atrial diameter and EF improvement.
4.2. Catheter-Based Renal Denervation as a New Strategy for AF Management
Despite a long history of medical exploration of AF, many aspects of the management of AF remain controversial [21] . The long-term efficacy of currently available antiarrhythmic drugs for the prevention of AF recurrence is far from ideal because of limited efficacy and potential side effects, particularly proarrhythmia [22] . Since Haissaguerre et al. reported that triggers arising from the pulmonary veins are responsible for the majority of paroxysmal AF [23] , catheter ablation of AF is now a realistic therapeutic option across a broad spectrum of patients—from patients with paroxysmal AF to those with long-lasting persistent AF [24] . Although the superiority of catheter ablation over antiarrhythmic drug therapy has been demonstrated in middle-aged patients with paroxysmal AF, the role of the procedure in other patient subgroups such as those with long-standing persistent AF has not been well-defined [25] [26] . Currently, ganglionated plexi (GP) ablation has been employed for both paroxysmal and persistent AF, and GP ablation appears to be an efficacious method to improve pulmonary vein (PV) isolation in patients with AF [27] [28] . However, ablation-induced atrial or ventricular proarrhythmia has been reported and anatomic GP modification appears to carry a higher risk of iatrogenic left atrial tachycardias than PV isolation [29] [30] . Fat pad ablation did not achieve long-term suppression of AF induction in canine model [31] [32] . We demonstrated in the present study that inducibility of AF can be decreased by renal sympathetic denervation during short-time rapid atrial pacing. The effectiveness and safety of catheter-based renal denervation had been demonstrated in patients in recent years [33] [34] .
5. Summary
Both cholinergic and adrenergic stimulation can promote the occurrence of AF, although adrenergic stimulation is much less effective than cholinergic stimulation. Interaction between adrenergic and cholinergic tone may play an important role in initiating and perpetuating AF. Simultaneous adrenergic stimulation has been shown to facilitate the induction of Ach-mediated AF. About 20% of the time atropine completely prevents catecholamine mediated AF, indicating an important role of cholinergic tone in these AF episodes. Catecholamine administration decreases the threshold of Ach concentration for AF induction and increased AF duration. In vivo, acute ANS stimulation shortens atrial and PV refractoriness, and significantly changes atrial conduction times, promoting AF induction and prolonging the arrhythmia. This underscores the importance of acute variations in ANS tone and its interactions in the pathophysiology of AF. The autonomic nerve system, particularly the vagal component, plays an important role in AF. ANS activation can result in significant changes of atrial electrophysiology and facilitate the induction of AF by both reentry and triggered activity, probably through Ca-mediated mechanisms. Modification of cardiac ANS inputs might be effective in AF control. Clinical observations show that enhanced parasympathetic tone contributes to some cases of paroxysmal AF, which were clinically referred to vagal AF. The elctrophysiological mechanisms of vagal AF mainly comprise atrial APD/ERP shortening and increased dispersion of atrial refractoriness via activation of IKACh. Interactions between vagal and adrenergic tone may also play a role in AF. It has been reported that ablation of the renal sympathetic nerves result in a marked reduction in renal nor-epinephrine spillover with reduction of rennin production, aid natriuresis, an increase in renal blood flow and sustained reduction in systemic blood pressure and effectively treat HTN.The effectiveness and safety of catheter-based renal denervation had been demonstrated in patients with refractory hypertension in recent years. We demonstrated in the present study that inducibility of AF can be decreased by renal sympathetic denervation during rapid atrial pacing. The present findings further support the assertion that ablation of extracardiac nerves can be an effective alternative to ablation of intracardiac nerves by atrial ablation for the treatment of AF. The most important, we observed that the time course of RD effect on inhibition of AF, i.e. the on time of RD effect is at 4 hours after RD procedure, and the RD effect on AF reduction could last for 20 hours after RD procedure.
Funding
The study was supported by grant from Shanghai Nature & Science Foundation (10411956900).
References
- Zhao, Q.Y., Yu, S.B., Zou, M.F., Dai, Z.X., Wang, X.L., Xiao, J.P. and Huang, C.X. (2012) Effect of Renal Sympathetic Denervation on the Inducibility of Atrial Fibrillation during Rapid Atrial Pacing. Journal of Interventional Cardiac Electrophysiology, 35, 119-125. http://dx.doi.org/10.1007/s10840-012-9717-y
- Wijffels, M.C., Kirchhof, C.J., Dorland, R. and Allessie, M.A. (1995) Atrial Fibrillation Begets Atrial Fibrillation. A Study in Awake Chronically Instrumented Goats. Circulation, 92, 1954-1968. http://dx.doi.org/10.1161/01.CIR.92.7.1954
- Everett, T.H., Li, H., Mangrum, J.M., McRury, I.D., Mitchell, M.A., Redick, J.A., et al. (2000) Electrical, Morphological, and Ultrastructural Remodeling and Reverse Remodeling in a Canine Model of Chronic Atrial Fibrillation. Circulation, 102, 1454-1460. http://dx.doi.org/10.1161/01.CIR.102.12.1454
- Ausma, J., Wijffels, M., Thone, F., Wouters, L., Allessie, M. and Borgers, M. (1997) Structural Changes of Atrial Myocardium Due to Sustained Atrial Fibrillation in the Goat. Circulation, 96, 3157-3163. http://dx.doi.org/10.1161/01.CIR.96.9.3157
- Tsai, C.T., Lai, L.P., Lin, J.L., Chiang, F.T., Hwang, J.J., Ritchie, M.D., et al. (2004) Renin-Angiotensin System Gene Polymorphisms and Atrial Fibrillation. Circulation, 109, 1640-1646. http://dx.doi.org/10.1161/01.CIR.0000124487.36586.26
- Goette, A., Staack, T., Rocken, C., Arndt, M., Geller, J.C., Huth, C., et al. (2000) Increased Expression of Extracellular Signal Regulated Kinase and Angiotensin-Converting Enzyme in Human Atria during Atrial Fibrillation. Journal of the American College of Cardiology, 35, 1669-1677. http://dx.doi.org/10.1016/S0735-1097(00)00611-2
- Healey, J.S., Baranchuk, A., Crystal, E., Morillo, C.A., Garfinkle, M., Yusuf, S., et al. (2005) Prevention of Atrial Fibrillation with Angiotensin-Converting Enzyme Inhibitors and Angiotensin Receptor Blockers: A Meta-Analysis. Journal of the American College of Cardiology, 45, 1832-1839. http://dx.doi.org/10.1016/j.jacc.2004.11.070
- Ehrlich, J., Hohnloser, S. and Nattel, S. (2006) Role of Angiotensin System and Effects of its Inhibition in Atrial Fibrillation: Clinical and Experimental Evidence. European Heart Journal, 27, 512-518. http://dx.doi.org/10.1093/eurheartj/ehi668
- Kumagai, K., Nakashima, H., Urata, H., Gondo, N., Arakawa, K. and Saku, K. (2003) Effects of Angiotensin II type 1 Receptor Antagonist on Electrical and Structural Remodeling in Atrial Fibrillation. Journal of the American College of Cardiology, 41, 2197-2204. http://dx.doi.org/10.1016/S0735-1097(03)00464-9
- Elvan, A., Wylie, K. and Zipes, D.P. (1996) Pacing-Induced Chronic Atrial Fibrillation Impairs Sinus Node Function in Dogs. Electrophysiological Remodeling. Circulation, 94, 2953-2960. http://dx.doi.org/10.1161/01.CIR.94.11.2953
- Nakashima, H., Kumagai, K., Urata, H., Gondo, N., Ideishi, M. and Arakawa, K. (2000) Angiotensin II Antagonist Prevents Electrical Remodeling in Atrial Fibrillation. Circulation, 101, 2612-2617. http://dx.doi.org/10.1161/01.CIR.101.22.2612
- Chiou, C.W., Eble, J.N. and Zipes, D.P. (1997) Efferent Vagal Innervation of the Canine Atria and Sinus and Atrioventricular Nodes the Third Fat Pad. Circulation, 95, 2573-2584. http://dx.doi.org/10.1161/01.CIR.95.11.2573
- Schauerte, P., Scherlag, B.J., Pitha, J., Scherlag, M.A., Reynolds, D., Lazzara, R., et al. (2000) Catheter Ablation of Cardiac Autonomic Nerves for Prevention of Vagal Atrial Fibrillation. Circulation, 102, 2774-2780. http://dx.doi.org/10.1161/01.CIR.102.22.2774
- Tan, A.Y., Zhou, S., Ogawa, M., Song, J., Chu, M., Li, H., et al. (2008) Neural Mechanisms of Paroxysmal Atrial Fibrillation and Paroxysmal Atrial Tachycardia in Ambulatory Canines. Circulation, 118, 916-925. http://dx.doi.org/10.1161/CIRCULATIONAHA.108.776203
- Lu, Z., Scherlag, B.J., Lin, J., Niu, G., Fung, K., Zhao, L., et al. (2008) Atrial Fibrillation Begets Atrial Fibrillation Autonomic Mechanism for Atrial Electrical Remodeling Induced by Short-Term Rapid Atrial Pacing. Circulation: Arrhythmia and Electrophysiology, 1, 184-192. http://dx.doi.org/10.1161/CIRCEP.108.784272
- Goette, A., Honeycutt, C. and Langberg, J.J. (1996) Electrical Remodeling in Atrial Fibrillation: Time Course and Mechanisms. Circulation, 94, 2968-2974. http://dx.doi.org/10.1161/01.CIR.94.11.2968
- Tieleman, R.G., De Langen, C., Van Gelder, I.C., de Kam, P.J., Grandjean, J., Bel, K.J., et al. (1997) Verapamil Reduces Tachycardia-Induced Electrical Remodeling of the Atria. Circulation, 95, 1945-1953. http://dx.doi.org/10.1161/01.CIR.95.7.1945
- Touyz, R.M., Sventek, P., Lariviere, R., Thibault, G., Fareh, J., Reudelhuber, T., et al. (1996) Cytosolic Calcium Changes Induced by Angiotensin II in Neonatal Rat Atrial and Ventricular Cardiomyocytes Are Mediated via Angiotensin II Subtype 1 Receptors. Hypertension, 27, 1090-1096. http://dx.doi.org/10.1161/01.HYP.27.5.1090
- Laszlo, R., Bentz, K., Konior, A., Eick, C., Schreiner, B., Kettering, K., et al. (2010) Effects of Selective Mineralocorticoid Receptor Antagonism on Atrial ion Currents and Early Ionic Tachycardiainduced Electrical Remodelling in Rabbits. Naunyn-Schmiedeberg’s Archives of Pharmacology, 382, 347-356. http://dx.doi.org/10.1007/s00210-010-0553-2
- Xiao, H.D., Fuchs, S., Campbell, D.J., Lewis, W., Dudley, S.C., Kasi, V.S., et al. (2004) Mice with Cardiac-Restricted Angiotensin-Converting Enzyme (ACE) Have Atrial Enlargement, Cardiac Arrhythmia, and Sudden Death. American Journal of Pathology, 165, 1019-1032. http://dx.doi.org/10.1016/S0002-9440(10)63363-9
- Nattel, S. and Opie, L.H. (2006) Controversies in Atrial Fibrillation. Lancet, 367, 262-272. http://dx.doi.org/10.1016/S0140-6736(06)68037-9
- Wyse, D.G., Waldo, A.L., DiMarco, J.P., Domanski, M.J., Rosenberg, Y., Schron, E.B., et al. (2002) A Comparison of Rate Control and Rhythm Control in Patients with Atrial Fibrillation. New England Journal of Medicine, 347, 1825-1833. http://dx.doi.org/10.1056/NEJMoa021328
- Haissaguerre, M., Jais, P., Shah, D.C., Takahashi, A., Hocini, M., Quiniou, G., et al. (1998) Spontaneous Initiation of Atrial Fibrillation by Ectopic Beats Originating in the Pulmonary Veins. New England Journal of Medicine, 339, 659666. http://dx.doi.org/10.1056/NEJM199809033391003
- Shah, A.J., Liu, X., Jadidi, A.S. and Haïssaguerre, M. (2010) Early Management of Atrial Fibrillation: From Imaging to Drugs to Ablation. Natural Reviews Cardiology, 7, 345-354. http://dx.doi.org/10.1038/nrcardio.2010.49
- Dewire, J. and Calkins, H. (2010) State-of-the-Art and Emerging Technologies for Atrial Fibrillation Ablation. Natural Reviews Cardiology, 7, 129-138. http://dx.doi.org/10.1038/nrcardio.2009.232
- Oral, H., Chugh, A., Yoshida, K., Sarrazin, J.F., Kuhne, M., Crawford, T., et al. (2009) A Randomized Assessment of the Incremental Role of Ablation of Complex Fractionated Atrial Electrograms after Antral Pulmonary Vein Isolation for Longlasting Persistent Atrial Fibrillation. Journal of the American College of Cardiology, 53, 782-789. http://dx.doi.org/10.1016/j.jacc.2008.10.054
- Pokushalov, E., Romanov, A., Shugayev, P., Artyomenko, S., Shirokova, N., Turov, A., et al. (2009) Selective Ganglionated Plexi Ablation for Paroxysmal Atrial Fibrillation. Heart Rhythm, 6, 1257-1264. http://dx.doi.org/10.1016/j.hrthm.2009.05.018
- Katritsis, D.G., Giazitzoglou, E., Zografos, T., Pokushalov, E., Po, S.S. and Camm, A.J. (2011) Rapid Pulmonary Vein Isolation Combined with Autonomic Ganglia Modification: A Randomized Study. Heart Rhythm, 8, 672-678. http://dx.doi.org/10.1016/j.hrthm.2010.12.047
- Po, S.S., Nakagawa, H. and Jackman, W.M. (2009) Localization of Left Atrial Ganglionated Plexi in Patients with Atrial Fibrillation. Journal of Cardiovascular Electrophysiology, 20, 1186-1189. http://dx.doi.org/10.1111/j.1540-8167.2009.01515.x
- Kron, J., Kasirajan, V., Wood, M.A., Kowalski, M., Han, F.T. and Ellenbogen, K.A. (2010) Management of Recurrent Atrial Arrhythmias after Minimally Invasive Surgical Pulmonary Vein Isolation and Ganglionic Plexi Ablation for Atrial Fibrillation. Heart Rhythm, 7, 445-451. http://dx.doi.org/10.1016/j.hrthm.2009.12.008
- Oh, S., Zhang, Y., Bibevski, S., Marrouche, N.F., Natale, A. and Mazgalev, T.N. (2006) Vagal Denervation and Atrial Fibrillation Inducibility: Epicardial Fat Pad Ablation Does Not Have Long-Term Effects. Heart Rhythm, 3, 701-708. http://dx.doi.org/10.1016/j.hrthm.2006.02.020
- Zhao, Q.Y., Huang, H., Zhang, S.D., Tang, Y.H., Wang, X., Zhang, Y.G., et al. (2010) The Relation between Atrial Remodeling and Inducibility of Atrial Fibrillation after Epicardial Ganglionic Plexi Ablation. Europace, 12, 805-810. http://dx.doi.org/10.1093/europace/euq089
- Krum, H., Schlaich, M., Whitbourn, R., Sobotka, P.A., Sadowski, J., Bartus, K., et al. (2009) Catheter-Based Renal Sympathetic Denervation for Resistant Hypertension: A Multicentre Safety and Proof-of-Principle Cohort Study. Lancet, 373, 1275-1281. http://dx.doi.org/10.1016/S0140-6736(09)60566-3
NOTES

*Corresponding author.


