Open Journal of Organic Polymer Materials
Vol.3 No.4(2013), Article ID:38376,5 pages DOI:10.4236/ojopm.2013.34014
A Novel Approach of Dyeing Jute Fiber with Reactive Dye after Treating with Chitosan
1Department of Textile Engineering, Dhaka University of Engineering and Technology, Gazipur, Bangladesh
2Department of Applied Chemistry and Chemical Engineering, University of Dhaka, Dhaka, Bangladesh
3Department of Textile Engineering, Mawlana Bhashani Science and Technology University, Tangail, Bangladesh
Email: *arahman@duet.ac.bd, abushaid@duet.ac.bd, mahbub.mbstu@yahoo.com, papiahq@yahoo.com, hannan_tex62@yahoo.com
Copyright © 2013 M. A. Rahman Bhuiyan et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received July 13, 2013; revised August 13, 2013; accepted August 20, 2013
Keywords: Chitosan; Dyeing; Jute Fiber; Reactive Dye
ABSTRACT
Jute is generally not dyed with reactive dye though it is a cellulosic fiber. Reactive dye is extensively used to dye cotton, viscose and other cellulosic fibers whereas jute is dyed with basic dye. This paper presents a novel approach to dye the jute fiber with reactive dye after treating with chitosan. Jute fabric was treated with chitosan solution at different concentrations (0.5%, 1%, 2%, 3% and 4%) and then dyed with reactive dye. The depth and fastness of shade of dyed fabric were analyzed by comparing the chitosan treated samples with untreated dyed fabric samples. It has been found that, the dyebath exhaustion is increased with the increment of chitosan concentrations. The exhaustion percentages have found 36.79%, 41.59%, 48.33%, 54.46% and 58.75% for the fabric treated with 0.5%, 1%, 2%, 3% and 4% chitosan solution respectively, while the exhaustion of dyebath is only 23.15% for untreated fabric. The K/S values (at λmax = 540 nm) of dyed samples have found 4.93, 6.77, 10.5, 14.07, 15.57 and 2.37 for 0.5%, 1%, 2%, 3%, 4% and untreated fabric respectively. The color fastness to washing and rubbing of the dyed fabrics was also evaluated. In case of dry rubbing, both types of fabric have shown almost similar fastness ratings. However, chitosan treated fabrics have shown inferior fastness rating in case of wet rubbing and washing, particularly for the fabrics at higher chitosan concentrations.
1. Introduction
Jute is a natural cellulosic fiber obtained from the bark of jute plant. The main chemical constituent of jute fiber is α-cellulose along with hemicelluloses and lignin [1]. This fiber is popular due to its bio-degradability, high tensile strength, low extensibility and better breathability [2,3]. In recent times, due to the improvement of people’s living standards and need for environmental protection, the demand of natural biodegradable and eco-friendly fibers like jute is increasing day by day [4].
The coloration of jute fiber is mainly carried out with basic dye in slightly acidic medium. As acid degraded the cellulose [5], there is a possibility to decrease the strength of jute fiber. Moreover, the dyeing process of jute with basic dye is complicated and many of the basic dyes are not fast to acids, alkalis, washing and particularly to light [6]. Again, the achievable color ranging with basic dye is not as wide as the reactive dye which is popular dyestuff for cellulose dyeing. Reactive dye is the worldwide acceptable dye for the coloration of cotton goods due to their ease of applicability, cost, brilliancy of color and high wet fastness properties [7]. However, in spite of being a cellulosic fiber, jute is normally not dyed with reactive dye. Reactive dyeing of jute is not economical for its high crystallinity and high degree of orientation [8] which is the hindrance of high color yield. Again the dye exhaustion is low due to presence of comparatively less amount of cellulose (58% - 63%) [1] than cotton (94%) [9]. It is not possible to increase the amount of cellulose in the fiber. However, increasing the percentage of dyesite in the fiber is a logical principle to improve the dye exhaustion of jute fiber. According to Bashar and Khan [10], the introduction of cationic sites within the cellulose is an effective way to increase the dye adsorption. Cationic sites can be introduced either by aminization or cationization [11]. Treatment of jute with chitosan is an aminization technique to introduce cationic site within the fiber polymer (Figure 1) structure and
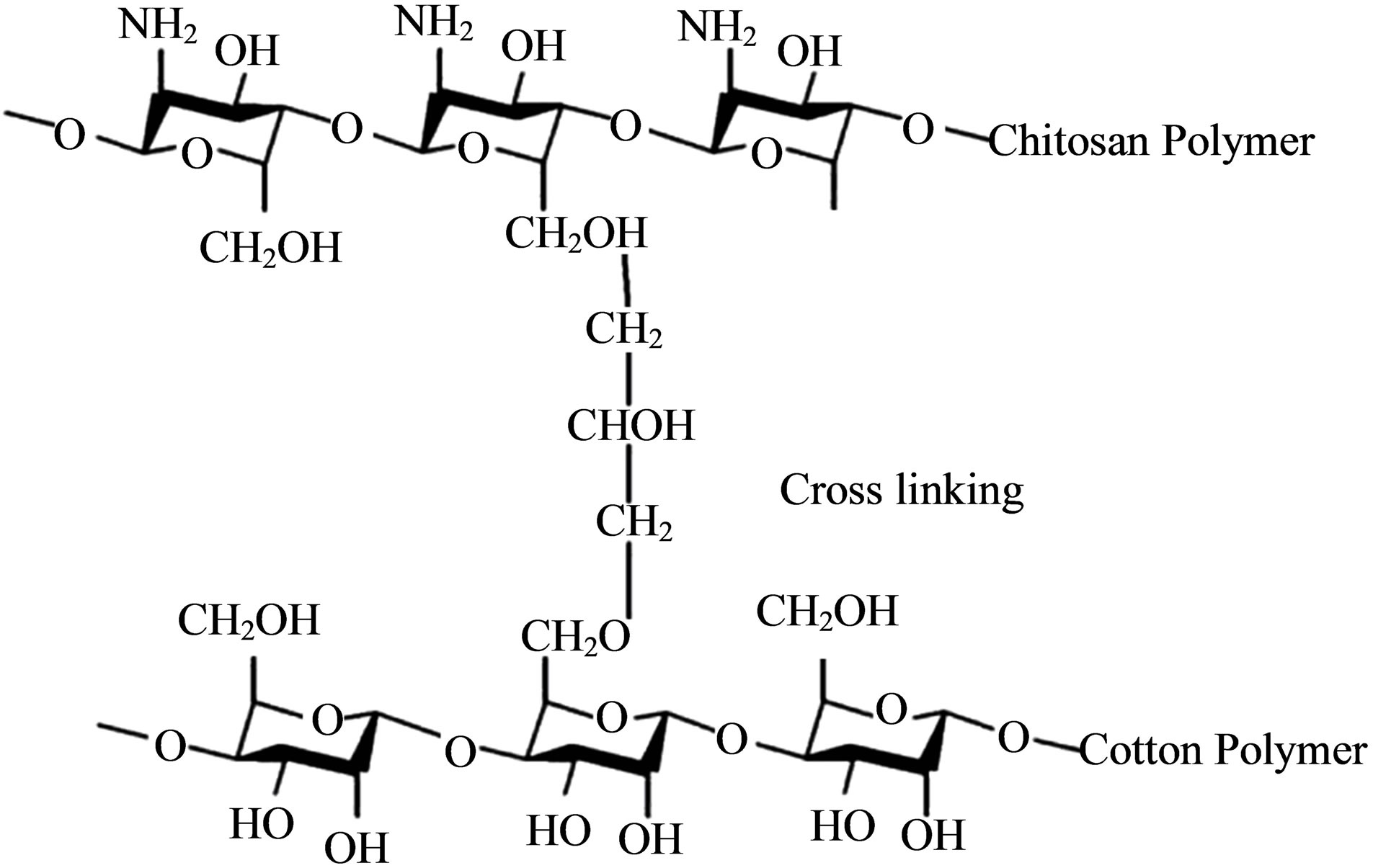
Figure 1. Cross-linking of Chitosan with Cotton fiber polymer [20].
increase the hydroxyl group in the fiber for dye absorption. Chitosan [β-(1-4)-2-amio-2-deoxy-D-glucopyranose] is a nontoxic and biodegradable [12] biopolymer, abundantly found in nature; specially in the exoskeletons of crustaceans [13,14] arthropods and mollusks [15,16] as well as the cell walls of certain fungi [13,17-19] in the form of chitin. Chitin is deacetylited in alkaline medium to obtain chitosan. Chitosan can be applied on cellulosic fiber in various methods [7]. Cellulosic fibers form a crosslink with chitosan [20], resulting positive dyesites on the fiber surface. Chitosan can easily adsorb anionic dyes such as direct, acid and reactive dyes by electrostatic attraction due to its cationic nature [10].
The application of chitosan on jute fiber offers the possibility of increasing the hydroxyl group for the formation of covalent bond with reactive dye and develop desired level of exhaustion. The current paper investigated the dyeability and color performance of chitosan treated jute fabric dyed with reactive dye and compared the results with the fabric dyed without chitosan treatment.
2. Experimental
2.1. Sample Preparation
Scoured, bleached 100% plain hessian jute fabric (261 GSM) was used in all the experiment. The fabric samples were treated with 4 g/l caustic soda solution for 20 minutes at 90˚C prior to dying and neutralized with acetic acid solution. Chitosan chips (color off white, deacetylation > 70%) were collected from the laboratory of Institute of Radiation and Polymer Technology (IRPT), Bangladesh Atomic Energy Commission, Dhaka, Bangladesh. Chitosan solution was prepared by dissolving chips in distilled water in the presence of 2% acetic acid. Then the fabric samples were treated in chitosan solution for 60 minutes at 60˚C temperature, squeezed to remove excess solution and dyed. The dyeing was carried out in Sandolab infrared lab dyeing machine (Copower Technology Ltd., Taiwan) by using a commercial reactive dye (Novacron Red FN2BL form Swiss Colours BD). Detergent (Imeron PCLF) and leveling agent (Drimagen E3R) were collected from (Clariant, Bangladesh) and used as received. Sodium hydroxide, soda ash and acetic acid were used as received and all were commercial grade. For the ease of identification, all the test fabrics were coded as shown in Table 1.
2.2. Recipe Formulation
Fabric samples were dyed with Novacron Red FN2BL at 1% (on the weight of fabric) shade. Five different concentrations of chitosan (0.5%, 1%, 2%, 3% and 4%) were used for fabric treatment. Recipes are given in Tables 2 and 3.
2.3. Dyeing Procedure
3.0 g (± 5%) fabric samples were dyed treating with chitosan and without chitosan in exhaust dyeing method. The dyeing procedure is shown in Figure 2.
2.4. Dyeing Performance Test
The color properties of dyed goods were analyzed by spectrophotometer (Data color D650). Colorfastness to washing and rubbing was measured according to ISO 105 C03 and ISO 105 X 12 [22]. Wash fastness tester (Gyrowash model no: 415/8), rubbing fastness tester (Crock meter, model no: 670) from James H Heal & Co, UK were used for the respective fastness testing.
3. Results and Discussions
3.1. Dyebath Exhaustion
Dye exhaustion may define as the leaving of a dye from
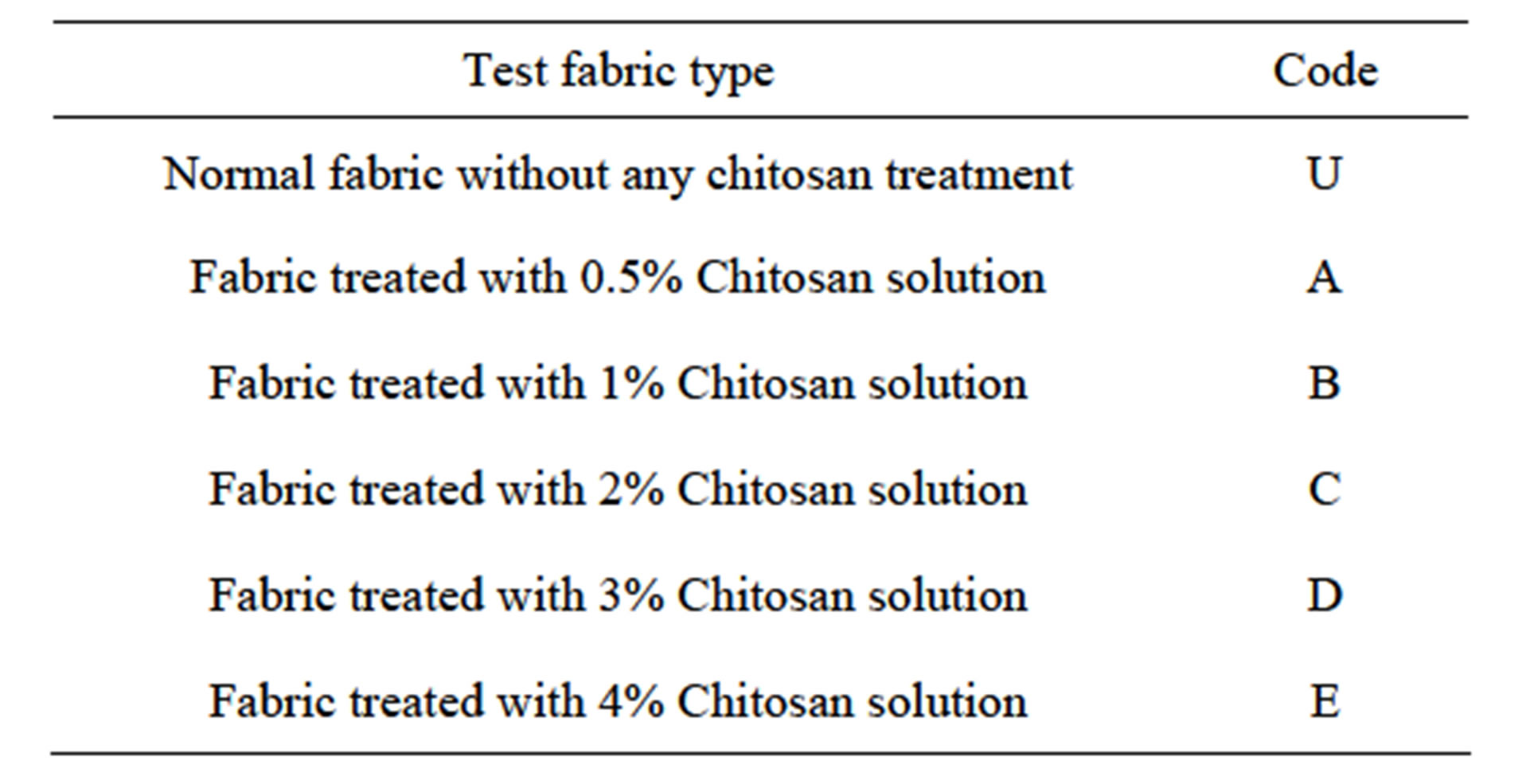
Table 1. Test fabric coding.
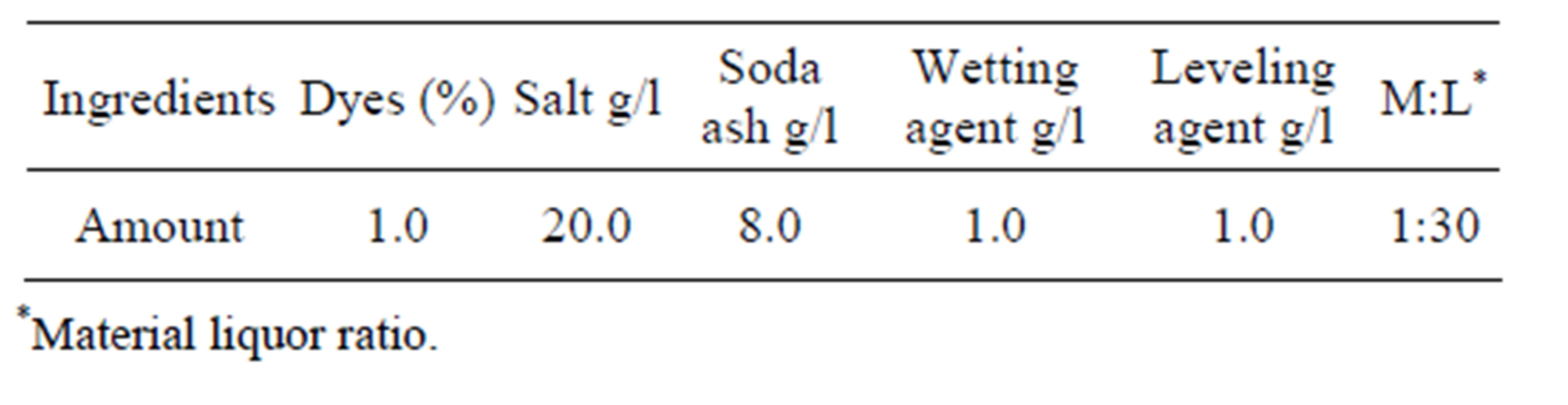
Table 2. Recipe for 1.0% (on the weight of fabric) shade without treatment the fabric with chitosan.
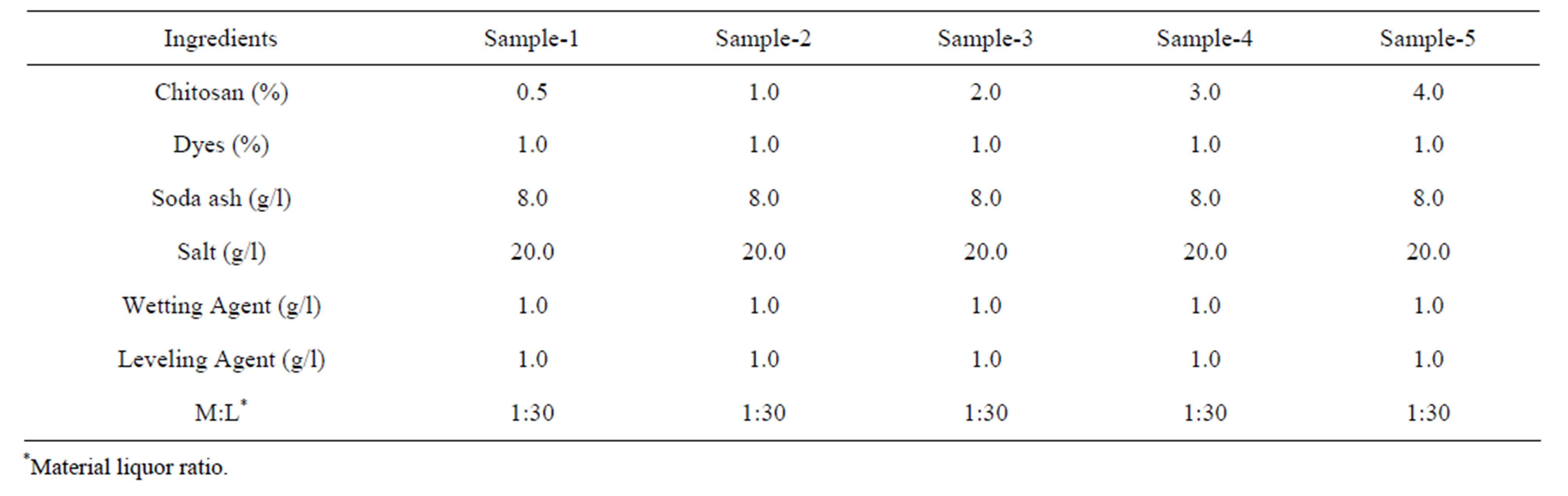
Table 3. Recipe for 1.0% (on the weight of fabric) shade treating the fabric with chitosan.
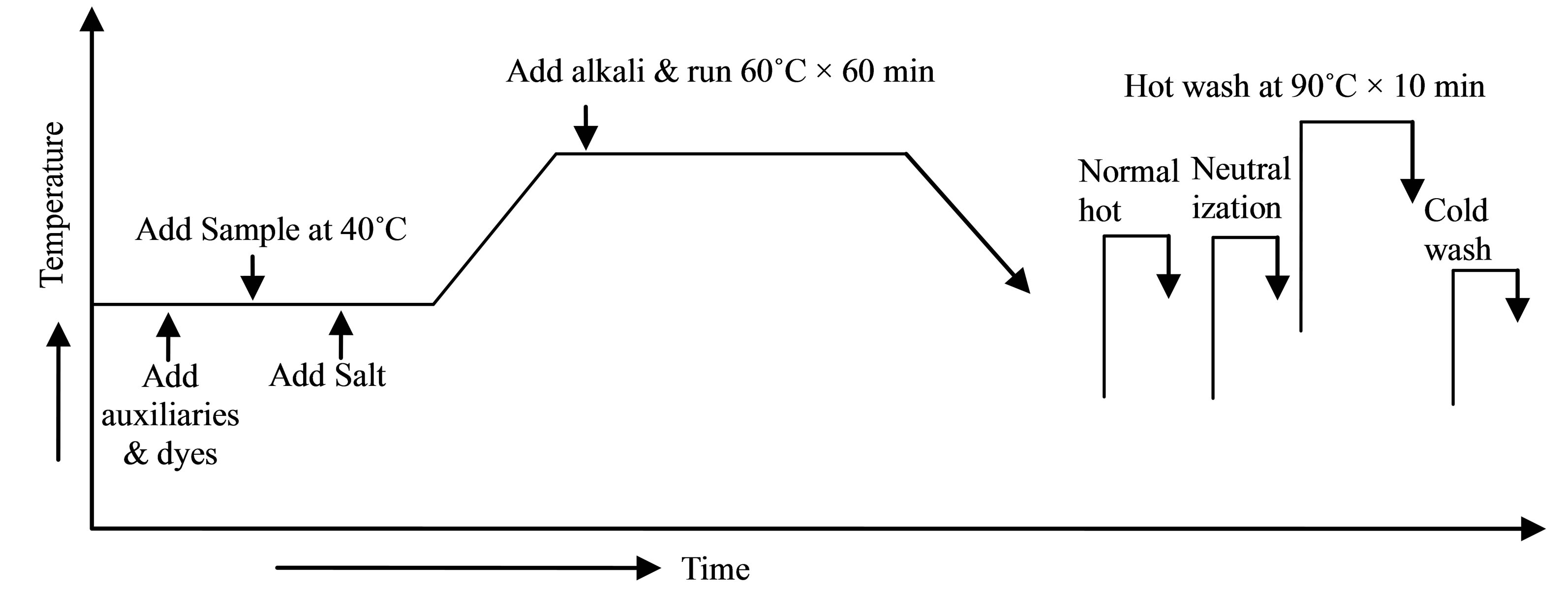
Figure 2. Dyeing scheme.
the dye bath and attachment to the fiber being dyed. For instance, 70% exhaustion would mean that 70% of the total amount of dye has attached to the fiber, and 30% is still in solution. Dyebath exhaustion can be calculated as the mass of dye taken up by the material divided by the total initial mass of dye in the bath, for a bath of constant volume [21].
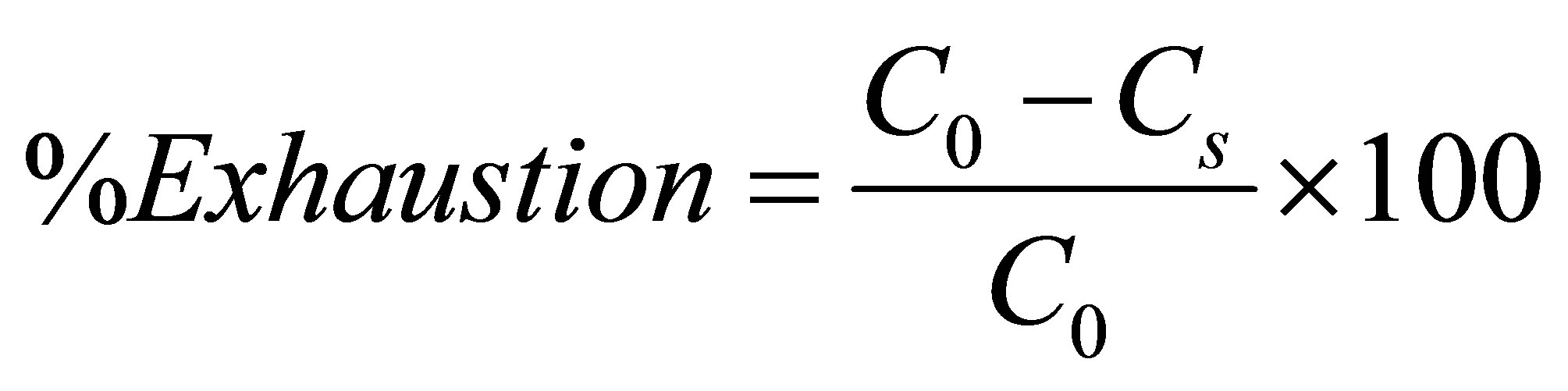 (1)
(1)
Where, C0 and Cs are the concentrations of dye in the dyebath initially and at some time during the process, respectively.
The treatment of fabric with chitosan has enhanced the dyesite in cellulose macromolecule of jute fiber. As a result, the treated fabric absorbed more dyestuff than the untreated sample and this absorption has increased the exhaustion percentage of dye in the treated fabric. The improved dyebath exhaustion has shown by the longer bar diagram in Figure 3 where all the chitosan treated fabric samples have shown longer bar than the untreated fabric sample. From the statistical analysis, it has been found that, the values of exhaustion percentages of chitosan treated fabric has fluctuated more than the untreated fabric sample, resulting bigger error bar in the bar
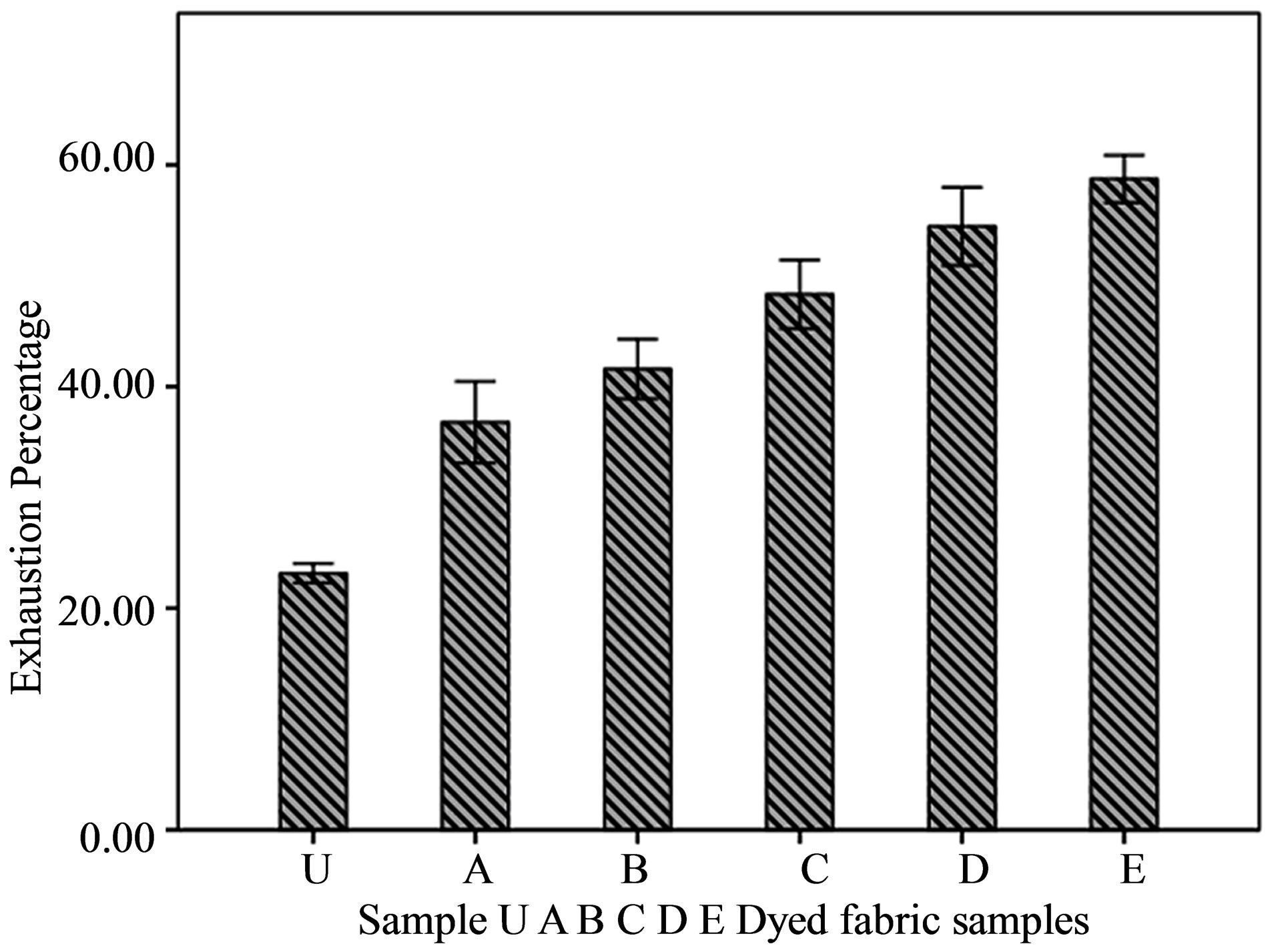
Figure 3. Dye exhaustion percentage of chitosan treated and untreated samples.
diagram. One possible reason for comparatively higher error bar in treated fabric may be the unequal chitosan absorption by different samples.
3.2. Depth of Shade
Color depth of the dyed fabrics was analyzed by measuring the K/S values of samples. Higher the value of K/S more dye will absorb in the fabric. Color measuring instrument (spectrophotometer) determines the K/S value of a given fabric through Kubelka-Munk equation as follows [21].
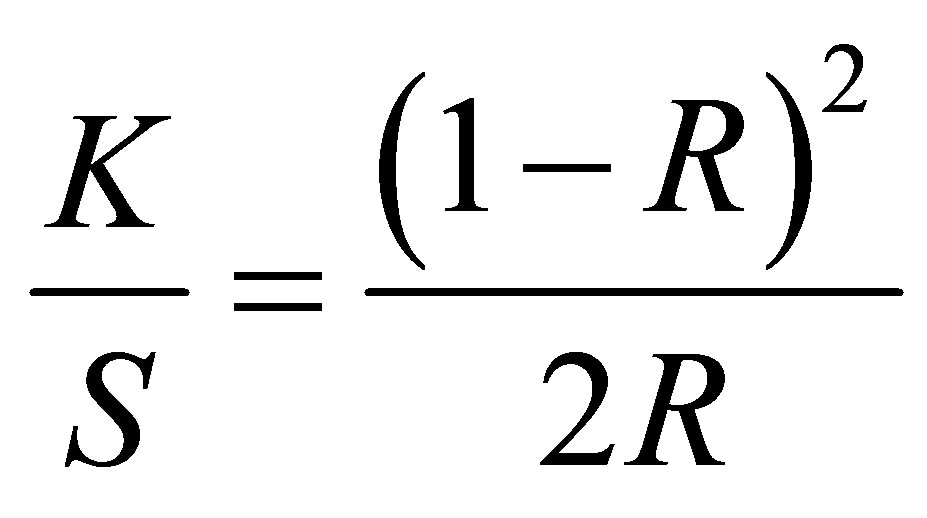 (2)
(2)
Where R = reflectance percentage, K = absorption and S = scattering of dyes. The values found for all the dyed fabrics are shown in Figures 4 & 5.
It has been found that the chitosan treated fabric samples have absorbed significantly higher amount of dyes than normal untreated fabric. The dyebath parameters such as shade %, amount of salt and alkali, dyeing time and temperature were constant for all the samples. The increased K/S value in the chitosan treated fabric indicates the presence of higher amount of the dye absorbed in the chitosan treated fabric.
3.3. Fastness Properties
The fastness to washing and rubbing of the dyed samples were also analyzed. Wash fastness had assessed for color change and color staining with respect to medium cellulosic wash [22]. Rubbing fastness was evaluated in dry and wet condition. Fastness ratings of different types of test samples are presented in the Table 4.
Dry rubbing of all type of samples have shown almost similar rating whereas wet rubbing and wash fastness to color change of treated samples represent inferior rating (U = 4/5 and D, E = 3). The staining of color has also
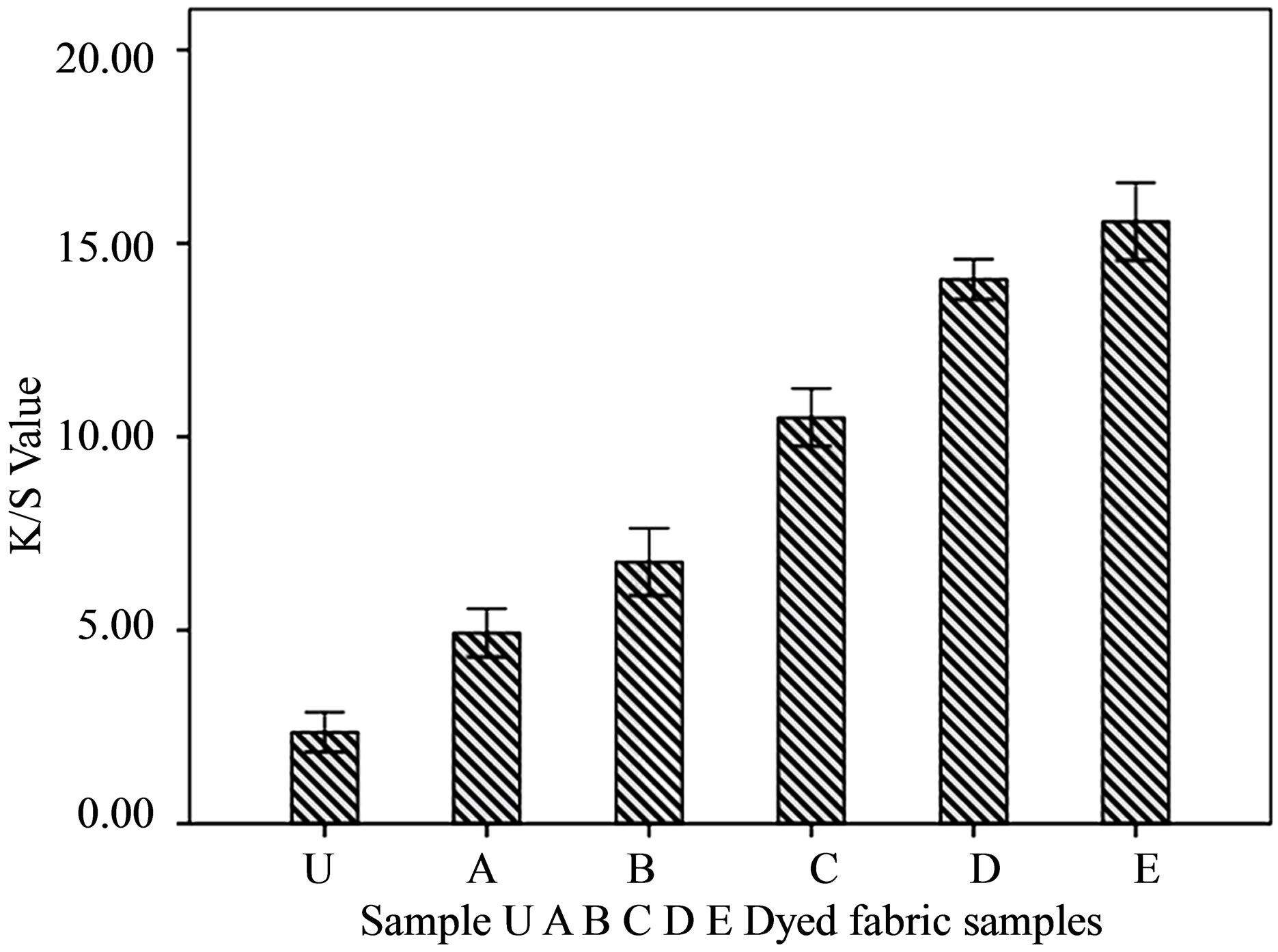
Figure 4. The gradual increment of dye absorption (K/S value) with the increment of chitosan concentration.
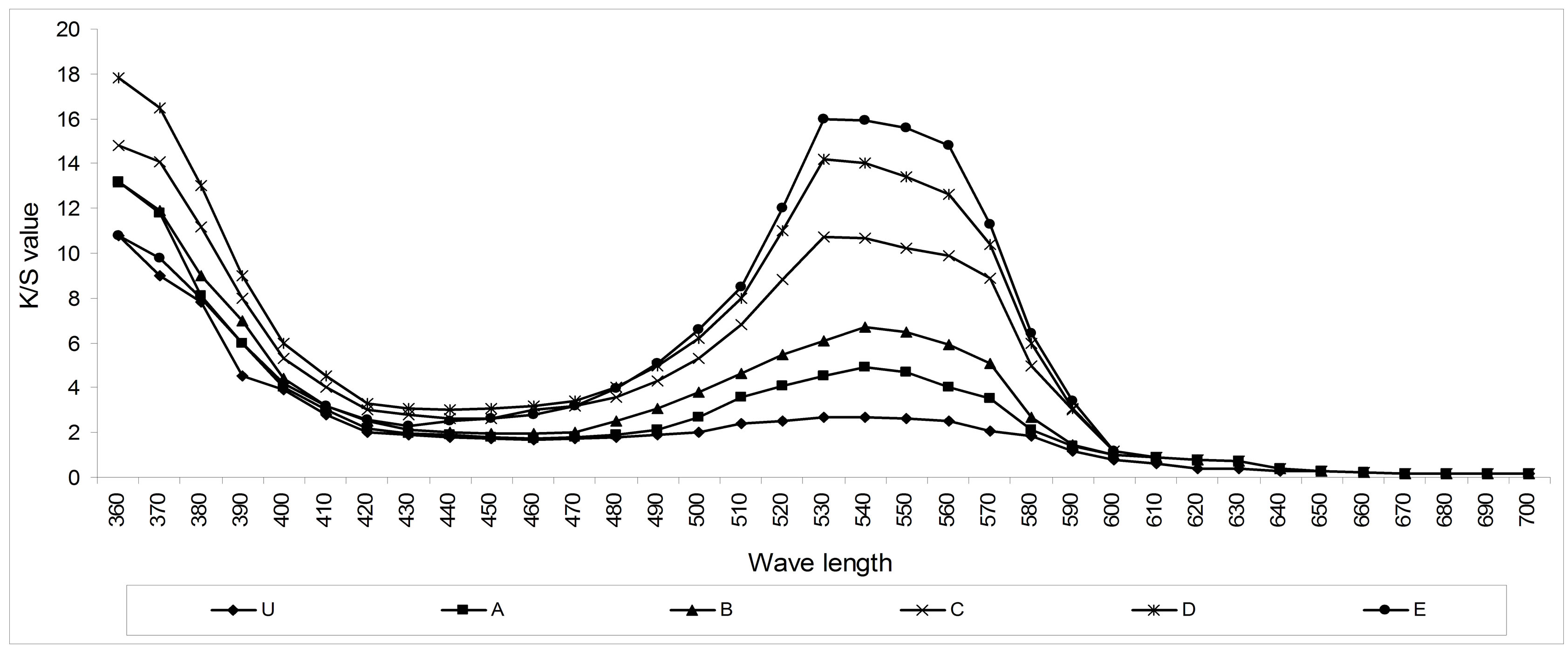
Figure 5. The dye absorption or K/S value (at λmax = 540 nm) of treated and untreated dyed samples.
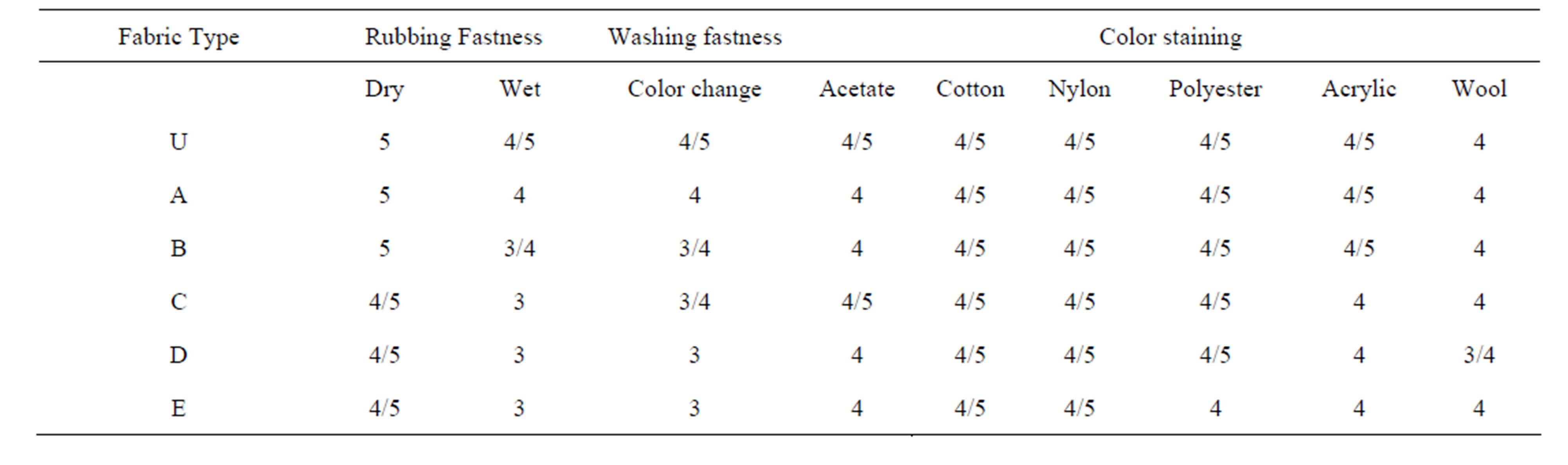
Table 4. Fastness Rating of different test fabrics after dyeing with and without Chitosan.
found lower in few chitosan treated samples (D, E) particularly in wool and acrylic fiber. In general the deeper shade shows inferior fastness than a lighter one on similar type of fabric. As discussed above, the chitosan present in the fabric enhance the dyesite causing deeper shade. Hence, as a general consequence of achieving deeper shade, the chitosan treated fabric samples have shown slightly lower fastness rating in comparison to lighter untreated fabric.
4. Conclusion
This study is intended to improve the absorption of reactive dye by jute fiber after treating with chitosan. The application of chitosan on jute fiber enhances the cationic sites for dye adsorption and also increases the hydroxyl group for fixation. Accordingly, dye exhaustion and depth of shade improve in the treated fabric compared to the untreated fabric. Though the fastness rating is lower in case of wet rubbing and washing, the overall fastness rating is still satisfactory. So the treatment of jute with chitosan can be an effective way for the coloration of jute fabric with reactive dye.
REFERENCES
- M. Lewin, “Handbook of Fiber Chemistry,” 3rd Edition, Taylor & Francis, New York, 2010.
- H. Q. Yu, M. Dang and C. W. Yu, “A Preliminary Study on Chemical Degumming of Jute and Kenaf Fibers,” Plant Fiber and Products, Vol. 25, No. 1, 2003, pp. 190- 192.
- P. Ghosh, A. K. Samanta and G. Basu, “Effect of Selective Chemical Treatments of Jute Fiber on Textile-Related Properties and Processible,” Indian Journal of Fibers &Textile Research, Vol. 29, No. 1, 2004, pp. 85-89.
- W.-M. Wang, Z.-S. Cai and J.-Y. Yu, “Study on the Chemical Modification Process of Jute Fiber,” Journal of Engineered Fibers and Fabrics, Vol. 3, No. 2, 2008, pp. 1- 11.
- E. P. G. Gohl and L. D. Vilensky, “Textile Science,” 2nd Edition, Longman Cheshire, New Delhi, 1983.
- V. A. Shenai, “Chemistry of Dyes and Principles of Dyeing,” 4th Edition, Sevak, Mumbai, Vol. 2, 1983.
- S. Houshyar and S. H. Amirshahi, “Treatment of Cotton with Chitosan and Its Effect on Dyeability with Reactive Dyes,” Iranian Polymer Journal, Vol. 11, No. 5, 2002, pp. 295-302.
- W. Wang, B. Xu, Z. Cai and J. Yu, “Study on Chemical Modification and Dyeing Properties of Jute Fiber,” Journal of the Textile Institute, Vol. 101, No. 7, 2010, pp. 613-620. http://dx.doi.org/10.1080/00405000802638651
- J. G. Cook, “Handbook of Textile Fibres: Natural Fibres,” 5th Edition, Woodhead, Cambridge, 2005. http://dx.doi.org/10.1533/9781855734852
- M. Mahbubul Bashar and M. A. Khan, “An Overview on Surface Modification of Cotton Fiber for Apparel Use,” Journal of Polymers and the Environment, Vol. 21, No. 1, 2012, pp. 1-10. http://dx.doi.org/10.1007/s10924-012-0476-8
- A. H. M. Renfrew, “Reactive Dyes for Textile Fibres,” Society of Dyers & Colourists, West Yorkshire, 1999.
- Q. Li, E. T. Dunn and E. W. Grandmaison, “Applications and Properties of Chitosan,” Journal of Bioactive and Compatible Polymers, Vol. 7, No. 4, 1992, pp. 370-397. http://dx.doi.org/10.1177/088391159200700406
- E. S. Abdou, K. S. A. Nagy and M. Z. Elsabee, “Extraction and Characterization of Chitin and Chitosan from Local Sources,” Bioresource Technology, Vol. 99, No. 5, 2008, pp. 1359-1367. http://dx.doi.org/10.1016/j.biortech.2007.01.051
- F. A. Sagheer, M. A. Al-Sughayer and S. Muslim, “Extraction and Characterization of Chitin and Chitosan from Marine Sources in Arabian Gulf,” Carbohydrate Polymers, Vol. 77, No. 2, 2009, pp. 410-419. http://dx.doi.org/10.1016/j.carbpol.2009.01.032
- K. V. Harish Prashanth, F. Kittur and R. Tharanathan, “Solid State Structure of Chitosan Prepared under Different N-Deacetylating Conditions,” Carbohydrate Polymers, Vol. 50, No. 1, 2002, pp. 27-33. http://dx.doi.org/10.1016/S0144-8617(01)00371-X
- C. Peniche and W. Argüelles-Mona, “Chitin and Chitosan: Major Sources, Properties and Applications,” Monomers, Polymers and Composites from Renewable Resources, Oxford, 2008, p. 517.
- J. Kučera, “Fungal Mycelium—The Source of Chitosan for Chromatography,” Journal of Chromatography B, Vol. 808, No.1, 2004, pp. 69-73. http://dx.doi.org/10.1016/j.jchromb.2004.05.023
- S Chatterjee, M. Adhya, A. K. Guha and B. P. Chatterjee, “Chitosan from Mucor rouxii : Production and Physico-Chemical Characterization,” Process Biochemistry, Vol. 40, No. 1, 2005, pp. 395-400. http://dx.doi.org/10.1016/j.procbio.2004.01.025
- W. Suntornsuk, P. Pochanavanich and L. Suntornsuk, “Fungal Chitosan Production on Food Processing By-Products,” Process Biochemistry, Vol. 37, No. 7, 2002, pp. 727-729. http://dx.doi.org/10.1016/S0032-9592(01)00265-5
- S. H. Lee, M. J. Kim and H. Park, “Characteristics of Cotton Fabrics Treated with Epichlorohydrin and Chitosan,” Journal of Applied Polymer Science, Vol. 117, No. 2, 2010, pp. 623-628. http://dx.doi.org/10.1002/app.31351
- A. D. Broadbent, “Basic Principles of Textile Coloration,” Society of Dyers and Colourists, West Yorkshire, 2001.
- B. P. Saville, “Physical Testing of Textiles,” Woodhead Publishing Limited, Cambridge, 1999. http://dx.doi.org/10.1533/9781845690151
NOTES
*Corresponding author.

