World Journal of Cardiovascular Diseases
Vol.3 No.1(2013), Article ID:26541,7 pages DOI:10.4236/wjcd.2013.31002
Impact of cytochrome P450 2C19*2 polymorphism on the clinical cardiovascular events after stent implantation in patients receiving clopidogrel of a southern Tunisian region*
![]()
1Cardiology Department, Hedi Chaker Hospital, Medicine University of Sfax, Sfax, Tunisia
2CBS Center, Sfax, Tunisia
Email: #leilaabidt@yahoo.fr
Received 27 July 2012; revised 27 September 2012; accepted 24 November 2012
Keywords: Clopidogrel; CYP2C19*2 Polymorphism; MACE; Stent Thrombus; Percutaneous Coronary Intervention
ABSTRACT
Introduction: The concept of clopidogrel resistance, first described in biology is being strengthened by recent data from clinical epidemiology. The cardiologists have been sensitized to this concept because of its possible involvement in the occurrence of coronary stent thrombosis. Purpose of the study: The purpose of this study was to investigate the genetic variant of the gene CYP 2C19 in our population and to assess the involvement of this genetic profile in the occurrence of major cardiovascular events (MACE) during the follow-up period. Methods: Our prospective study was conducted between May 2009 and September 2010 including 100 patients admitted to the cardiology department for percutaneous coronary stenting. The patients were divided into 2 groups: those with at least one CYP2C19*2 allele (*2 carriers) and noncarriers. Results: The mean age of our patients was 56.7 years ± 10, 5. No remarkable differences in the baseline characteristics were noted between the two groups. The prevalence of CYP2C19*2 allele in our population was 11.5%. Hospital mortality was estimated at 3%. No statistically significant differences were noted between the two groups regarding the occurrence of intra hospital MACE. The mean follow up was 7.5 ± 4.87 months for the entire study population. The rate of MACE during the follow-up of patients receiving clopidogrel was 8.2% throughout the study population: 5.3% in the *2 non-carriers versus 18.2% in the *2 carriers with a statistically significant difference (p = 0.075) at the risk of error of 10%. Concerning the occurrence of stent thrombosis, there was no significant statistical difference between the two study groups. Conclusion: From these results it is suggested that CYP2C19*2 polymorphism is associated with increase in the occurrence of MACE among Tunisian patients receiving clopidogrel. A larger study is needed to assess the role of genotyping in the evaluation of the phenomenon of clopidogrel resistance.
1. INTRODUCTION
Percutaneous coronary stenting (PCI) was a major therapeutic advance. The central role of platelets in the occurrence of arterial thrombotic complications is now well documented. The antiplatelet drugs have become essential for coronary disease. Clopidogrel plays a significant role in the prevention of thrombotic events in patients undergoing PCI, but a substantial number of such events still occur [1], which can be partially explained by the phenomenon of drug resistance, up to 30% of patients in initial studies [2,3]. Oh et al. [4] reported that the CYP2C19*2 allele carrier status was associated with an increased incidence of adverse clinical events.
To exert an antiplatelet effect, clopidogrel requires conversion to an active metabolite by hepatic cytochrome P450 (CYP) enzymes [5,6]. The two-step hepatic cytochrome P450 (CYP)-dependant oxidative metabolism of the prodrug appears to be of particular importance. Pharmacogenomic analyses have identified loss-of-function variant alleles of CYP 2C19 and specifically the 2C19*2 allele, to be the predominant genetic mediators of the antiplatelet effect of clopidogrel. Carriers were have been shown to have lower active metabolite levels of clopidogrel, higher platelet reactivity and associated poorer outcomes. The mechanisms of the variability of the biological response to clopidogrel are very numerous. While some are non modifiable as the polymorphism of cytochrome P450 or the existence of diabetes, others may vary over time such as the presence of acute coronary syndrome or platelet turnover.
The objectives of the present study are: to compare the occurrence of major cardiovascular events between the two groups: the *2 carriers (the allele with reduced function (group A)) and the *2 non carriers (group B). As a consequence to assess the relationship between the CYP2C19*2 polymorphism and the frequency of cardiac events in Tunisian patients receiving clopidogrel after stent implantation.
2. METHODS
Inclusion criteria for patients’ selection: Patients admitted for all types of ACS undergoing PCI with stent implantation.
Consecutive patients admitted for ACS to the Department of Cardiology of Sfax (Tunisia) from May 2009 to September 2010 were eligible for this prospective study. We enrolled 100 patients with all types of ACS undergoing PCI with stent implantation and who were treated with aspirin and clopidogrel according to the current guidelines. All patients received a loading dose of 250 mg of aspirin followed by a daily regimen of 75 to 250 mg and a loading dose of either 300 or 600 mg of clopidogrel followed by either 75 mg or 150 mg daily.
Exclusion criteria were: patients with a problem of platelet aggregation. Data concerning demographic parameters, coronary heart disease risk factors and treatment were collected for each patient. All interventions were performed according to current clinical guidelines. The type of stent implanted and the use of Glycoprotein IIb/IIIa inhibitors were let at discretion of the operator. Weight adjusted intra-procedural unfractionated heparin was administered during the procedure and was routinely continued for at least 24 hours. Clinical endpoints measured were the composite of cardiovascular death, nonfatal MI, TLR, definite or probable stent thrombosis or cerebrovascular accident (CVA) at 1-3-6 and 12 months follow-up. Cardiovascular death was defined as death from acute myocardial infarction, coronary artery disease (CAD), or congestive heart failure. Stent thrombosis was classified by the academic research consortium (ARC) definitions as definite, probable, or possible and as acute (within 24 h from procedure), subacute (1 to 30 days), or late (30 days to 1 year) [7].
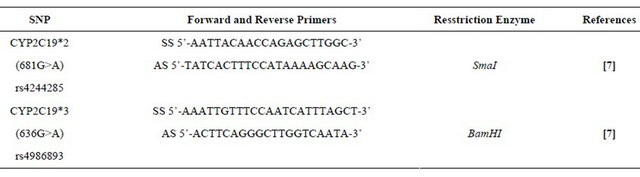
3. GENETIC SCREENING
Each patient underwent on the inclusion a blood sample in EDTA tube. Table 1 summarizes the primers used to identify the single nucleotide polymorphism (SNP). Genomic deoxyribonucleic acid (DNA) was extracted from peripheral blood leukocytes by the salting-out method. Genotyping for CYP2C19*2 (rs4244285) and CYP2C19*3 (rs4986893) was performed by allele-specific polymerase chain reaction, 2 allele-specific primers by reaction, or restriction fragment length polymorphism. The polymerase chain reaction products were visualized on 2% agarose gels stained with 1 μg/μL ethidium bromide. The variant CYP2C19*2 with loss of function is secondary to a G A nucleotide substitution at position 681 at the junction of intron 4 and exon 5. This genetic polymorphism is causing a shift of reading frame responsible for aberrant splicing. The transcript of the codon is stopped prematurely resulting in the formation of a nonfunctional truncated protein responsible for the phenotype hypo responder to clopidogrel. The CYP2C19*3 variant with loss of function is a G A nucleotide substitution at position 636 of exon 4 creates a premature stop codon at the origin of a truncated protein metabolically inactive.
4. STATISTICAL ANALYSIS
All statistical analyses were performed using SPSS (version 17). Continuous variables are presented as mean ± SD. Differences in continuous parameters between the 2 groups were calculated using a 2-tailed unpaired t-test. Categorical variables are presented as frequency counts. Comparison of categorical variables between the 2 groups was performed using the chi-square test. Values were
Table 1. The primer pairs used.
considered statistically significant at p < 0.05. However, in certain cases (that we will indicate), we considered the difference significant if p < 0.10. Survival curve were established by using Kaplan Meier method and compared by the log rank test for univariate analysis of survival.
5. RESULTS
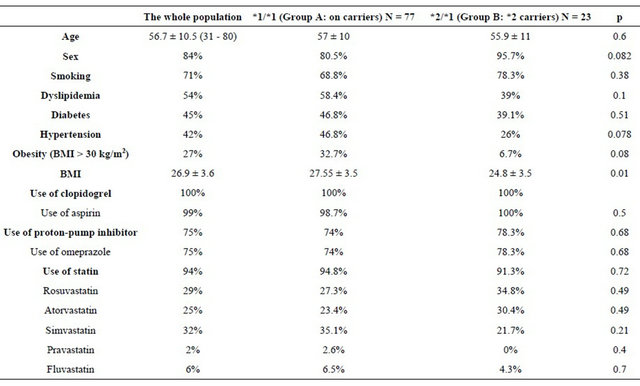
One hundred patients who underwent PCI were included in our study. Of the 100 patients, 23 had at least one *2 allele (*2 carriers) and the remaining 77 were CYP2C19 wild-type homozygotes (*1/*1) (non-carriers). For the variant CYP2C19*2, we obtained 23 heterozygous (*1/ *2). But no cases of type homozygous (*2/*2) were found, giving a prevalence of the mutated form (*2) of 11.5%. The variant CYP2C19*3 did not present any polymorphism in our population. Baseline characteristics of the study population are listed in Table 2. No significant differences in patient characteristics were noted between the 2 groups.
47 patients had an acute coronary syndrome with persistent ST segment elevation (47% of cases) including 5 (10.4%) with severe left ventricular failure (Killi p ≥ 3) (Killip 3:2 patients, and Killip 4:3 patients). 35 patients (35%) had an acute coronary syndrome without ST elevation. Stable angina was found in 18 patients (18% of cases).
Analysis of the angiographic data according to the genetic profile showed no differences in the quantitative coronary angiography data between the 2 groups. Concerning the procedural characteristics, there was no significant difference. The left anterior descending artery (LAD) was the most dilated artery (58% of cases in the entire study population, 55.8% in group A and 65.2% in group B, p = 0.42) (Table 3). All patients received aspirin except one patient who was allergic to aspirin and in whom a previous attempt of desensitization has failed, the patient belongs to the group A. Unfractionated heparin was administered in per procedural for all patients. Nineteen patients (19% of the population of the study) were also given an anti GPIIbIIIa, this more frequently in the group B (34.8% versus 14.3%, p = 0.028). A loading dose of 600 mg of clopidogrel was prescribed in eleven cases (11% of the study population) with no statistically significant difference between the two groups. This dose was administered during the acute phase of STEMI. Using a double maintenance dose of clopidogrel (150 mg) was noted in 27% of cases (28.6% in the group A versus 21.7 % in the group B (p = 0.5). This attitude was especially observed in diabetic patients, if drug-eluting stent is used and in case of laborious angioplasty (77% of patients receiving a double dose of clopidogrel were diabetic and 70% were implanted with a drug-eluting stent).
6. CLINICAL OUTCOMES
Table 4 illustrates the incidence of cardiovascular out comes during the hospitalization period.
Table 2. Baseline characteristics of the study population.
The incidence of in-hospital MACE did not differ significantly between the 2 groups during the hospitalization. *2 carrying does not appear to be a predictor of inhospital MACE. The mean follow was 7.5 ± 4.87 months for the entire study population (range between 1 and 17 months). It was 7 ± 5 months in the group A and 8.9 ± 4.4 months in the group B without statistically significant difference. The average intake of clopidogrel was 6.67 ± 6.38 months for the whole population (range between 1 and 24 months). It was 6.9 ± 6.44 months in the group A and 5.9 ± 6.25 months in the group B without significant difference (p = 0.12). The rate of MACE during followup of patients receiving clopidogrel: was 8.2% in the entire study population. It was higher in the group of *2 carriers: 5.3% in group A versus 18.2% in the group B with a statistically significant difference (p = 0.075) at the risk of error of 10%. Details of MACE during follow-up period of patients receiving clopidogrel according to the genetic profile are listed in the Table 5 and Figure 1.
The stent thrombosis: Three patients presented during the follow-up stent thrombosis. The first patient in the group A presented 48 hours after angioplasty, a certain and early stent thrombosis treated by primary balloon angioplasty. The second patient in the group B had presented six months after the angioplasty a late and likely stent thrombosis treated by thrombolysis. Both patients were on clopidogrel during the occurrence of stent thrombosis. A third patient belonging to the group A presented three months after an angioplasty a late stent thrombosis treated by primary angioplasty. The latter patient was not on clopidogrel at the onset of thrombosis. A study of aspirin resistance has been performed in this patient showed a resistance profile in the PFA 100 test. No significant increase in the incidence of stent thrombosis was noted in our study according to genetic profile.
7. Discussion
There has been increasing evidence for a considerable interindividual variability of anti platelet drug response to clopidogrel associated with an increased risk for thromboischemic complications after coronary intervention [8-10]. The prevalence of clopidogrel resistance remains uncertain; it ranges from 5% to 44% as reported in
Table 3. Quantitative coronary angiography and PCI data.
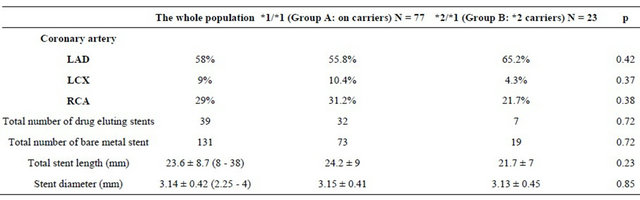
LAD, left anterior descending artery; LCX, left circumflex artery; RCA, right coronary artery.
Table 4. Cardiovascular outcomes during the hospitalization.
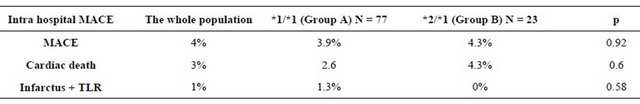
Table 5. Details of MACES during follow-up under clopidogrel according the genetic profile.
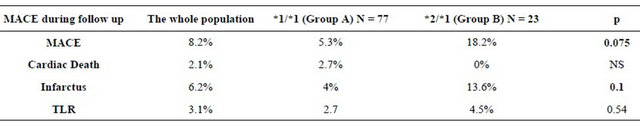
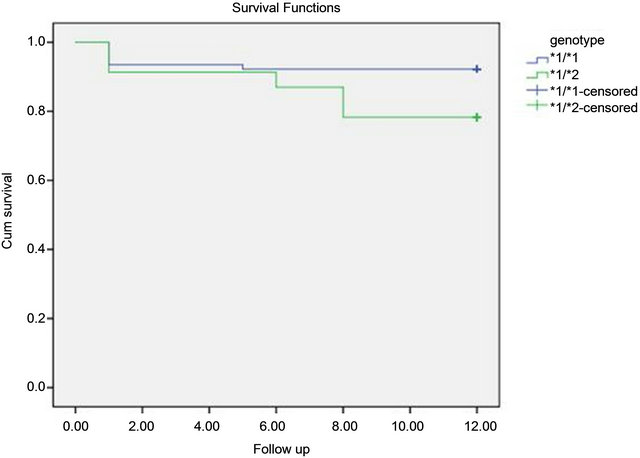
Figure 1. Curves compared cumulative survival without MACE in patients receiving clopidogrel according to the genetic profile.
different studies [11-13]. This wide ranging variability is probably due to the lack of a standardized definition of the term “clopidogrel resistance”. Another important and still unresolved issue is the difference between “clinical” and “laboratory” resistance. The clinical definition of resistance is based on the failure to prevent the adverse events while patients are “on treatment”. It is generally accepted that low response to antiplatelet agents correlates with more events, but it would be inaccurate to lay the entire blame for on-treatment clinical events on clopidogrel resistance, since platelets respond to several agonists via different pathways. With these considerations in mind, the term “clinical resistance” should be avoided and a more appropriate term would be “treatment failure”. The variability of response to anti-platelet agents can not be explained only by clinical risk factors, because we know that this variability was also observed in healthy subjects [14] and there is variability in platelet aggregation pre-existing, even in patients who have never antiplatelet agents [15]. In this context, a genetic cause has been particularly sought. Cytochrome 2C19 (CYP2C19) is known to be involved in hepatic metabolism of clopidogrel, absorbed as a pro drug. Several allelic variants of CYP2C19 have been identified, the sequences leading to aberrant splicing of messenger in the synthesis of the protein, thus resulting in a loss of the function of the cytochrome. The present study describes a significant association between poor response to clopidogrel of patients with coronary artery disease undergoing PCI and carrying at least one allele of the CYP2C19*2 loss of fonction polymorphism. The genetic polymorphism for cytochrome 2C19 has a very important ethnic variation. The allele frequencies in our Tunisian sample were compared with other studies related to different populations (Figure 2). To our knowledge, our study is the first who tried to determine the prevalence of genetic polymorphism of CYP2C19 in Tunisia through a sample of 100 patients. It has been estimated at 11.5% for the CYP2C19*2. By calculating the prevalence and confidence intervals corresponding to different populations (Figure 2), we can see that this prevalence is closer to the European [16] population as the Afro American [17]. Asian populations [18] have similar prevalences that are higher than our population. CYP2C19*3 was absent in our study population, a concordant data with the Canadian [19], European-American, Portuguese [20], Swedish [21], and Afro American. The largest prevalence of CYP2C19*3 was noted in the Asian population (4.5% to 11%).
Several studies have demonstrated an association between the CYP2C19 genotype and a diminished platelet response to clopidogrel and more cardiovascular events after stent implantation [22-25]. Trenk et al. reported an association between the CYP2C19*2 polymorphism and high on-treatment platelet reactivity after the administration of clopidogrel [26]. The presence of the CYP2C19*2
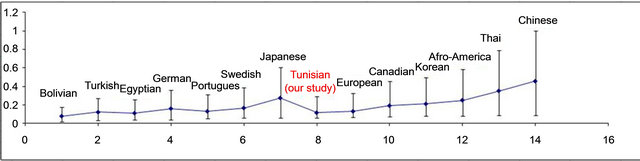
Figure 2. Inter-ethnic comparison of the prevalence of the CYP2C19*2 allele [2,12,13,20].
polymorphism is significantly associated with an increased risk of stent thrombosis following implantation of a coronary stent [24]. In the present study we demonstrated a significantly higher incidence of MACE in *2 carriers (18.2%) than in non-carriers (5.3%) but no significant increase in the incidence of stent thrombosis.
There were several limitations to the present study. Only patients who revisited hospital were enrolled in the study. Therefore, some selection bias was likely. The small sample size did not provide sufficient power to identify differences in the clinical outcome between *2 carriers and non-carriers. Further, because of the small sample size, it remains unclear whether the CYP2C19*2 polymorphism is a real risk factor for stent thrombosis. Although a previous report demonstrated that the CYP2C19*2 polymorphism affects responsiveness to Clopidogrel [25,27] we did not assess platelet aggregation in this population. Therefore, it is unclear whether the CYP2C19*2 polymorphism inhibits the antiplatelet effect of clopidogrel in the present study population.
8. CONCLUSION
In the present study, the CYP2C19*2 genetic profile leads to an increase in the occurrence of MACE in the follow-up of patients receiving clopidgrel. The lack of statistically significant differences in stent thrombosis is secondary to the small size of our sample. A larger study is needed to better assess the role of genotyping in the evaluation of the phenomenon of clopidogrel resistance.
![]()
![]()
REFERENCES
- Mehta, S.R., Yusuf, S., Peters, R.J., Bertrand, M.E., Lewis, B.S., Natarajan, M.K., et al. (2001) Effects of pretreatment with clopidogrel and aspirin followed by longterm therapy in patients undergoing percutaneous coronary intervention: The PCI-CURE study. The Lancet, 358, 527-533. doi:10.1016/S0140-6736(01)05701-4
- Angiolillo, D.J., Fernandez-Ortiz, A., Bernardo, E., Alfonso, F., Macaya, C., Bass, T.A., et al. (2007) Variability in individual responsiveness to clopidogrel: Clinical implications, management, and future perspectives. Journal of the American College of Cardiology, 49, 1505- 1516. doi:10.1016/j.jacc.2006.11.044
- Wang, T.H., Bhatt, D.L. and Topol, E.J. (2006) Aspirin and clopidogrel resistance: An emerging clinical entity. European Heart Journal, 27, 647-654. doi:10.1093/eurheartj/ehi684
- Oh, I.Y., Park, K.W., Kang, S.H., Park, J.J., Na, S.H., Kang, H.J., et al. (2012) Association of cytochrome P450 2C19*2 polymorphism with clopidogrel response variability and cardiovascular events in Koreans treated with drug-eluting stents. Heart, 98, 139-144. doi:10.1136/hrt.2011.227272
- Gurbel, P.A. and Tantry, U.S. (2007) Clopidogrel resistance? Thrombosis Research, 120, 311-321. doi:10.1016/j.thromres.2006.08.012
- Savi, P., Pereillo, J.M., Uzabiaga, M.F., Combalbert, J., Picard, C., Maffrand, J.P., et al. (2000) Identification and biological activity of the active metabolite of clopidogrel. Thrombosis and Haemostasis, 84, 891-896.
- Mauri, L., Hsieh, W.H., Massaro, J.M., Ho, K.K., D’Agostino, R. and Cutlip, D.E. (2007) Stent thrombosis in randomized clinical trials of drug-eluting stents. The New England Journal of Medicine, 356, 1020-1029. doi:10.1056/NEJMoa067731
- Chaudhry, A.S., Kochhar, R. and Kohli, K.K. (2009) Importance of CYP2C19 genetic polymorphism in the eradication of Helicobacter pylori in north Indians. Indian Journal of Medical Research, 130, 437-443.
- Geisler, T., Langer, H., Wydymus, M., Göhring, K., Zürn, C. and Bigalke, B. (2006) Low response to clopidogrel is associated with cardiovascular outcome after coronary stent implantation. European Heart Journal, 27, 2420- 2425. doi:10.1093/eurheartj/ehl275
- Hochholzer, W., Trenk, D., Bestehorn, H.P., Fischer, B., Valina, C.M. and Ferenc, M. (2006) Impact of the degree of peri-interventional platelet inhibition after loading with clopidogrelon early clinical outcome of elective coronary stent placement. Journal of the American College of Cardiology, 48, 1742-1750. doi:10.1016/j.jacc.2006.06.065
- Lev, E.I., Patel, R.T., Maresh, K.J., Guthikonda, S., Granada, J., DeLao, T. and Bray, P.F., (2006) Aspirin and clopidogrel drug response in patients undergoing percutaneous coronary intervention: The role of dual drug resistance. Journal of the American College of Cardiology, 47, 27-33. doi:10.1016/j.jacc.2005.08.058
- Gurbel, P.A., Bliden, K.P., Hayes, K.M., Yoho, J.A., Herzog, W.R. and Tantry, U.S. (2005) The relation of dosing to clopidogrel responsiveness and the incidence of high post-treatment platelet aggregation in patients undergoing coronary stenting. Journal of the American College of Cardiology, 45, 1392-1396. doi:10.1016/j.jacc.2005.01.030
- Dziewierz, A., Dudek, D., Heba, G., Rakowski, T., Mielecki, W. and Dubiel, J.S. (2005) Interindividualvariability in response to clopidogrel in patients with coronary artery disease. Kardiologia Polska, 62, 108-117.
- Serebruany, V.L., Steinhubl, S.R., Berger, P.B., Malinin, A.I., Bhatt, D.L. and Topol, E.J. (2005) Variability in platelet responsiveness to clopidogrel among 544 individuals. Journal of the American College of Cardiology, 45, 246-251. doi:10.1016/j.jacc.2004.09.067
- Michelson, A.D., Linden, M.D., Furman, M.I., Li, Y., Barnard, M.R., Fox, M.L., et al. (2007) Evidence that preexistent variability in platelet response to ADP accounts for “clopidogrel resistance”. Journal of Thrombosis and Haemostasis, 5, 75-81. doi:10.1111/j.1538-7836.2006.02234.x
- Xie, H.G., Stein, C.M., Kim, R.B., Wilkinson, G.R., Flockhart, D.A. and Wood, A.J. (1999) Allelic, genotypic and phenotypic distributions of S-mephenytoin 4’-hydroxylase (CYP2C19) in healthy Caucasian populations of European descent throughout the world. Pharmacogenetics, 9, 539-549. doi:10.1097/00008571-199910000-00001
- Goldstein, J.A., Ishizaki, T., Chiba, K., Morais, S.M., Bell, D., Krahn, P.M., et al. (1997) Frequencies of the defective CYP2C19 alleles responsible for the mephenytoin poor metabolizer phenotype in various Oriental, Caucasian, Saudi Arabian and American black populations. Pharmacogenetics, 1, 59-64. doi:10.1097/00008571-199702000-00008
- Takakubo, F., Kuwano, A. and Kondo, I. (1996) Evidence that poor metabolizers of (S)-mephenytoin could be identified by haplotypes of CYP2C19 in Japanese. Pharmacogenetics, 6, 265-267. doi:10.1097/00008571-199606000-00011
- Nowak, M.P., Sellers, E.M. and Tyndale, R.F. (1998) Canadian native Indians exhibit unique CYP2A6 and CYP2C19 mutant allele frequencies. Clinical Pharmacology & Therapeutics, 64, 378-383. doi:10.1016/S0009-9236(98)90068-6
- Ruas, J.L. and Lechner, M.C. (1997) Allele frequency of CYP2C19 in a Portuguese population. Pharmacogenetics, 7, 333-335. doi:10.1097/00008571-199708000-00009
- Chang, M., Dahl, M.L., Tybring, G., Tharson, E. and Bertilsson, L. (1995) Use of omeprazole as aprobe drug for CYP2C19 phenotype in Swedish Caucasians: Comparison with S-mephenytoin hydroxylation phenotype and CYP2C19 genotype. Pharmacogenetics, 5, 358-363. doi:10.1097/00008571-199512000-00004
- Collet, J.P., Hulot, J.S., Pena, A., Villard, E., Esteve, J.B., Silvain, J., et al. (2009) CytochromeP450 2C19 polymorphism in young patients treated with clopidogrel after myocardial infarction: A cohort study. Lancet, 373, 309- 317. doi:10.1016/S0140-6736(08)61845-0
- Schuldiner, A.R., O’Connell, J.R., Bliden, K.P., Gandhi, A., Ryan, K., Horenstein, R.B., et al. (2009) Association of cytochrome P450 2C19 genotype with the antiplatelet effect andclinical efficacy of clopidogrel. The Journal of the American Medical Association, 302, 849-857. doi:10.1001/jama.2009.1232
- Sibbing, D., Stegherr, J., Latz, W., Koch, W., Mehilli, J., Dorrler, K., et al. (2009) Cytochrome P450 2C19 loss-offunction polymorphism and stent thrombosis following percutaneous coronary intervention. European Heart Journal, 30, 916-922. doi:10.1093/eurheartj/ehp041
- Mega, J.L., Close, S.L., Wiviott, S.D., Wiviott, S.D., Shen, L., Hockett, R.D., et al. (2009) Cytochrome P450 polymorphism and response to clopidogrel. The New England Journal of Medicine, 360, 354-362. doi:10.1056/NEJMoa0809171
- Trenk, D., Hochholzer, W., Fromm, M.F., Chialda, L.E., Pahi, A., Valina, C.M., et al. (2008) Cytochrome P450 2C19 681G>A polymorphism and high on-clopidogrel platelet reactivity associated with adverse 1-year clinical outcome of elective percutaneous coronary intervention with drug-eluting or bare-metal stents. Journal of the American College of Cardiology, 51, 1925-1934. doi:10.1016/j.jacc.2007.12.056
- Jinnai, T., Horiuchi, H., Makiyama, T., Tazaki, J., Tada, T., Akao, M., et al. (2009) Impact of CYP2C19 polymorphism on the antiplatelet effect of clopidogrel in an actual clinical setting in Japan. Circulation Journal, 73, 1498-1503. doi:10.1253/circj.CJ-09-0019
NOTES
*Ethical approval for this research was given par ethical comity of university hedi chaker hospital.
#Corresponding author.

